A theoretical insight about co-pyrolysis reaction of natural gas and coal
Mingjun Pan, Chengkai Jin, Bingying Han, Runping Ye, Rongbin Zhang,, Gang Feng,,
1 School of Future Technology, School of Chemistry and Chemical Engineering, Institute of Applied Chemistry, Nanchang University, Nanchang 330031, China
2 School of Chemistry and Chemical Engineering, Nanchang University, Nanchang 330031, China
3 State Key Laboratory of Clean and Efficient Coal Utilization, Taiyuan University of Technology, Taiyuan 030024, China
Keywords: Natural gas Thermodynamics Hydrocarbons Co-pyrolysis Gibbs free energy Density functional theory
ABSTRACT The co-pyrolysis of natural gas and coal is a promising way for the production of acetylene due to its high efficiency for energy and hydrogen utilization. This work investigated the thermodynamics for the copyrolysis reaction of natural gas and coal using density functional theory. The favorable reaction conditions are presented in the form of phase diagrams.The calculation results show that the extra amount of methane may benefit the production of acetylene in the co-pyrolysis reaction, and the C/H ratio of 1:1,temperature around 3000 K and pressure at 0.1 MPa are most favorable.The results would provide basic data for related industrial process for the production of acetylene.
1. Introduction
Acetylene is an important platform molecule in coal and natural gas industry[1]. Coal and natural gas occupy a large proportion in the energy market. With relatively abundant coal in China, development of acetylene chemical industry is expected to relieve the dependence on petroleum. The main industrial methods for the production of acetylene in China are calcium carbide method,methane partial oxidation method [2], methane arc cracking method, and plasma method [3–5]. Compared to above methods,plasma method has advantages of concentrated energy, high temperature and high speed as well as low CO2emission [6,7]. It is reported that in the plasma jet with high temperature and high enthalpy, the volatile matter or even fixed carbon in coal can be directly converted into acetylene [8]. In addition, plasma jet can provide an efficient method of activating methane, even the C—H bond of methane has an average bond energy as high as 413 kJ∙mol-1[9].
Previous research related to production of acetylene with plasma method usually used coal and natural gas separately as raw materials[10–13].Conversion of coal to acetylene with plasma method started in the 1960s at Sheffield University,UK.The superiority of this method attracted further research [6,7,14]. Relevant research started in China in the 1990[15].In 2002,Xinjiang Tianye(Group) Co. Ltd. (China) collaborated with Russian scientists and Chinese scholars to develop a 2 MW hydrogen plasma generator and coal pyrolysis reactor. In 2006, big breakthrough was made at the 2 MW industrial platform: the reactor was continuously operated for 95 mins [16]. Conversion of natural gas to acetylene in plasma method was carried out representatively in the National Engineering and Environmental Laboratory of Idaho (INEEL) and National Energy Technology Laboratory of Pittsburgh (NETL) [3].The best result they published in 2002 is that the plasma power is 60 kW, the cracking of natural gas is 7.25 m3∙h-1, with the natural gas conversion rate of 96%, and acetylene yield of 93%. Based on this calculation, for every 1 ton of acetylene produced,1550 m3of natural gas should be consumed, and the energy consumption per unit product is 12.9 kW∙h∙kg-1.
Extensive previous works has investigated the thermodynamics for the conversion of coal and methane to acetylene. Chenet al.[17] and Yanet al.[18] separately established the coal pyrolysis model in plasma. Chen’s model revealed the non-uniform flow field and temperature field under the unique design of the Vshaped plasma. Yan’s model described the complex gas-particle reacting flow of coal pyrolysis under ultrahigh temperature with millisecond(ms) reaction time in hydrogen plasma reactors. Baddouret al.[19] calculated the equilibrium diagram for system of C and H2at 0.1 MPa, and found that acetylene content reached a maximum near 3800 K. Holmenet al.[20] calculated the free energy changes for the conversion of methane, and their results showed that the conversion of methane to acetylene is possible by the fact that, at high temperature, the free energy of formation of ethyne is lower than that of methane (and other saturated hydrocarbons). Wuet al.[7] evaluated the chemical and phase equilibria of coal pyrolysis , and found that both increasing C/H ratio and additional methane could improve the production of acetylene. Though previous works reported that high temperature accelerates the production of acetylene, they didn’t consider the effect of temperature and pressure on acetylene yield at the same time.
