Graphene quantum dots doped poly(vinyl alcohol) hybrid membranes for desalination via pervaporation
Yunyun Wan, Lulu Yao, Peng Cui
School of Chemistry and Chemical Engineering, Hefei University of Technology, China
Anhui Province Key Laboratory of Controllable Chemical Reaction & Materials Chemical Engineering, Hefei, 230009, China
Keywords: Pervaporation Desalination Graphene oxide quantum dots Poly(vinyl alcohol) Membranes
ABSTRACT Pervaporation desalination by highly hydrophilic materials such as poly(vinyl alcohol) (PVA) based separation membrane is a burgeoning technology of late years. However, the improvement of membrane flux in pervaporation desalination has been a difficult task. Here, a novel hybrid membrane with doped graphene oxide quantum dots(GOQDs)which is rich in hydrophilic groups and small size into the matrix of PVA was prepared to improve the membrane flux. The membranes structures were described by field emission scanning electron microscopy(FESEM),atomic force microscopy(AFM),Fourier transform infrared (FT-IR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA) and X-ray diffraction (XRD). And more, Water contact angle, swelling degree, and pervaporation properties were carried out to explore the effect of GOQDs in PVA matrix. In addition, GOQDs content in the hybrid membrane,NaCl concentration,and feed temperature were investigated accordingly.Moreover,the hydrogen bonds between PVA chains were weakened by the interaction between GOQDs and PVA chains.Significantly,the hybrid membrane with optimized doped GOQDs content, 200 mg∙L-1, displays a high membrane flux of 17.09 kg∙m-2∙h-1 and the salt rejection is consistently greater than 99.6%.
1. Introduction
As the global population and urbanization continuously grows,water scarcity has become a major challenge facing around the world. Many efforts had been taken to seek technical solutions to improve drinking water supplies. Accordingly, desalination technology,which removes salt from non-potable sources such as saltwater and brine,has proven to be an effective way to address such issue. Currently, seawater desalination is mainly realized through membrane separation technology by virtue of its high efficiency,energy saving,and high operational stability features[1].Pervaporation(PV)has received much attention because of its highly selective purification for water with high salt concentration. However,the lower flux is a major problem in PV process [2]. Generally,PV is driven by the chemical potential gradient that is generated by the pressure difference across the membrane.The performance of PV is directly related to the differential pressure of each component [3]. The recognized mechanism of pervaporation process is the dissolution-diffusion principle, which consists of three steps:(1) the feed liquid components are adsorbed and dissolved on the membrane surface; (2) the dissolved components are diffused through the membrane;(3)the components are desorbed from the permeation side [4].
Till now, polymeric membranes, inorganic membranes, and hybrid membranes have been widely utilized for seawater desalination. However, the application of polymeric membranes has been restricted by several problems, including low mechanical and biostability and low rejection rate [5,6]. For inorganic membranes, their further application was limited by high-cost production. Therefore, it has found that the hybrid membranes prepared by the combination of polymers and inorganic fillers can be better used for PV.For polymer-based matrixes,they must be chemically stable, easily to prepare, highly hydrophilic, and stain resistant[7,8]. Commonly, the polymers adopted as seawater desalination hybridization membranes are mainly including cellulose acetate(CA) [9], polyamide (PA) [10] and polyvinyl alcohol (PVA) [11],etc.With excellent forming ability and good hydrophilic properties,PVA has been one of the most extensively utilized PV membrane materials for desalination. However, because of its water-soluble nature, PVA should be further modified to avoid dissolution in water during PV process [12].
The inorganic fillers in hybridized membranes are graphene oxide quantum dots (GOQDs) [11], and multi-walled carbon nanotubes (MWCNTs) [13], graphene oxide (GO) [14], and covalent organic frameworks(COF) [15]. Among them, GO is one of the fillers that have received much attention.It has been demonstrated that GO membranes enable water molecules to penetrate freely and rapidly, while blocking other molecules [4]. This is mainly because the formation of the hydrophilic area by hydrophilic groups such as hydroxyl, carboxyl and epoxy groups and the hydrophobic area by sp2-hybridized carbon ring. They serve to adsorb water molecules and reduce the resistance to the flow of water molecules, respectively, while forming a channel for water movement.However,GO tends to be arranged parallel in polymers.This could be a barrier against permeates, resulting in a relatively high resistance to mass transfer of GO-modified hybrid membranes [16]. Therefore, it is believed that by shortening the lateral dimensions of the GO sheets, the transport path of the permeate molecules can be shortened with reduced mass transfer resistance,thus ensuring faster permeation.As a result,GOQDs with the similar structure and less than 100 nm lateral dimensions[11]became an excellent candidate of PV membrane for desalination. Because of the smaller size, the permeation path of the infiltrating molecules could be shorter than that of GO.And with the GO-like structure, sp2carbon structures and hydrophilic functional groups(—OH, —COOH, and C—O—C) in GOQDs could provide a ‘‘highway”to improve water flux[17].Typically,GOQDs have been used to prepare hybrid membranes for various separation processes such as nanofiltration [18], positive osmosis [19] and reverse osmosis [20]. A thin-membrane nanocomposite with tannic acid(TA) mixed with GOQDs to enhance water flux and nanofiltration antifouling properties has been prepared by Zhanget al.[18]. In addition, Songet al.[21] prepared a reverse osmosis membrane from polyamide (PA), and the pollution resistance and chlorine resistance can be enhanced by the incorporation of GOQDs. However, the development of hybrid membranes with GOQDs for desalination is still scarce to date.
