Pt-Ni core–shell structure with Pt-skin and electronic effect on catalytic performance
Chong Yao, Dandan Ma, Jie Luo, Yixin Chen, Min Tian, Haoxuan Xie, Chunshan Lu, Feng Feng,Xiaoliang Xu, Qingtao Wang, Qunfeng Zhang, Xiaonian Li
State Key Laboratory Breeding Base of Green Chemistry Synthesis Technology, College of Chemical Engineering, Zhejiang University of Technology, Hangzhou 310014, China
Keywords: Nitrobenzene p-Aminophenol Pt-Ni/C bimetallic catalyst Electronic effect Pt(5d)-Ni(3d) coupling
ABSTRACT In order to improve the catalytic performance of the nitrobenzene hydrogenation rearrangement to prepare p-aminophenol,a bimetallic Pt-Ni/C(PNC)catalyst was synthesized.Taking advantage of the synergistic effect of Ni and Pt to enhance product selectivity and catalytic performance stability,the electrons in Ni are moved to Pt by the electron effect,which affects the catalyst’s ability to activate H2 as well as the amount of hydrogen activated.Furthermore,due to the strong Pt(5d)-Ni(3d)coupling effect,Ni can effectively maintain Pt stability in the acidic system and reduce Pt dissolution.The stability of the PNC can be found to be greatly enhanced compared to the Pt/C(PC)catalyst,and p-aminophenol selectivity is greatly enhanced, showing excellent catalytic performance.
1. Introduction
p-Aminophenol (PAP) is a major organic intermediate, and it is widely used in medicine, dyestuff, and as an anti-ageing agent for rubber [1,2]. In the traditional process, PAP is prepared by reduction ofp-chloronitrobenzene andp-nitrophenol with iron powder in several steps [3,4]. However, it is difficult to control the rate of reaction reduction using this method, and will produce a large quantity of iron sludge and waste water, leading to severe pollution of the environment [5,6]. Therefore, we prepared PAP by a one-step reduction of nitrobenzene,primarily using a Pt-based catalyst for hydrogenation and using sulphuric acid as a catalyst for Bamberger rearrangement reaction, and the reaction system is a four phase system.In addition, Pt based catalysts have the following two limitations in the system:the selectivity ofp-aminophenol is not high, which may be due to the Pt-based catalysts having a high capacity to activate hydrogen and a high adsorption of phenylhydroxylamine (PHA) to the intermediate, leading to the easy formation of aniline [4,7–10]. Pt-based catalysts are prone to Pt loss in the sulfuric acid system, leading to a decrease in the stability of the catalyst.
The bimetallic Pt-M (M = Ni, Fe, Co, Ru,etc.) catalysts, which have been employed in numerous catalytic reactions, have attracted a great deal of attention because of their synergistic effects, which could improve the performance of catalysts, such as Pt-Ni [11–14], Pt-Fe [15–17], Pt-Co [18–20], Pt-Ru [21,22], and other catalysts. Gonget al. [11] synthesized a unique Pt-Ni crossdouble dumbbell type of Pt–Ni nanostructures with increased catalytic activity for oxygen reduction reactions (ORR) and methanol oxidation reactions (MOR). They attributed the excellent performance of electrooxidation to the synergistic interaction between the Pt atoms and Ni atoms. Tianet al. [12] designed onedimensional clustered nanocages of Pt-Ni alloys with Pt-Skin structure for the oxygen reduction reaction,which exhibited high quality and high specific activity . The influence of Pt-Ni on hydrogen adsorption and hydrogenation of cyclohexene was explored by Humbertet al. [13] through the possibility of replacing the bulk Pt in the Pt-Ni-Pt (block) structure by anchoring Pt-Ni on the WC substrate. The influence of octahedral PtxNi1–xnanocrystals was investigated by Liet al. [14], models of truncated octahedra and tetrahedra on the catalytic performance of the heterogeneous hydrogenation reaction.