Though both coal and nature gas could be used separately as feed for acetylene production using thermal plasma method. The point is that there is a surplus of hydrogen when methane is cracked alone in plasma method,vs.shortage of hydrogen when coal is cracked alone.In addition,the cracking reaction of methane is strongly endothermic [3],vs.high enthalpy is beneficial to improve the conversion of coal cracking reaction[6].It is expected to make full use of the energy and improve the atomic utilization of hydrogen and carbon if the two reactions could be combined into one plasma reactor system. More acetylene can be obtained more efficiently by the co-pyrolysis of natural gas and coal compared with the pyrolysis of natural gas and coal separately.
Investigation into the co-pyrolysis of natural gas and coal has been reported for a few decades.The researches on the conversion of natural gas and coal to acetylene with plasma method mainly focused on technical and engineering issues for the industrialization,e.g.,how to design suitable reactors to control problem of coking,separation of cracked gas costing less energy,control of stable power and effective quenching technology,etc. However, few researches have investigated the effects of feed ratio, temperature and pressure to the yield of acetylene.This work is aimed to investigate the feed and energy balance for the co-pyrolysis reaction of nature gas and coal. The results would provide basic data for related industrial process for the production of acetylene.
2. Calculation Methods
All calculations were performed with the PBE function in the TURBOMOLE software. The basis set of atomic attributes was def-TZVP. And RI-density functional theory (RI-DFT) was used. The convergence criteria were 1.0 × 10–6Hartree (1 Hartree = 2625.5 kJ∙mol-1) for the self-consistent field (SCF) energy. Multiplicity was set as automatic (RHF/UHF).
Firstly,a series of possible products,which could be obtained by cracking methane and coal were selected: acetylene, ethylene,ethane, propylene, 1-butene, 2-butene, 2-methylpropene, 1-pentene, 2-pentene, 2-methylbutene, 2-methyl-2-butene, 3-methyl-1-butene, benzene and graphite (C34) for the calculations.In order to simplify the process,coal is approximatively presented by C16H10(pyrene).
For frequencies analysis, translational, rotational and vibrational contributions are considered for all molecules. There is no imaginary frequency for all optimized structures.
The Gibbs free energies are calculated by the following Equation:
where theEscf, ZPE and Δμ(T,p) are calculated self-consistent field energies, zero-point energies and change of chemical potential,respectively.
The change of chemical potential is calculated by the following equation:
where theNAandkBare Avogadro constant and Boltzmann constant, respectively (pθ= 101.325 kPa).
We have considered as many reactions as possible and calculated the Gibbs free energy change for each reaction.
Finally, thermodynamic phase diagrams are plotted and the thermodynamically favorable reaction is screened by phase diagrams (Fig. 1).