In this study, a bottom-up approach, direct pyrolysis of citric acid (CA), was used to synthesize GOQDs. The hybrid membranes for pervaporation desalination were prepared by dispersing GOQDs into polyvinyl alcohol(PVA)matrix.Notably,the dehydration separation performance of the hybrid membranes was improved thanks to the incorporation of GOQDs. Also, the effects of GOQDs on membrane morphology, structure, hydrophilicity, swelling,and dehydration properties were systematically characterized and evaluated.
2. Experimental
2.1. Materials and reagents
Citric acid (CA), Analytical Purity, Aladdin Reagents (Shanghai)Co., Ltd., Polyvinyl Alcohol (PVA), Sodium Hydroxide (NaOH),Sodium Chloride (NaCl), Glutaraldehyde (25%), and Concentrated Hydrochloric Acid (HCl), all of them are analytically pure, Sinopharm Chemical Reagent Co. Ltd. Dialysis bag (MWCO = 1000)Sinopharm Chemical Reagent Co.Ltd.Polyvinylidene fluoride(pore size 200 nm), Jinteng Experimental Equipment Co. Ltd. Deionized water laboratory self-made.
2.2. Preparation of hybrid membranes
GOQDs were synthesized by common ‘‘bottom-up” approach[22], just as direct pyrolysis of citric acid in Supplementary Material shows. A certain amount of homemade GOQDs powders were sonically dispersed into 50 ml deionized water for 30 min, then 1.5 g PVA(3%(mass)in water)was added into the dispersion.After stirring at room temperature for 12 h,the dispersion was heated to 363 K and kept 2 h to dissolve. Finally, a certain amount of glutaraldehyde (25%) and concentrated hydrochloric acid (about 20 μl)were added into the dispersion at 333 K to form crosslinked casting solutions.
A certain amount of deformed PVA-GOQDs-Xsolutions(whereXdenotes the concentration of GOQDs in solution, mg∙L-1)were covered dropwise onto PVDF porous membrane which placed on the glass plate and then dry at 313 K. The membrane samples without porous support were prepared with the corresponding solutions on clean glass plate and dry at 313 K.
2.3. Structural characterization of the membrane
In this study, Scanning electron microscopy (SEM, SU8020,Japan)was used to observe the surface and cross-section morphology of the membranes, which required gold spraying of the samples. The surface morphology of the PVA-GOQDs-Xhybrid membranes were tested by atomic force microscopy(AFM,Dimension, Germany). The chemical structure of the PVA-GOQDs-Xhybrid membranes were analyzed by fourier transform infrared spectroscopy (FT-IR, Nicolet 67, USA) in the scan range of 4000–500 cm-1.The glass transition temperatures(Tg)of the membrane materials were measured by differential scanning calorimetry(DSC, Q2000, USA). Thermal performance was obtained in a temperature range of 313–1073 K (heating rate 10 K∙min-1) uising thermogravisince analysis (TGA, STA 449F5, Germany). The dspace variation of the composite membrane could be calculated by X-ray diffraction meter (XRD, X’Pert PRO MPD, Netherlands),where the radiation source was Cu Kα, wavelength λ = 0.15418 nm, scanning range: 5°–60° (10 (°)∙min-1).The static contact angle meter (CA, GR001PC130D, China) was used to measure the membranes surface hydrophilicity.
2.4. Characterization of membrane properties
2.4.1. Swelling degree
The swelling degree (SD) of the membranes were measured by soaking the membrane samples without porous support into pure water at room temperature. The initial membrane samples were weighed, and then weighed again after adsorption equilibrium in water for 48 h. The calculation formula (1) of SD is as follows:
HereWdandWsare the mass of the membrane samples before and after soaking in water, respectively.
2.4.2. Testing of pervaporation properties
The pervaporation (PV) properties of PVA-GOQDs-Xhybrid membranes were evaluated by a self-made device in the laboratory which effective area is 9.62×10–4m2.The pervaporation test was operated at least 3 times for each membrane, each test was performed 30 min,and the final results were averaged.The feed liquid of water/sodium chloride solution flow were controlled about 25 L∙h-1, the pressure on the downstream side of the membrane is made to no more than 400 Pa by a vacuum pump. As operation steady,the permeate vapor was collected in a cold trap submerged in liquid nitrogen and then weighed. The conductivity in the feed solution and permeate was measured with a benchtop conductivity meter (DDS-307A, Shanghai Yidian Scientific Instruments Co.,Shanghai, China), and then the salt concentration in the permeate was obtained from the calibration curve of conductivity versus salt solution concentration. According to the pervaporation data, the permeation flux (J, kg∙m-2∙h-1) and the salt rejection (R, %) could be calculated by Eqs. (2) and (3), respectively.
Here,Q(g)is the permeate mass of the peameated sample,t(h)is the operation time,A(m2)is the effective area of the membrane,cpandcfrepresent the salt concentrations of the permeate and feed, respectively.
3. Results and Discussion
3.1.Microscopic characterization of PVA-GOQDs-X hybrid membranes
3.1.1. FESEM
The surface and the cross-section morphologies of the PVAGOQDs-X membranes were observed by SEM as Fig. 1. It can be seen clearly that both the surface and cross-section of the PVAGOQDs-0 membrane(pure PVA)exhibit uniform and smooth from Fig. 1(a) and (b). And there was no deformation could be investigated. This is no difference with the common characteristic of cross-linked PVA dense membranes [23]. The surface and crosssectional morphology of PVA-GOQDs-100/200 hybrid membranes showed uniformly and smooth like PVA-GOQDs-0 membrane as Fig. 1(c)–(f). That is mainly because that GOQDs were compatible with PVA and could well be dispersed so it exhibits uniform and smooth. But from the surface and cross-section morphology of PVA-GOQDs-300 hybrid membrane in Fig. 1(g) and (h), it can be deduced that the GOQDs agglomeration occurs with the content increase [16]. The water-absorbing ability of membrane should be weakened because of the GOQDs agglomeration,further leaded to flux decrease. This agrees to the desalination properties described below.The thickness of PVA-GOQDs-Xmembranes were about 14.5 μm from cross-section SEM images.