On the basis of improved product selectivity and catalyst stability, a Pt-Ni/C bimetallic (PNC) catalyst with a Pt-skin was synthesized by wet impregnation method. The synthesis path way and catalyst model are shown in Fig. 1(a). The dissolved Ni content after sulfuric acid treatment is shown in Fig. 1(b), and no increase in the Ni content of the solution is observed after 0.5 h. Coprecipitation of Pt-Ni was initially formed by the metal precursor under the precipitation and complexation of the ammonia. It is then reduced under the action of hydrazine hydrate, complexed and reprecipitated, and filtered and dried to form precursor Pt-Ni bimetallic catalyst(PPNC)with a Ni enriched surface.PPNC formed a Pt-Ni bimetallic catalyst with Pt-skin after treatment with 17%sulphuric acid (nPt:nNi=24:1, Fig. 1(c)). Improvements in catalyst performance can be caused by the following two parts: Firstly,the Pt-skin formed due to the change in surface atomic morphology, the catalyst surface formed wrinkles and protrusions [23–25]; The second possibility is that Pt(5D)-Ni(3D) strong coupling effect is formed between Pt and Ni,and the electron effect between Pt and Ni affects the performance of the catalyst[26–28].Our work is primarily concerned with the effect of electronic effects between Pt and Ni on the catalytic.
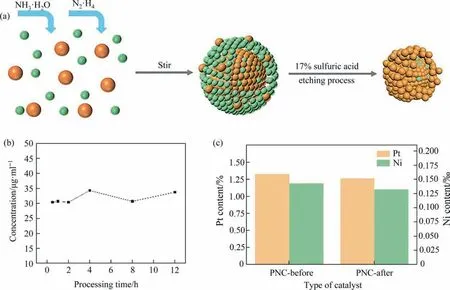
Fig. 1. Catalyst preparation process and metal content. (a) Catalyst wet impregnation method process. Atomic absorption spectrometer (AAS) results of (b) relationship between sulfuric acid treatment time of PPNC and Ni content (c) metal content before and after PNC reaction.
Ni is chosen as the second metal modifier as other metals (Ru,Cu, Co, La,etc.) are found to be less selective to aminophenol than the modified Ni catalyst Pt(as shown in the Supplementary Material Table S3).The inclusion of Ni in XRD results in a decrease in the lattice parameters of Pt, the change in the crystalline phase of Pt brought about by the addition of a second metal [29], the diffraction peak of Pt face-centred cubic (fcc) crystalline phase at 39.8°,and the addition of Ni leads to the formation of an fcc Pt phase with a change in lattice constant and a shift of the diffraction peak towards a higher angle [30,31].
2. Experimental
2.1. Chemicals
Reagents used in the synthesis of catalysts include chlorplatinic acid hexahydrate (H2PtCl6∙6H2O, Pt ≥37.5%, Shaanxi Rock New Materials Co., Ltd.), nickel nitrate hexahydrate (Ni(NO3)3∙6H2O,98%, Aladdin), ammonia (NH3∙H2O, 25%–28%, Sinopharm), hydrazine hydrate(N2H4,80%in water,General),activated carbon(powder, Macklin). Reagents required for catalyst evaluation include nitrobenzene (C6H5NO2, 99%, Macklin), sulfuric acid (H2SO4,95%-98%, Sinopharm), cetyltrimethyl ammonium chloride (CTAC,97%, Macklin),p-aminophenol (C6H7NO, ≥98%, HPLC, Macklin),aniline (C6H7N, ≥99.9%, GC, Sinopharm), 4,4′-diaminodiphenyl ether (C12H12N2O, 98%, Macklin),o-aminophenol (C6H7NO, 98%,Sinopharm),nitric acid(HNO3,65%-68%,Sinopharm),hydrochloric acid(HCl,36%–38%,General),Commercial Pt/C catalyst(PC,Sigma-Aldrich).
2.2. Catalyst preparation
Pt-Ni /C bimetallic catalyst was prepared by wet impregnation method according to the following process. First, 2.80 ml H2PtCl6aqueous solution (H2PtCl6∙6H2O concentration 0.03 g∙ml-1) and 1.30 ml Ni(NO3)3aqueous solution (Ni(NO3)3∙6H2O concentration 0.06 g∙ml-1) were added to 30 ml deionized water and stirred and premixed for 1 h. Add 1 g activated carbon and stir for 4 h.Then add ammonia water until pH = 9, and stir thoroughly for 2 h.Then add hydrazine hydrate 400 μl,and stir for 6 h.Finally,filter,rinse with a large amount of deionized water until the filtrate is neutral, and vacuum dry at 60 °C for 8 h to obtain PPNC. The prepared PPNC was treated with 100 ml of 17% sulfuric acid solution for 24 h, and the treated catalyst was filtered, washed, and dried under vacuum at 60 °C for 8 h to obtain PNC.