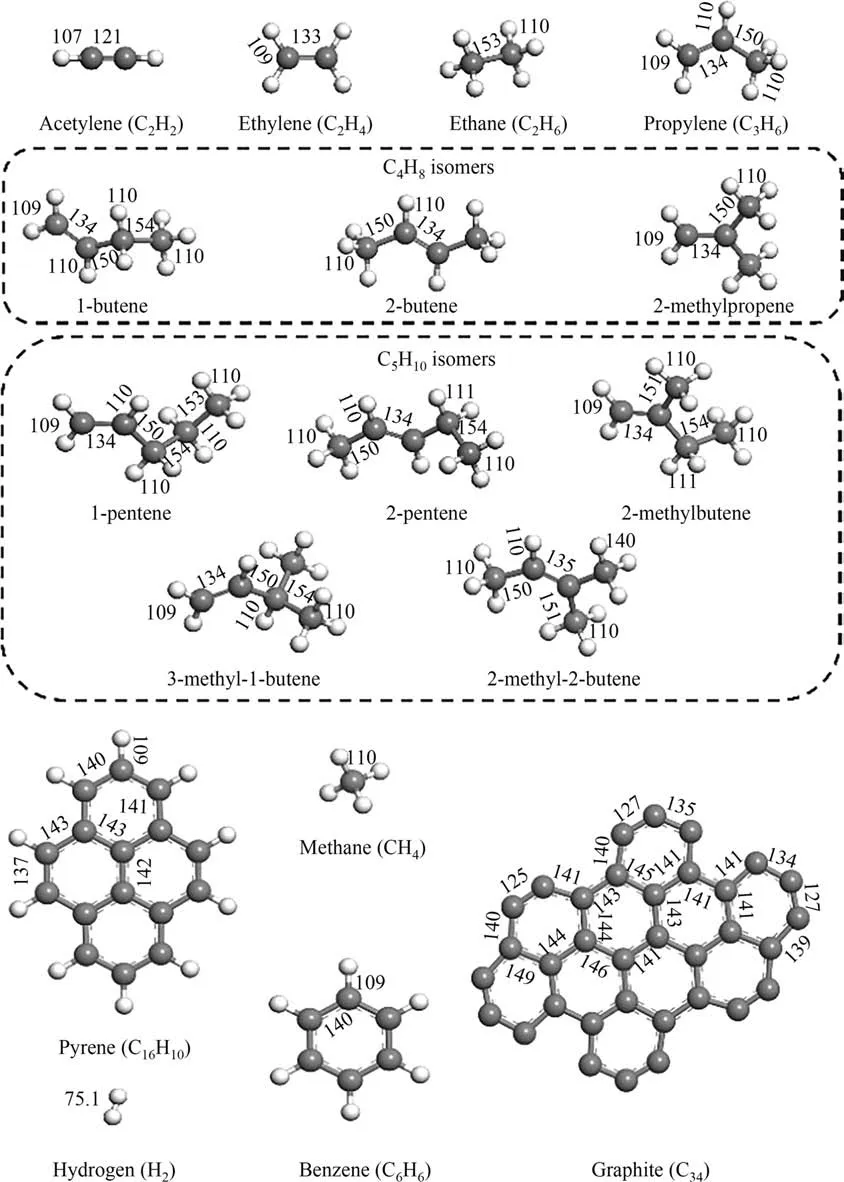
Fig. 1. The structure and bond length of the molecules involved in the reaction (unit: pm).
3. Results and Discussion
This work explored the reaction conditions of co-pyrolysis of natural gas and coal from the view of thermodynamics. The thermodynamics for the pyrolysis of natural gas and coal are separately discussed in Sections of 3.1 and 3.2, and the case for the copyrolysis of natural gas and coal is discussed in Section 3.3.
3.1. Methane decomposition
Our calculated enthalpy(298 K, 0.1 Pa, Table 1) for the conversion of methane to acetylene, ethylene and ethane are 401, 209.8 and 63.8 kJ∙mol-1,vs.the reported experimental data of 376.5,202.3 and 65.1 kJ∙mol-1[21], which confirms that our calculation is reliable. When methane is used as feedstock for the pyrolysis,the equilibrium formation of acetylene requires as high temperatures around 3300 K [3]. Since the theoretical enthalpy change for the conversion of methane to acetylene is 376.5 kJ∙mol-1, the production of acetylene is endothermic by 4.03 kW∙h∙kg-1-C2H2[22]. It is approximate to our calculated value, which is 401 kJ∙mol-1and can be converted to 4.28 kW∙h∙kg-1-C2H2. Our calculated results are the most ideal values by calculating the heat of reaction only from the point of view of thermodynamic enthalpy. The lowest energy consumption for the conversion of methane to acetylene in the experiments designed by the Industrial Plasma Engineering Laboratory, Korea Institute of Machinery and Materials is about 9 kWh∙kg-1-C2H2. The large difference between experimental and theoretical data is mainly because we do not consider the electrothermal conversion efficiency, the heat loss caused by reactor structure and energy consumption caused by the formation of by-products,etc. [23]. It should be mentioned that additional hydrogen is added into the reaction system in their experiments. Besides, the three moles of hydrogen molecules are produced by the two moles of methane. As a pollution-free, efficient, and inexpensive energy carrier [24–26], the effects of the additional hydrogen are: (1) inhibit the formation of soot which is the main by-product, (2) inhibit the conversion of methane and results in the lower methane conversion [5] and (3) increase the selectivity to C2H4[23].