3.1.2. AFM
The effect of GOQDs incorporation on the surface morphology of PVA-GOQDs-Xmembranes was further investigated by AFM on membranes, as shown in Fig. 2.
It is obviously that significant difference of surface roughness occurred between PVA-GOQDs-0 membrane and PVA-GOQDs-200 hybrid membrane. Therefore, the dimples on the surface of PVAGOQDs-0 membrane were sharp and dense while on the surface of PVA-GOQDs-200 hybrid membrane were gentle and sparse.Table 1 shows theRaandRqof PVA-GOQDs-0 membrane and PVA-GOQDs-200 hybrid membrane. It can be seen that theRaandRqvalues were increased from 0.386 nm and 0.762 nm(PVA-GOQDs-0) to 7.51 nm and 9.77 nm (PVA-GOQDs-200), indicating that the surface roughness of the hybrid membrane increased significantly [24,25].
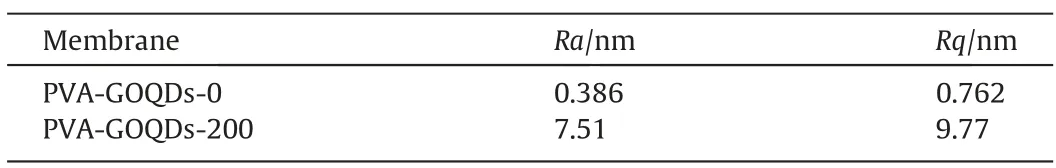
Table 1Surface roughness of PVA-GOQDs-0 and PVA-GOQDs-200 membrane
3.2. Structural characterization of PVA-GOQDs-X hybrid membranes
3.2.1. FTIR
Fourier transform infrared spectroscopy (FTIR) was performed by attenuation total reflection (ATR) to characterize the chemical structure of PVA-GOQDs-Xhybrid membranes. As Fig. 3(a) shows,the characteristic peaks of the PVA-GOQDs-X hybrid membranes are similar to PVA-GOQDs-0 membrane,with the stretching vibration peak of –OH appearing around 3260 cm-1, C—O appearing around 1250 cm-1, and —C—O—C— appearing around 910 cm-1[26]. This suggests that GOQDs were distributed in the interior of the membrane and are not enriched in the surface of the membrane [22]. However, it can be seen from Fig. 3 (b) that with the addition of GOQDs the stretching vibration peaks of –OH appear at 3259 cm-1(PVA-GOQDs-0), 3266 cm-1(PVA-GOQDs-100),3269 cm-1(PVA-GOQDs-200) and 3260 cm-1(PVA-GOQDs-300).This is mainly attributed to the change in hydrogen bonding,where the oxygen-containing groups on GOQDs are hydrogen bonded to the —OH on PVA, which leads to a decrease in hydrogen bonding between the hybrid membrane chain segments. However, as the more GOQDs lead to agglomeration occurs, the exposed oxygencontaining groups of GOQDs decrease and finally lead to the enhancement of hydrogen bonding between the PVA chain segments [22,27,28]. This is also evidenced by DSC results.
3.2.2. DSC
The glass transition temperature (Tg) of the hybrid membranes were characterized by DSC, as shown in Fig. 4. It could be confirmed from DSC that theTgof PVA-GOQDs-0 membrane was 380.7 K, while theTgof hybrid membranes were reduced to 375.8 K(PVA-GOQDs-100)and 364.6 K(PVA-GOQDs-200),but that of PVA-GOQDs-300 hybrid membrane increased to 381.8 K. The decrease ofTgindicates the hydrogen-bond between PVA chains was weakened, that mainly caused by the competition of the hydrogen-bond between GOQDs and PVA. The subsequent decrease ofTgindicates the enhancement of hydrogen-bond between PVA chains, that mainly because GOQDs agglomeration attenuated the hydrogen-bond between GOQDs and PVA [29]. At the same time, it has been reported that the addition of nanofillers inhibits the migration of polymer chains and prevents ‘‘free radical transfer”, thus improving thermal stability [13,30].
3.2.3. TG
The results of thermogravisince analysis(TGA)analysis of PVAGOQDs-Xmembranes were shown as Fig.5.Below 423 K,the PVAbased membranes material was thermally stable, and the main thermal weight loss at this stage was due to the volatilization of water adsorbed in the membrane; between 423–603 K, the thermal mass loss was attribute to the thermal decomposition of PVA side chain groups (—OH); above 603 K, the thermal mass loss was mainly due to the thermal decomposition of the PVA main chain. The thermal decomposition of the PVA main chain was divided into two stages: in the range of 603–703 K, thermal mass loss was mainly the decomposition of the PVA carbon chain; in the range of 703–823 K, thermal mass loss was glutaraldehyde and other carbon compounds resulting from thermal decomposition [31]. For the thermal decomposition stage of PVA side chain groups(—OH),the addition of GOQDs was the change of the starting thermal decomposition temperature of PVA-based membranes from 448.9 K (PVA-GOQDs-0) to 450.5 K (PVA-GOQDs-100),451.9 K (PVA-GOQDs-200), 450.7 K (PVA-GOQDs-300). It could be seen that the hydrogen bonding between the GOQDs and the—OH in the side chain of PVA slowed down the thermal decomposition of—OH and improved the thermal stability of the side chain.However, the aggregation of GOQDs weakened this effect and made PVA-GOQDs-300 less thermally stable. This could also be indicated by the mass of the pyrolysis residue. At 603–703 K, the PVA-GOQDs-Xmembranes had almost the same thermal decomposition properties,which shown that the effect of GOQDs on the PVA carbon chain was not obvious.The ash content after decomposition and stabilization at 703–823 K showed that PVA-GOQDs-0 > PVAGOQDs-100 > PVA-GOQDs-300 > PVA-GOQDs-200, which was caused by the doping of GOQDs to reduce the reaction of–OH with glutaraldehyde.