The Ni/C (NC) catalyst was prepared in the same way as described above for PNC, using 1.30 ml of Ni(NO3)3aqueous solution (Ni(NO3)3∙6H2O concentration 0.06 g∙ml-1) for the metal precursor, and without 17% sulfuric acid treatment.
2.3. Catalyst characterizations
Transmission electron microscopy(TEM)Tecnai G2 F30 S-Twin uses 300KM acceleration voltage and Oxford EDS Xplore80. The X-ray diffraction spectra(XRD)of the samples were obtained using the Empyrean instrument from PNAlytical company in the Netherlands. Cu Kα (0.1541 nm) was used as the X-ray source. The operating voltage was 45 kV and the operating current was 40 mA. Xray photoelectron spectroscopy(XPS)using the Shimadzu KRATOS AXIS Ultra DLD instrument,can use monochromatic Al target X-ray source and dual anode Al/Mg target X-ray source. It can provide chemical information about the surface and interface of samples from 1 to 10 nm and semi-quantitative analysis of relative element content (accuracy is about 10%).
Argon ion sputtering XPS was tested by X-ray photoelectron spectrometer (Thermo Fischer, ESCALAB Xi +, USA ), vacuum of the analytical chamber 8 × l0–10Pa, excitation source using Al Kα ray (hv = 1486.6 eV), working voltage of 12.5 kV, filament current of 16 mA, test pass energy of 100 eV, narrow spectrum of 20 eV,step length of 0.05 eV, stopping time 40–50 ms. The sample was thinned by etching with an argon ion gun with an etching speckle size of 1.5 mm and an etching voltage of 300 eV. There are always two etchings, each 2 nm every time.
The H2temperature programmed desorption was conducted by PCA-1200 chemisorption instrument of Builder company, and the QGA quantitative gas analysis mass spectrometer (H2-TPD-MS) of HAPR0128 of Hiden Company was used as the detector. The catalyst was vacuum-dried at 110°C for 6 h before H2-TPD-MS.Firstly,H2is adsorbed at 50 °C in 10% H2/Ar atmosphere for 40 min, and the flow rate is 30 ml∙min-1(the following flow rate is 30 ml∙min-1).Subsequently,temperature-programmed desorption was carried out. At room temperature, He purging was used for 30 min to remove the H2in the physical adsorption state.The baseline of MS was leveled and the temperature was raised from room temperature to 600 °C at a rate of 10 °C∙min-1.
Elemental content was determined by atomic absorption spectrometer (AAS) using PERSEE’s flame graphite furnace integrated TAS-990AFG atomic absorption spectrophotometer. The sample should be treated with aqua regia (volume ratio,VHCl:VHNO3=3:1)before testing. Take 20 mg catalyst and place it in 2 ml aqua regia for ultrasonic treatment for 0.5 h,then stand for 24 h.Take 1 ml of the standing solution and dilute to 50 ml with deionized water.The Pt and Ni loadings of the catalysts are shown in the Supplementary Material Table S1.
2.4. Catalytic tests
Evaluation of catalysts selective hydrogenation of nitrobenzene was carried out in a 250 ml Hastelloy reactor. 126 ml deionized water, 14 ml 98% concentrated sulfuric acid (initial sulfuric acid mass concentration is 17%), 0.25 g CTAC, 0.4 g catalyst, 25 g nitrobenzene were added in the reactor in sequence. The reaction conditions were controlled as follows: temperature 75 °C, hydrogen pressure 1.0 MPa, speed 1000 r∙min-1. High performance liquid chromatography (HPLC) was used for quantitative analysis.Agilent 1260 Infinity TYPE ii chromatography was used for HPLC.The chromatographic column was Eclipse XDB-C18. The main and by-products, includingp-aminophenol, aniline, 4,4′-diaminodiphenyl ether,o-aminophenol,and the reactant nitrobenzene, were quantified by external standard method.
The applied catalyst needs to be washed with deionized water for several times after the reaction and then vacuum dried at 80°C for 5 h.