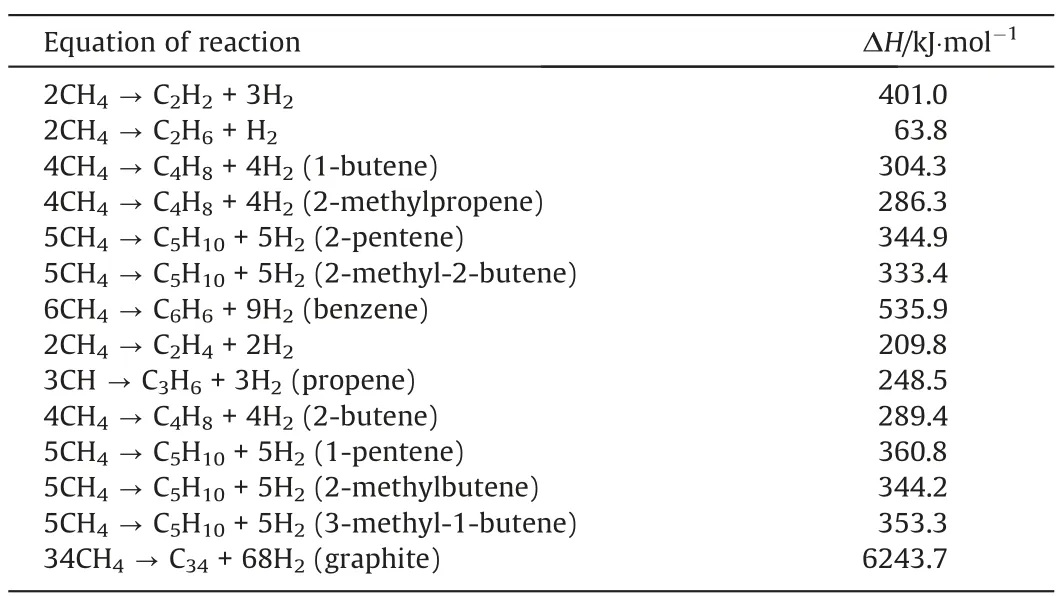
Table 1The enthalpy changes for relevant chemical reaction equations in 298 K, 0.1 MPa for methane cracking reaction
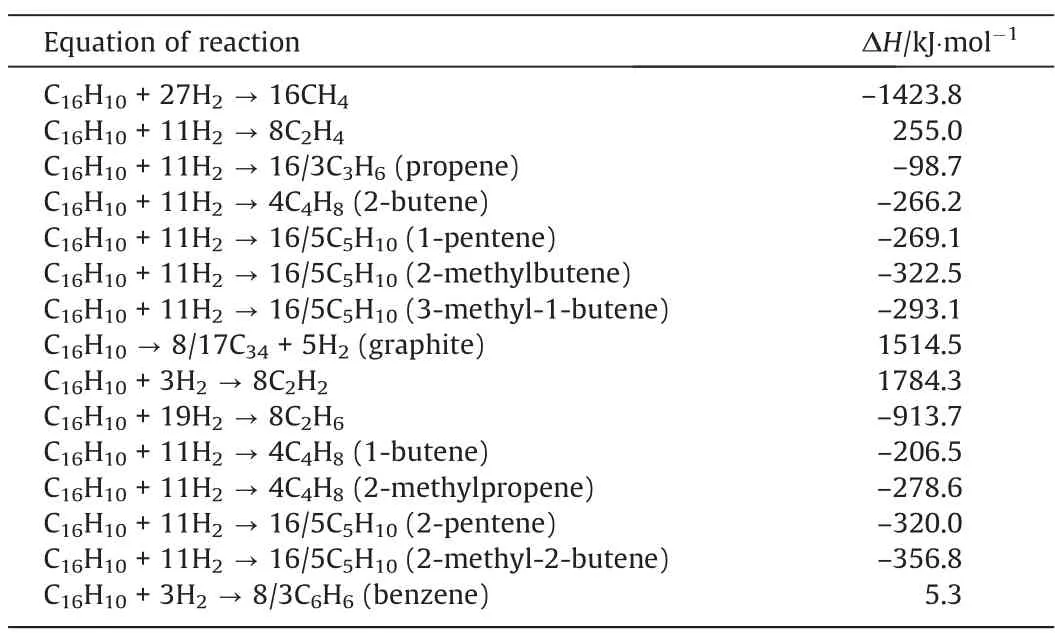
Table 2The enthalpy changes for relevant chemical reaction equations in 298 K, 0.1 MPa for coal cracking reaction
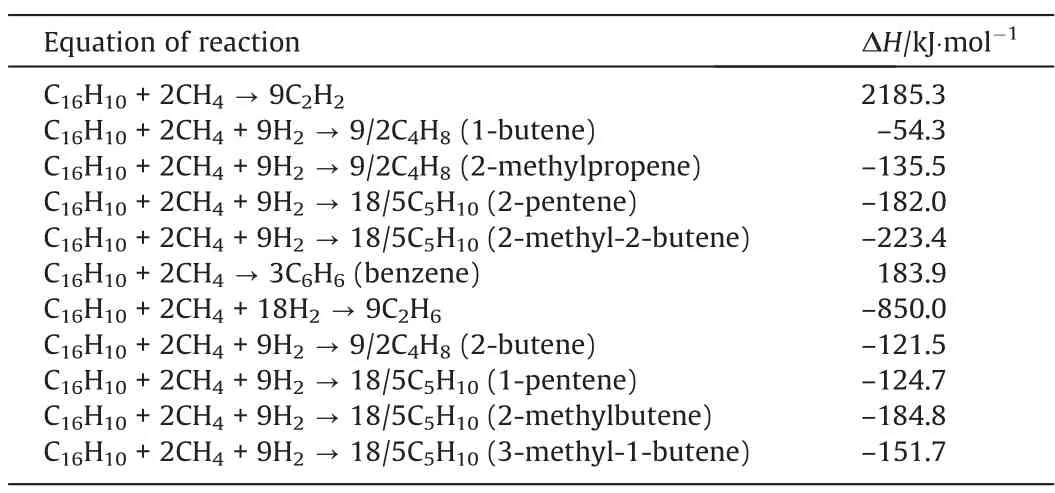
Table 3The enthalpy changes for relevant chemical reaction equations in 298 K, 0.1 MPa for co-pyrolysis of methane and coal
The plotted phase diagram for the pyrolysis of methane is shown in Fig. 2. Our calculation results show that the Gibbs free energy change(ΔG)of the pyrolysis reaction decreases as the temperature rises when the temperature is higher than 1000 K. It means that as the temperature rises, the reaction tends to occur positively. Fig. 2 presents the curves of the conversion of methane to all possible products with the ΔGof 0. On the right side of each cracking reaction curve,the ΔG<0,which means the reaction can occur. On the left side of each cracking reaction curve, the ΔG> 0,which means the reaction is thermodynamically unfavorable. The right side of cracking reaction curve of acetylene,which presented in the red area in Fig. 2, is considered to be the most favorable for the formation of acetylene, without the byproducts of propylene,butene and pentene. However, ethylene, benzene and graphite are still formed in this region. It was reported that the standard free energy of formationof acetylene,ethylene and benzene are close at around 1650 K [20], which indicated that these products may be produced at the same time. In addition, theof acetylene gradually decreased, and theof ethylene and benzene gradually increased. When the temperature was higher than 1750 K, theof acetylene was lower than that of benzene and ethylene, indicating that acetylene tended to be formed, which agreed well with our calculation results.