3.2.4. XRD
The d-space value can be confirmed by XRD as Fig.6 and reflect the denseness of the polymer structure [32], and this has some connection with the degree of swelling of the hybrid membrane.The d-space can be considered as the average gap of the polymer chains and smaller d-space value implies tighter connected chain filling. From the Fig. 6, with the increase of GOQDs content, the 2θ values varied from 19.7° (PVA-GOQDs-0) to 19.6° (PVAGOQDs-100), 19.1° (PVA-GOQDs-200) and 19.5° (PVA-GOQDs-300). The d-space value shows a trend of increasing then decreasing again. As the amount of GOQDs was increased to 200 mg∙L-1,the d-space of the hybrid membrane reached the maximum and the inter-chain segments were well dispersed with GOQDs.At this time, the free space between PVA chains reached the largest and the membrane showed the most swelling degree. This is also consistent with the pervaporation properties later.
3.3. Interaction between GOQDs and PVA
The mechanism of PVA doping with GOQDs is shown in the Fig. 7. Hydrogen bonds are formed between the hydroxyl groups on the PVA chain segments without GOQDs,while also maintaining certain chain spacing.When GOQDs are dispersed in PVA,oxygencontaining groups on GOQDs can form hydrogen bonds with —OH bonds on PVA.That result to reduction of hydrogen bonds between polymer chain segments. At the same time, the spacing between the chain segments increases accordingly and the oxygencontaining groups can improve the membrane hydrophilicity.However, as the content of GOQDs increases and agglomeration occurs,the oxygen-containing groups exposed to GOQDs decrease,which eventually leads to the enhancement of hydrogen bonding between PVA chain segments.
3.4. Hydrophilic properties of PVA-GOQDs-X hybrid membranes
Hydrophilic properties of PVA-GOQDs-Xmembranes were described by contact angle and the swelling degree as Fig.8 shows.From Fig. 8(a), the contact angle of PVA-GOQDs-0 hybrid membranes was 70°, the PVA-GOQDs-100 and PVA-GOQDs-200 hybrid membrane was reduced to 60° and 55°, respectively. And the PVA-GOQDs-300 hybrid membrane was increased to 63°.The contact angle decrease indicated the hydrophilicity increase, while subsequent angle increase indicated decrease in hydrophilicity.This was exactly consistent with the result of the swelling degree.Because the addition of GOQDs provides abundant hydrophilic groups (such as —OH, —COOH, C—O—C,etc., shown in Fig. S2),the hybrid membrane hydrophilicity can be enhanced. But the agglomeration of excessive GOQDs would become more and more obvious. So, the exposed effective hydrophilic groups of GOQDs were greatly reduced, leading to the hybrid membrane hydrophilicity decreased.
From Fig. 8(b), the swelling degrees of PVA-GOQDs-0 membrane was 340%, with the addition of GOQDs, the swelling degree was promoted to 407% (PVA-GOQDs-100) and 410% (PVAGOQDs-200). In accordance with the contact angle results, the moderate GOQDs with abundant hydrophilic groups could increase the membranes swelling degree, while the excessive GOQDs agglomeration should restrict the act of hydrophilic groups so that the swelling degree would decrease.And more,the swelling degree could be enhanced by d-space grown.According to the above XRD patterns, the trend of d-space is fully consistent with the dissolution rate.
3.5. The pervaporation properties of the hybrid membranes
Fig. 9 shows the pervaporation results of PVA-GOQD-Xhybrid membranes. The pervaporation tests were performed by 3.5%(mass) NaCl solution at 333 K. Salt rejections were maintained no less than 99.9% for each membrane, and the fluxes were 7.28 kg∙m-2∙h-1(PVA-GOQDs-0), 9.53 kg∙m-2∙h-1(PVA-GOQDs-100), 10.82 kg∙m-2∙h-1(PVA-GOQDs-200) and 8.26 kg∙m-2∙h-1(PVA-GOQDs-300), respectively. Based on the above results, it can be deduced that the rise in flux was mainly due to the incorporation of GOQDs in the PVA. So, the hybrid membranes with higher hydrophilic ability could adsorb more water molecules than PVAGOQDs-0 membrane, and water molecules can be transported across the membrane through the ‘‘high-way” channels brought from GOQDs inside the hybrid membrane. Another reason is that the d-space was increased by doping the PVA with appropriate GOQDs.This made the space between the chain segments became larger and more favorable for water molecules passage. However,the hydrophilicity of the hybrid membrane decreases and common space between chain segments becomes smaller as the GOQDs aggregation. These phenomena lead to hybrid membrane defects increase and hinder the diffusion of water molecules [26].