3. Results and Discussion
3.1. Structural and morphological analysis
Analysis of catalyst morphology and structure was carried out using electron transmission microscopy (TEM). Fig. 2(a) showed the Pt-Ni catalyst alloy particles.The catalyst particle size was primarily distributed between 2.5–4.5 nm, with a mean particle size of approximately 3.8 nm (Fig. 2(d)). The lattice of PNC alloy is shown in Fig. 2(b), corresponding to the crystal faces of the PNC alloy,this corresponds to a spacing of 0.213 nm between the faces of the crystal(viaFFT of Fig.2(b),Fig.2(c)),compared to the(1 1 1)plane of Pt and the(1 0 1)plane of Ni(the crystal plane spacing is 0.225 nm and 0.201 nm). Compared with the (1 1 1) crystal plane spacing of Pt,the decrease indicates that the infiltration of Ni in the lattice of Pt makes the cell parameters of Pt smaller,so that the lattice spacing of Pt becomes smaller. Fig. 2(e) and 2(f) are the EDS surface scan and line scan spectra respectively. Based on the two spectra, it can be analyzed that the active Pt metal and the auxiliary Ni metal form a Pt-rich shell as well as the Pt-Ni alloy core–shell structure (core–shell structure as shown in Fig. 1(a)).
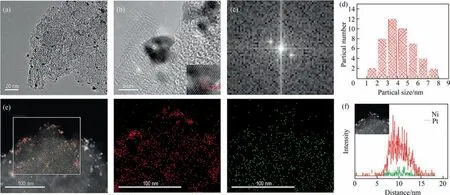
Fig. 2. Morphology and structure of PNC. (a) TEM. (b) HRTEM. (c) FFT image. (d) Particle size statistics for (a). (e) HAADF-STEM image and EDS mapping of Pt (red) and Ni(green). (f) EDS line scan mapping of Pt (red) and Ni (green).
Fig. 3(a) is a schematic of the XRD, through which the crystalline plane of the PNC was determined and the shape of the alloy formed by the bimetallic catalyst was indicated. PC does not have an obvious Pt diffraction peak due to its large dispersion. Diffraction peaks from the Pt (1 1 1) plane are observed at 40.16° in the PNC, and the Pt (1 1 1) plane at 39.76° in the standard PDF maps(PDF #70-2057). Compared with the standard diffraction peak of Pt in PNC, the diffraction peak of Pt shifts to a higher angle about 0.4°, indicating that there is a phenomenon of smaller lattice constant.It may be that the lattice parameters of Pt are reduced due to the Pt-Ni bond formed by the incorporation of Ni into the lattice of Pt. The XRD results are further supported by the smaller Pt lattice spacing in Fig. 2(b). XRD spectrum of PNC catalyst does not show Ni diffraction peak,there is not a single Ni crystal in the bimetallic catalysts, which exhibits the infiltration of metallic Ni into the Pt lattice. When combined with the results in Fig. 1(b) and Fig. 2(e)it can be seen that Ni is present in the bulk phase of the metallic particles.XRD spectrum analysis of Ni/C(NC)and activated carbon(Fig. S1) shows that there are disordered carbon peaks in the activated carbon that lie between 20°–30°and 42°–50°,and the major XRD diffraction peak of the Ni is coincident with that of the activated carbon between the 40°–45°, so Ni might not be present because of the large dispersion [32].