The effect of operation conditions to pervaporation properties were shown in Fig.10.It can be known from Fig.10(a)that the flux was influenced remarkably with the adjustment of the feed liquid NaCl concentration.At 333 K,with the feed liquid NaCl concentration enhanced from 1.5% (mass) to 3.5% (mass) and 5.5% (mass),PVA-GOQDs-0 membrane flux changed from 10.63 kg∙m–2h-1to 7.28 kg∙m-2∙h-1and 5.12 kg∙m-2∙h-1, while the PVA-GOQDs-200 hybrid membrane flux decreased from 11.11 kg∙m-2∙h-1to 10.82 kg∙m-2∙h-1, 8.14 kg∙m-2∙h-1. It is obviously that the flux decreased with the NaCl concentration increased for both PVAGOQDs-0 membrane and PVA-GOQDs-200 hybrid membrane.There are two main reasons: For one hand, the flux was closely related to the driving force, which was maintained by the partial vapor pressure of water. As a non-volatile molecule, NaCl concentration increasing led to the total vapor pressure of the feed decreased.And the higher temperature,the more significant of this relationship. So, the increase in the concentration of NaCl reduced the partial vapor pressure of water, which also could reduce the driving force. This was what made the change of flux [26,33]. For the other hand, NaCl enrichment on the membrane surface would lead to concentration polarization, which showed negative effect on membrane flux [34].
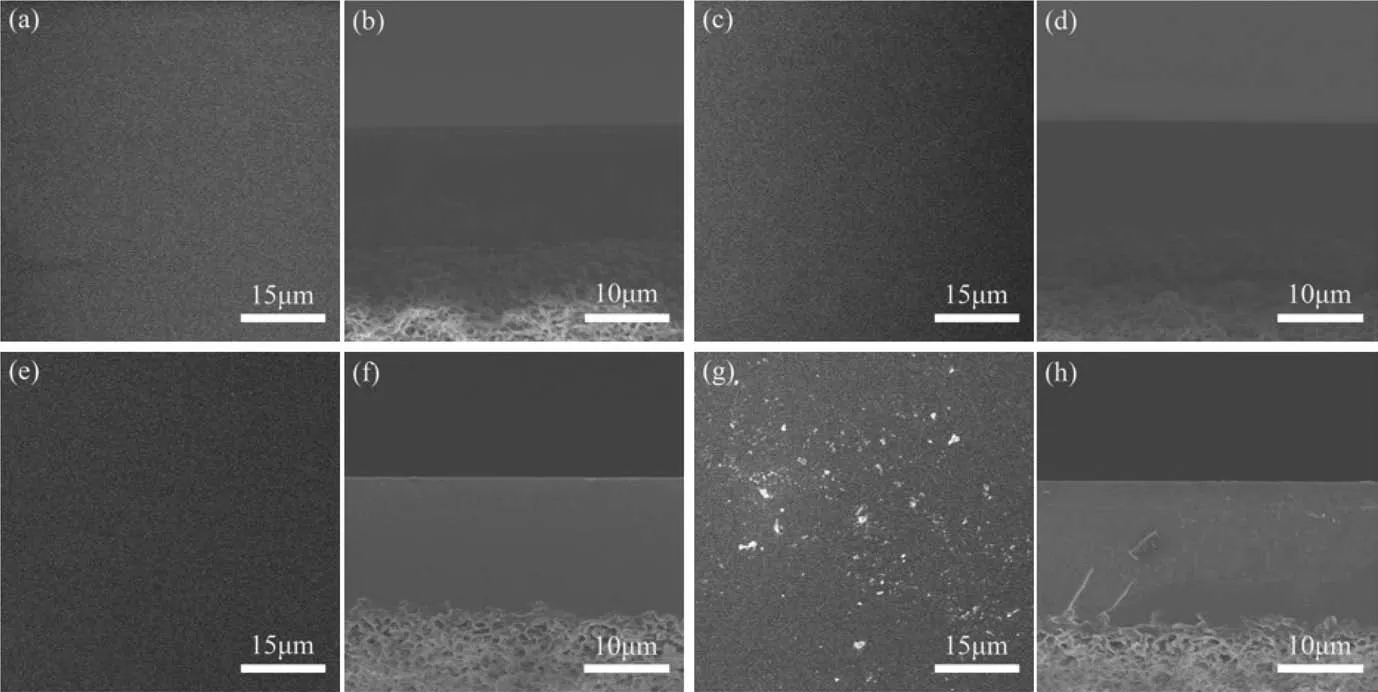
Fig.1. Surface and cross-section SEM images of PVA-GOQDs-0 membranes((a),(b)),PVA-GOQDs-100 hybrid membranes((c),(d)),PVA-GOQDs-200 hybrid membranes((e),(f)) and PVA-GOQDs-300 hybrid membranes ((g), (h)).
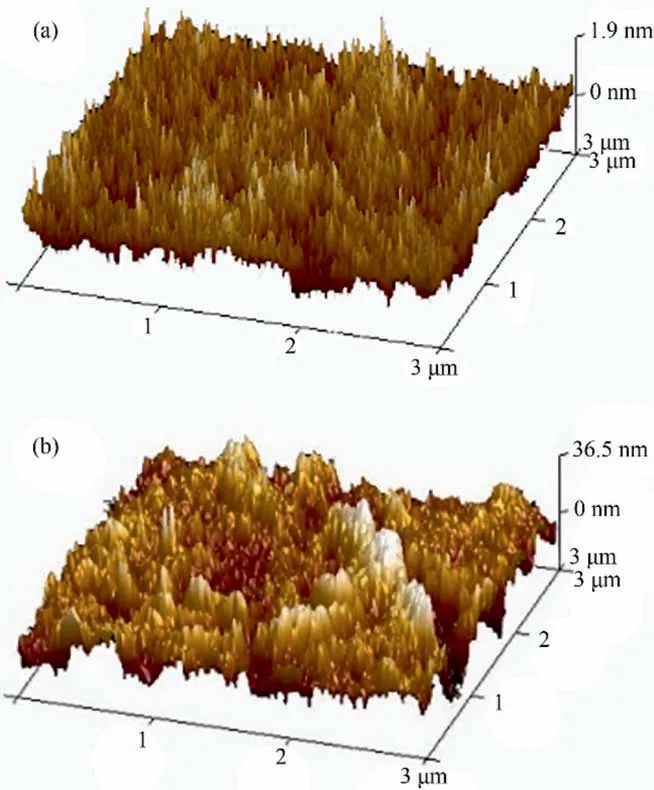
Fig. 2. AFM images of membrane: (a) PVA-GOQDs-0; (b) PVA-GOQDs-200.
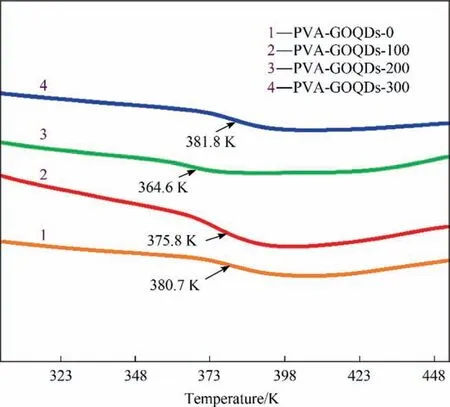
Fig. 4. DSC curves of PVA-GOQDs-X hybrid membranes.
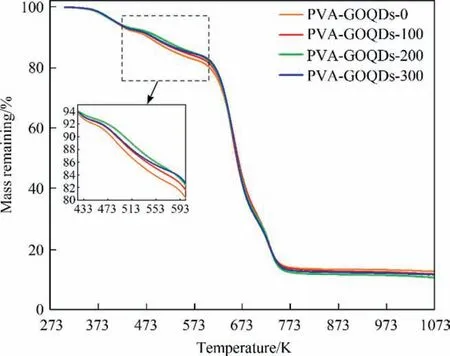
Fig. 5. TG curves of PVA-GOQDs-X hybrid membranes.
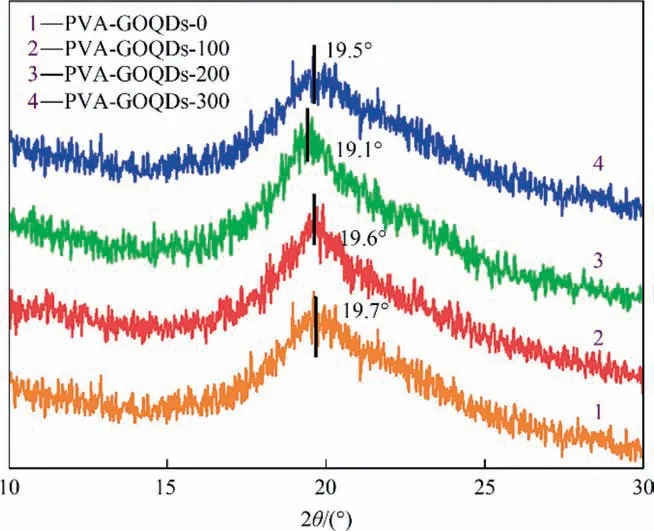
Fig. 6. XRD patterns of PVA-GOQDs-X hybrid membranes.
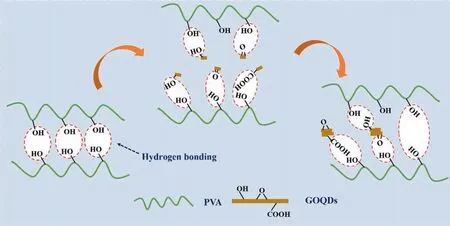
Fig. 7. Mechanism of interaction between GOQDs and PVA.
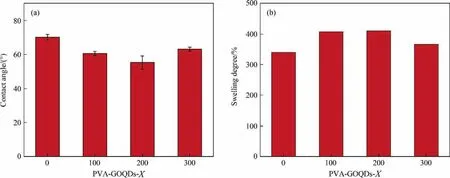
Fig. 8. Hydrophilic properties of PVA-GOQDs-X hybrid membranes: (a) water contact angle; (b) swelling degree.
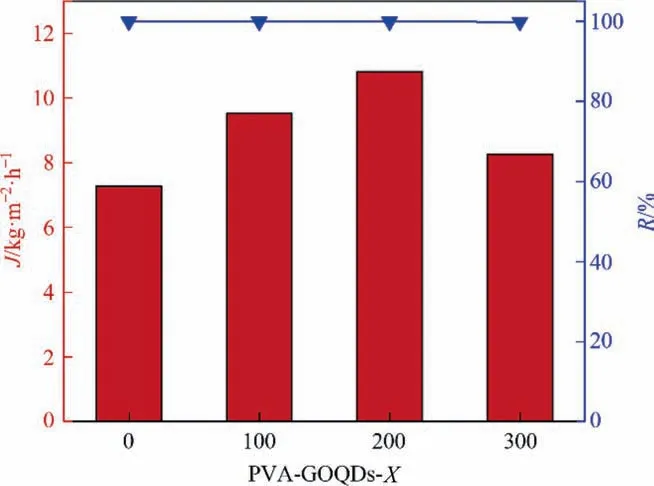
Fig. 9. PV flux and salt rejection of 3.5% (mass) NaCl solution at 333 K.