The XPS spectrum of Pt for the PNC catalyst is presented in Fig.3(b), with peaks 4f5/2and 4f7/2corresponding to 72.30 eV and 75.65 eV for Pt0(blue) and peaks 4f5/2and 4f7/2of 74.49 eV and 77.84 eV for Pt2+(pink). The PC peaks for Pt0(blue) are 72.59 eV and 75.94 eV, while the peaks for Pt2+(pink) are 74.88 eV and 78.23 eV for 4f5/2and 4f7/2. In the case of Pt in PNC, the peak is shifted by 0.29 eV toward the lower binding energy compared to PC indicating that Pt exhibits an electron gain in the bimetallic structure. The XPS wide spectra of PNC (Fig. 3(d)) display only the Pt metal peaks (Fig. S2(a) also demonstrates the absence of Ni),while PPNC(Fig.3(e))shows the two peaks of Pt and Ni metal,indicating that Ni is no longer present on the surface of the catalyst. The XPS spectra of PPNC (Fig. S2(b)) shows Ni 3p peak at 68.41 eV, 4f5/2peak and 4f7/2peak at 72.28 eV and 75.63 eV value for Pt0,and the 4f5/2and 4f7/2peaks at 75.24 eV and 78.59 eV in the case of Pt2+(pink). Furthermore, the presence of the Ni 3p peak in PPNC and its disappearance in PNC demonstrates that Ni is predominantly present in the bulk phase of the metal particles,forming the core layer structure of Pt-skin.The 2p3/2and 2p1/2of Ni0in PPNC (Fig. 3(c)) are found to be 856.17 eV and 873.57 eV, respectively, whereas the 2p3/2and 2p1/2of Ni0in the NC (Fig. 3(d)) is 855.95 eV and 873.35 eV, respectively, and PPNC exhibits a loss of electrons compared to NC. The wide spectra of PNC and PPNC(Fig. 3(d) and (e)) can demonstrate that following treatment with sulfuric acid, the Ni that had collected on the surface of the metal particles in the PNC was completely leached out and it was not possible to detect the presence of Ni using XPS. The presence of metallic Ni can be determined by AAS (Fig. 1(c)) and TEM (Fig. 2(e)) and it is therefore concluded that Ni in PNC is mainly present in the core of the metal,forming a shell on the surface in the form of Pt-Skin.The presence of a core shell structure of the catalyst can be shown by the results of the Ar ions Sputtering XPS(Fig.4(a)and(b)). The catalyst is etched using argon ions for 2 nm that etches a total of two. Comparison of the elemental composition of the etched catalyst surface showed that Ni increased after etching,and as etching the Ni relative proportion is increased (Fig. 4(a)).So it can be judged that the Ni was mostly concentrated inside the metal nanoparticles. The Ar ions Sputtering XPS of PNC peaks for Pt0(blue) are 72.23 eV and 75.58 eV (primary), 72.21 eV and 75.56 eV (First etching), and 72.15 eV and 75.50 eV (Second etching),the peak position is slightly shifted after second etching(Fig.4(b)). The 2p3/2and 2p1/2of Ni0in PNC (Fig. 4(a)) are found to 856.22 eV and 873.62 eV, compared with Ni0in the NC (Fig. 3(d)), the peak is shifted by 0.27 eV toward the higher binding energy.
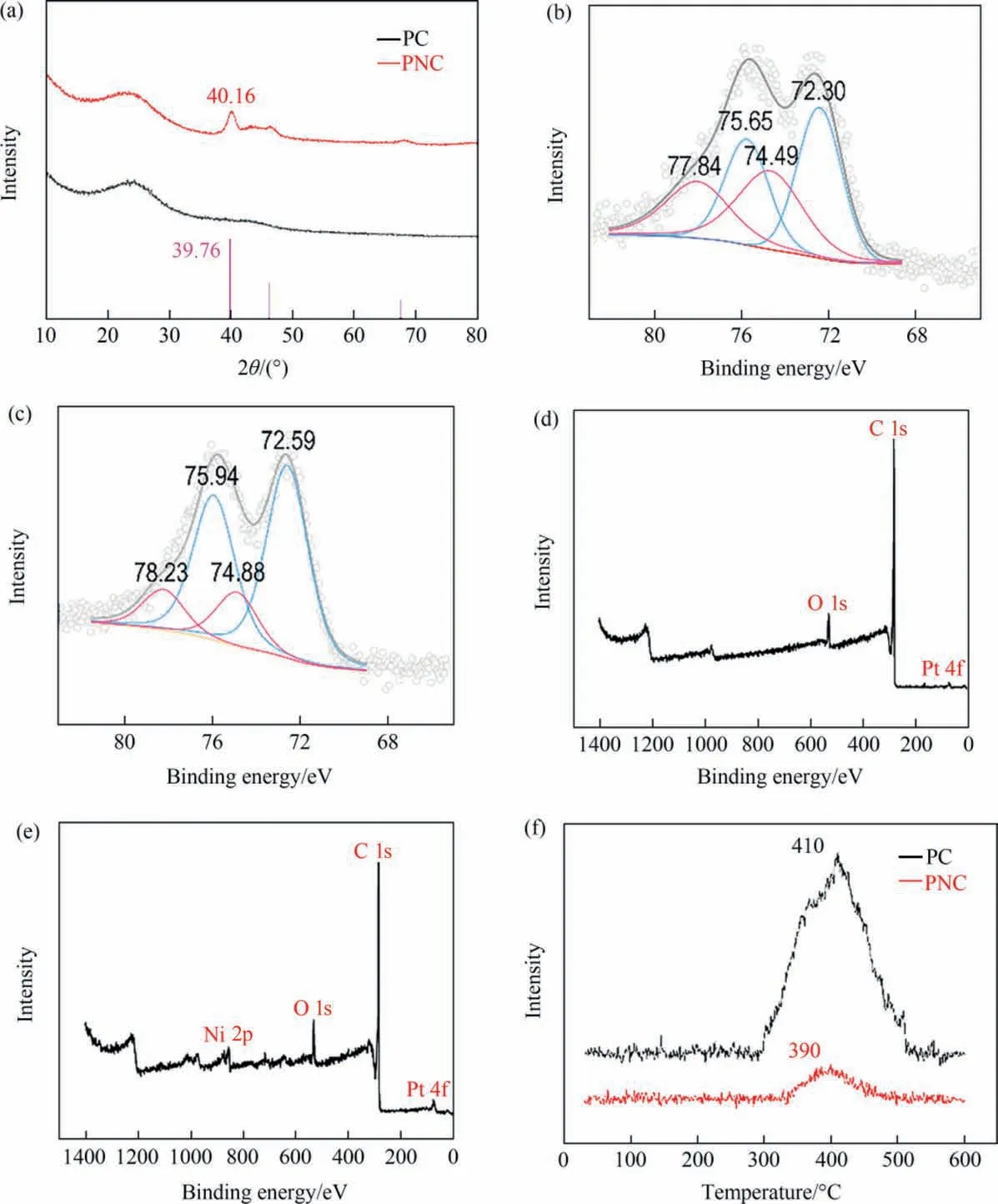
Fig.3. Characterization of PNC and PC:(a)XRD spectra of PNC(red)and PC(black);Pt 4f XPS spectra of(b)PNC and(c)PC;XPS wide spectra of(d)PNC and(e)PPNC;(f)H2-TPD-MS spectra of PNC (red) and PC (black).
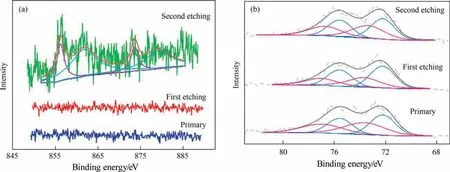
Fig. 4. Ar ions Sputtering XPS of PNC (a) Ni (b) Pt. And each etching is 2 nm for a total etching of 4 nm.
Fig.3(d)shows the results of H2-TPD-MS which showed that the hydrogen desorption peak of the PC catalyst was at 410°C,and that of PNC catalyst was at 390 °C, indicating that the adsorption strength of PNC for hydrogen was weakened. By comparing peak intensity and peak area for both catalysts,the amount of H2dissociation of the PC catalyst can be found to be much higher than that of the PNC,which is also why the PC catalyst is more likely to generate aniline as a by-product during the course of the reaction.The XPS and H2-TPD-MS results show that Pt has an enhanced ability to activate H2after gaining electrons from Ni and is capable of activating H2at a lower temperature, but the doping of Ni increases the particle size of the catalyst (Fig. S3) and reduces the catalyst active sites, thereby reducing the amount of hydrogen PNC adsorbed in the H2-TPD-MS results[33,34].Therefore,the maintenance of moderate hydrogen adsorption strength of the PNC catalyst is conducive to the improvement of PAP selectivity.We do not believe,of course,that the lower the strength of hydrogen adsorption,since in the reaction process the rate of diffusion of the intermediate PHA is closely related to the instantaneous concentration[8].In this reaction,hydrogenation is the decisive step,and the dissociation of hydrogen directly determines the instantaneous concentration of PHA [35]. If the instantaneous PHA concentration is too low, diffusion will be impeded. The adsorption state of the PHA intermediate is affected by a change in the electronic state of the Pt active center,which leads to a change in product selectivity [36].