Fig. 10(b) shows the effect of pervaporation temperature of PVA-GOQDs-0 membrane and PVA-GOQDs-200 hybrid membrane by NaCl concentration of 3.5% (mass) in the feed liquid. When the temperature of the feed changed, the result of desalination would also change. From Fig. 10 (b), the salt rejection could be maintained no less than 99.6%. In different feed temperature, the flux of PVA-GOQDs-0 membrane was 4.25 kg∙m-2∙h-1(313 K),6.83 kg∙m-2∙h-1(323 K),7.28 kg∙m-2∙h-1(333 K),11.16 kg∙m-2∙h-1(343 K) and 15.28 kg∙m-2∙h-1(353 K) respectively. Accordingly,the flux of PVA-GOQDs-200 hybrid membrane was 4.45 kg∙m-2∙h-1(313 K), 7.92 kg∙m-2∙h-1(323 K), 10.82 kg∙m-2∙h-1(333 K),13.08 kg∙m-2∙h-1(343 K) and 17.09 kg∙m-2∙h-1(353 K). So, for both PVA-GOQDs-0 and PVA-GOQDs-200 membranes, the flux increased along with the feed temperature increased. Several factors contribute to this phenomenon. Firstly, it was concluded above that PVA-GOQDs-200 hybrid membrane showed better hydrophilic properties than PVA-GOQDs-0 membrane. Second,increasing the feed temperature will increase the water vapor pressure on the feed liquid side, while the water vapor pressure on the permeation side could be considered to remain zero with vacuum. So, the increase in the driving force magnifies the water flux [33]. Third, according to the free volume theory, the instantaneous free volume is generated by the thermal motion of the polymer chain in the amorphous region.So,the temperature increasing caused the larger free volume of the polymer,and the water molecules were more likely to diffuse in the free volume [23]. In addition, according to the free volume theory, the rise of temperature could improve the diffusion rate of water molecules, which was conducive to the infiltration of water through the membrane [26].
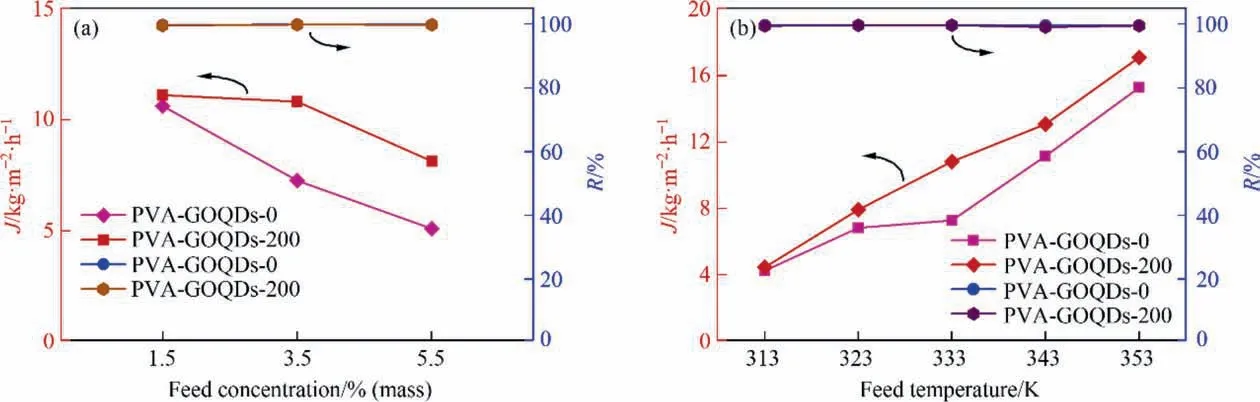
Fig. 10. Pervaporation condition effect: (a) feed concentration; (b) feed temperature.
In order to further understand the permeability of PVA-GOQDs-Xhybrid membranes at different feed temperature, the apparent activation energy of water through the membrane was calculated.The relationship between water flux and feed temperature can be quantitatively described Arrhenius-type relationship as shown in Eq. (4):
Here,Ji(kg∙m-2∙h-1) is the water flux (the water flux here is approximate to the flux,due to the high salt rejection of this work),Ai(kg∙m-2·h-1) is the pre-index factor, andR(kJ∙mol-1·K-1) is the gas constant,T(K) is the temperature, andEJ,i(kJ∙mol-1) is the apparent activation energy of water through the membrane during pervaporation desalination.
Fig.11 shows a typical Arrhenius plot of water volume and feed temperature for the treatment of a 3.5% (mass) NaCl solution. The curve shows that the natural logarithm of the water flux is linear with the reciprocal of the feed temperature.The slope of the Arrhenius diagram represents the apparent activation energy (EJ,W),which is a composite parameter. The activation energy is positive,indicating that the diffusion of water molecules increases with the increase of feed temperature [35].
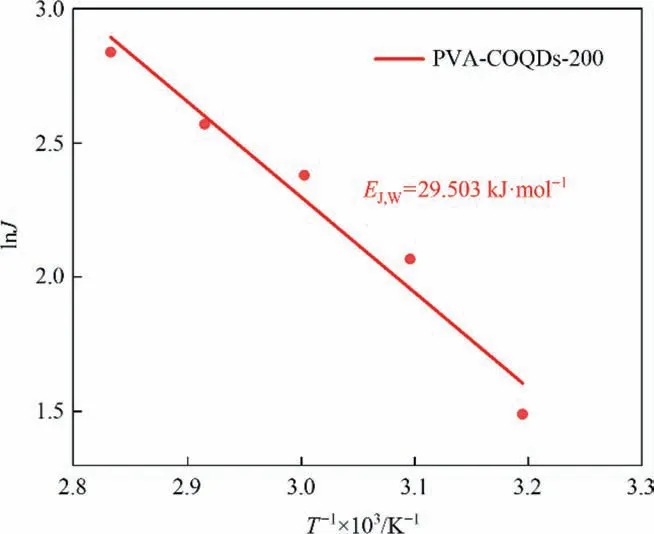
Fig.11. Alenius plot of water flux and feed temperature when treating 3.5%(mass)NaCl solution.