3.2. Hydrogenation performance and stability
The prepared PNC catalysts are of great importance in the hydrogenation of nitrobenzene intop-aminophenol. In order to compare the performance of the PC catalyst and the bimetal PNC catalyst in this reaction. Fig. 5(a) and (b) show that the PNC catalyst has a large improvement in the selectivity ofp-aminophenol compared to the PC catalyst. PC had a significantly higher activity than PNC, which also proved that the PC catalyst had a greater capacity for hydrogen dissociation in the reaction. This may be due to the lower amount of dissociated hydrogen from Pt due to the doping of Ni, and can alter the adsorption state of the PHA,leading to a change in its adsorption energy, which may cause PHA to fall away from the active center more rapidly and participate in Bamberger rearrangement,thereby improving the selectivity ofp-aminophenol[7].Fig.5(a)shows that 25 cycles to find were performed on the bimetallic catalyst, which in the rate of reaction and conversion, selectivity and performance in a stable range, the catalyst after numerous times of application in the sulphuric acid system still maintains good stability. As can be seen in Fig. 6(a)and (b), the TEM and EDS of the PNC after 25 repeats resulted in the lattice spacing of 0.213 nm, which was the same as that prior to the reaction. XRD characterization (as shown in Fig. 6(c)) of the cycle catalyst found that the XRD diffraction peak did not change after the catalyst was used 5 times and 25 times,indicating that the cycling catalyst maintained the same crystalline shape as the original catalyst. In the sulphuric acid system, corrosion of Pt by sulphuric acid is slowed after Pt and Ni form an alloy. Ni can effectively improve the resistance of Pt in sulfuric acid, so the stability of PNC catalyst can prove that the content of metal Pt and Ni basically remain unchanged before and after the reaction.Once the metal content has been detected across the AAS(as shown in Fig.6(d)),it was found that the content of metal Pt in PNC changed little after 25 times of application,although the PC loading was found to decrease significantly after 4 application times. And it was found that the Pt and Ni contents did not change significantly both before and after the PNC reaction(as shown in Fig.1(c))such that the PNC catalyst exhibited improved stability.The increased stability of the catalyst in the reaction system is a result of the Pt(5d)-Ni(3d)coupling, which reduces the dissolution of Pt into the sulfuric acid.Analysis of the XPS spectra of the PNC of after cycles reaction revealed that both the 4f5/2and 4f7/2peaks of Pt0(Fig. S4(a)) are 72.31 eV and 75.66 eV, and the peaks at 4f5/2and 4f7/2of Pt2+are 73.89 eV and 77.24 eV, respectively, which is consistent with the peak positions of the XPS spectra of Pt of PNC (Fig. 3(b)). The NC catalysts (Fig. S5) was also assessed, and the activity of the NC was very low compared to the PNC and the selectivity of conversion to NB and PAP was extremely low (Supplementary Material Table S2), losing activity after 2 h of reaction.
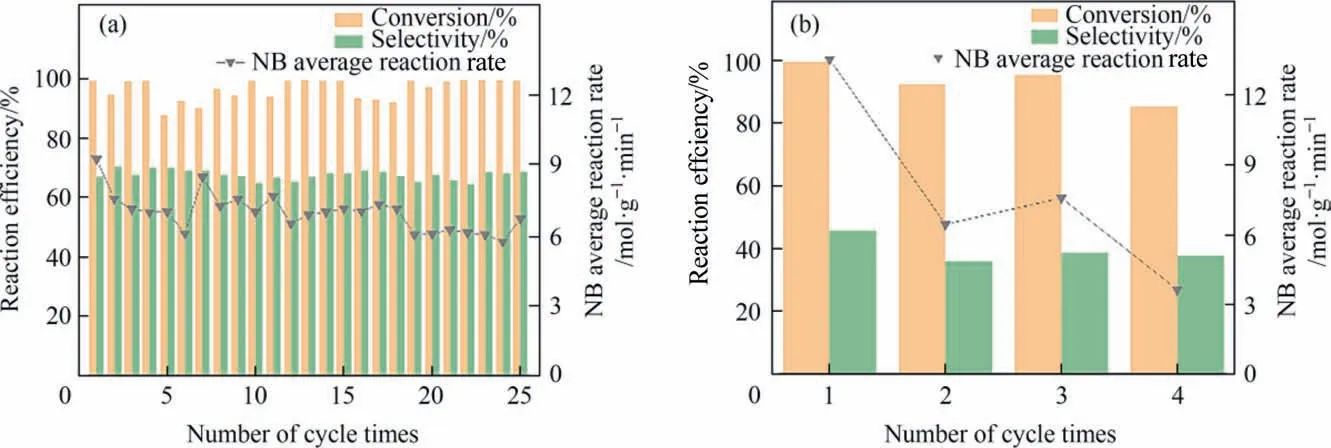
Fig. 5. Catalyst performance evaluation (a) PNC. (b) PC.