3.6. Long-term operation stability
Long-term operation of PVA-GOQDs-200 membrane was performed to observe its stability. As shown in Fig. 12, the PVAGOQDs-200 membrane was operated continuously for no less than 14 hours in the pervaporation desalination process using 3.5%(mass)NaCl feed solution at 343 K,and flux was maintained about 13 kg·m-2·h-1and the salt rejection was generally greater than 99.6%.
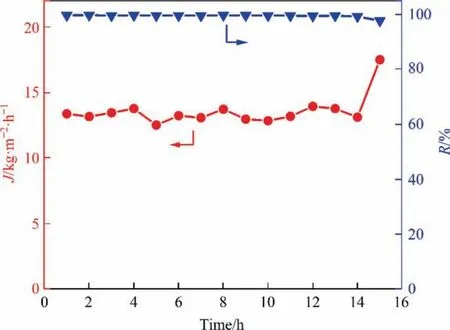
Fig. 12. Long-term operation evaluation of the PVA-GOQDs-200 membrane.
3.7.Comparison of PV performance with literature reports in this study
The PVA-GOQDs-Xhybrid membranes in this article was higher than 56.7% and 22.35% compared to the PVA/PSf and PVA/PES membranes reported in the literature [36,37], while it was higher than 73.90% and 88.88% compared to the PVA-g-GO/PAN and PVA/MWCNT membranes reported in the literature [38,39]. The comparison of specific the pervaporation properties is shown in Table 2. The separation performance of PVA based membranes showed good separation performance with the addition of GOQDs was 200 mg∙L-1.The potential for good pervaporation desalination performance was demonstrated at the feed concentration of 3.5%(mass) and the feed temperature of 343 K.
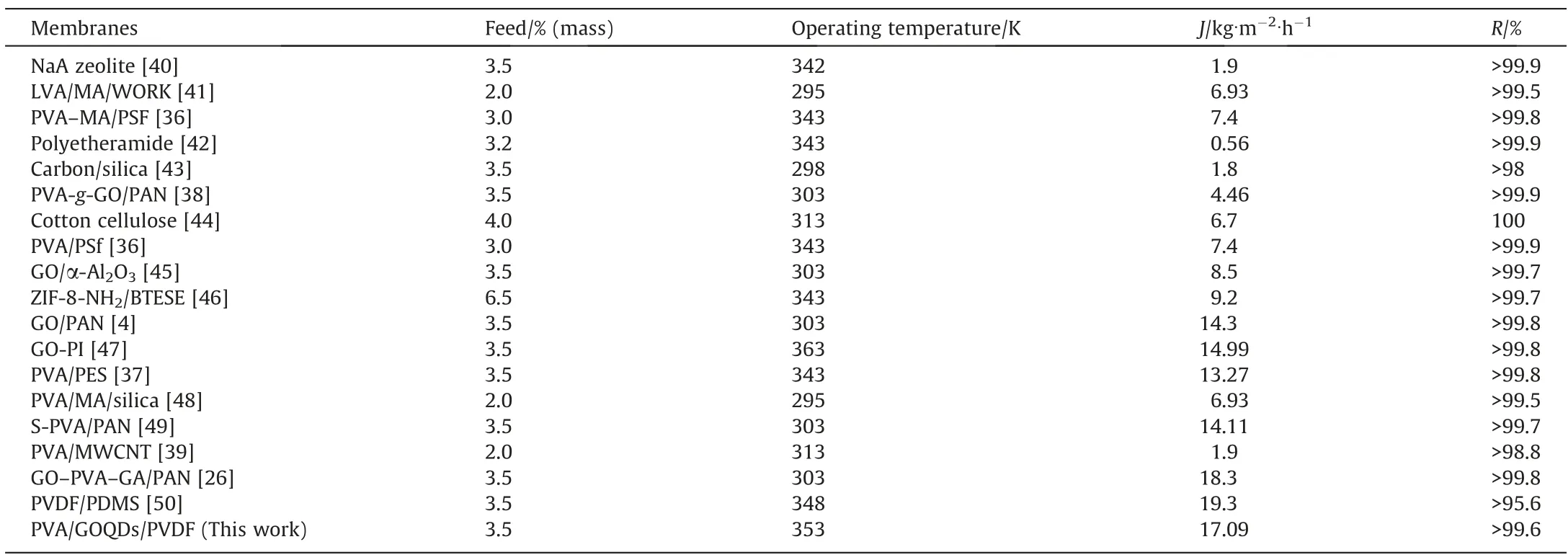
Table 2Different membrane pervaporation properties of water/NaCl solutions
4. Conclusions
To summarize, we prepared the poly(vinyl alcohol)-based hybrid membranes with doped graphene oxide quantum dots for pervaporation desalination. The hybrid membranes were characterized by various techniques including contact angle, swelling degree, and pervaporation. According to the results, the hydrophilicity of the PVA-GOQDs-Xhybrid membranes was improved compared to that of the PVA membrane.This was mainly because of the fact that the incorporation of GOQDs contributed more hydrophilic functional groups (such as —OH, —COOH and C—O—C,etc.). A large number of water molecules can be adsorbed in the highly hydrophilic hybrid membranes, and the water molecules could be transported across the membrane through the‘‘high speed” channels created by the GOQDs inside the hybrid membranes. And more, when GOQDs were added to PVA, its oxygencontaining functional groups created hydrogen bonds with the–OH on the PVA chains. The hydrogen bonds between PVA chains chould be weakened by the competitive effect. This result could increase the d-space of PVA with the appropriate doped GOQDs,thus could lead to more conducive to the passage of water molecules. However, excessive GOQDs showed reverse effects. Based on the results of pervaporation, the best flux of the PVA-GOQDs-200 hybrid membrane reaches 17.09 kg·m-2·h-1, which was 11.8%higher than the PVA-GOQDs-0 membrane with sufficient salt rejection.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.05.011.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