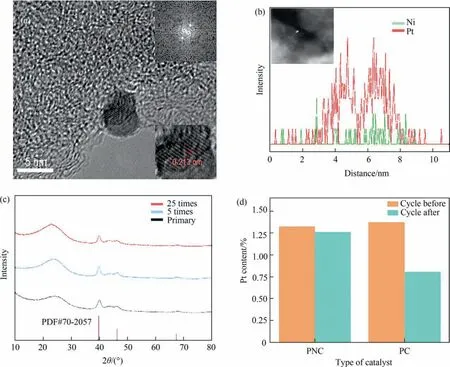
Fig. 6. Catalyst characteristics after reaction (a) TEM image of PNC. (b) HAADF-STEM image and EDS mapping of Pt (red) and Ni (green). (c) XRD spectra of PNC Primary catalyst, 5 times cycle and 25 times cycles. (d) AAS data before and after the reaction of PNC and PC.
The surface coverage of the activated hydrogen on the Pt-Ni catalyst surface of Pt-Skin is significantly reduced in comparison to Pt/C catalyst, which also explains the decrease in activity of the PNC catalysts[24].On the other hand,the decreased hydrogen activation capacity of PNC may inhibit excessive hydrogenation and improve PAP selectivity. The bimetallic Pt-Ni catalyst has significantly improved selective hydrogenation performance compared to the single metal Pt catalyst, which is primarily due to the synergistic effect between the bimetals improving product selectivity and catalyst stability [37,38]. Currently, there is a paucity of studies on the performance of Pt-M(M=Ni,Co,Fe and other transition metals) in strong acidic conditions, since the Ni on the surface of the particles will become unstable and easy to lose in strong acid. Therefore, we designed this bimetallic catalyst with Pt-Skin,there are two reasons for improving PNC catalyst stability:1) The formation of Pt-Skin can effectively prevent the dissolution of Ni inside the catalyst in sulfuric acid environment; 2) With the addition of Ni, sacrificial Ni atoms can be formed in the reaction process in order to inhibit the dissolution of the Pt atoms,in order for the PNC catalyst to exhibit higher catalytic stability than the commercial PC catalyst [19,39,40]. As a result, Pt-rich surface formation and Pt-Ni alloy corn characteristics are the two main contributors to the high PNC stability in sulfuric acid systems.
4. Conclusions
A bimetallic Pt-Ni catalyst was designed and prepared with the aim of efficiently improving the product selectivity and catalyst performance stability of the nitrobenzene hydrogenation rearrangement to prepare thep-aminophenol. The objective of this work was to compare the difference in performance between the Pt-Ni catalyst and the Pt-based, and assessed the effect of Ni on modifying Pt-based catalysts. Through characterization, electrons have been shown to flow from Ni to Pt, and electron-gaining Pt facilitates H2dissociation under softer conditions, but the amount of dissociated hydrogen decreases as a result of Ni doping decreasing the Pt active site. The product selectivity of the nitrobenzene hydrogenation reaction is greatly enhanced compared to the Ptbased catalyst due to the synergistic effect between Ni and Pt. As can be seen from the stability of the catalyst, there is no obvious quenching phenomenon after 25 cycles of application experiments.In parallel, through the aqua regia treatment experiment of the pre-reactor and post-reactor catalysts,it can be found that the loss of Pt in Pt-Ni bimetallic catalyst is much lower than the loss of Ptbased catalyst,and Ni may play an anti-corrosion role on Pt in the reaction. It is believed that some degree of alloying of a single metal with another metal or nonmetal is beneficial for improving the performance of the catalyst in a certain reaction process. Pt(5d)-Ni(3d)strong coupling is beneficial to the stability of metallic Pt in the reaction system.
CRediT Authorship Contribution Statement
Chong Yao: Data curation. Dandan Ma: Investigation. Jie Luo:Investigation. Yixin Chen: Investigation. Min Tian: Investigation.Haoxuan Xie: Investigation. Chunshan Lu: Investigation. Feng Feng: Investigation. Qingtao Wang: Investigation. Qunfeng Zhang:Supervision, Methodology, Investigation. Xiaonian Li: Supervision,Methodology, Investigation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (U20A20119, 22078292 and 22008212).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.026.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