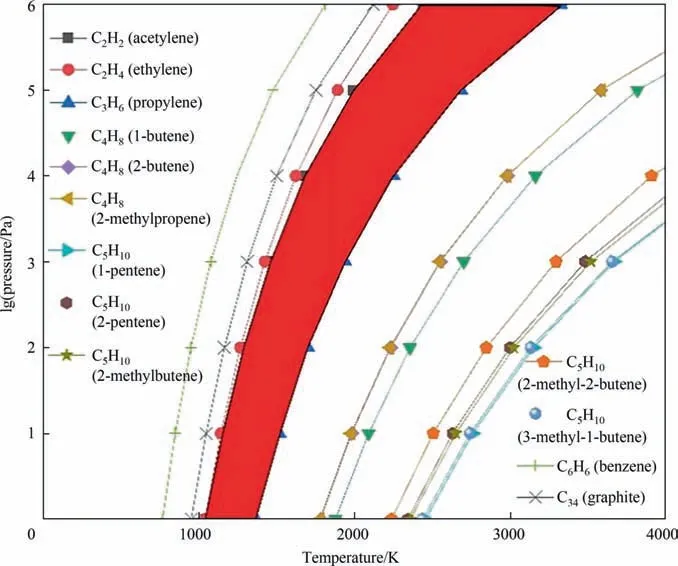
Fig. 2. Phase diagram of methane cracking reaction.
3.2. Coal decomposition
Yanet al.[6]explored the effects of coal properties and reaction conditions in coal pyrolysis.They used Ar/H2plasma and the maximum power input of plasma torch is 10 kW. In the hydrogen atmosphere, when heated to above 1800 K in within milliseconds,coal can be transformed into a gaseous mixture in which C2H2is the main product. Bondet al.[4] used Ar or Ar/H2plasma jet for the research and found that C2H2, H2and CO were main gas products. The research made by Nicholson and Littlewood [8] showed that the use of Ar/H2plasma could improve the production of acetylene. Yanet al.[6] found out that the existence of hydrogen in plasma gases could improve capability of reactor and increase the acetylene yield . The reason why the conversion of coal in Ar/H2plasma jet was much higher compared to using pure Ar is probably because H2is contributed to the conversion of carbon and therefore increased the yields of C2H2.
The plotted phase diagram for the pyrolysis of coal is shown in Fig. 3. The computational results show that the ΔGof the most pyrolysis reactions increase as temperature rises. Fig. 3 presents the curves of the conversion of coal to all possible products with ΔGof 0. On the left side of most cracking reaction curve, the ΔG< 0, which means the reaction can occur. On the right side of most cracking reaction curve,the ΔG>0,which means the reaction is thermodynamically unfavorable.However,the ΔGof coal pyrolysis to acetylene and graphite decrease as temperature rises. It means that as the temperature rises, the reaction of coal pyrolysis to acetylene tends to occur positively.On the right side of cracking reaction curve of acetylene, the ΔG< 0, which is considered to be the most favorable for the formation of acetylene. It was reported that when considered species of solid carbon and fixed C/H ratio to 6 [7], we could get the maximum acetylene content at around 3500 K. Compared to our reactions, for acetylene and benzene,we selected 12 as C/H ratio,vs.6 for other reactions. Excluding the effects of species of solid carbon and fixed C/H ratio to 6 [7],the maximum acetylene content was obtained from 1800–3000 K. With increasing C/H ratio, the mole fractions of acetylene and ethynyl radical increased. The temperature calculated by coal pyrolysis alone is slightly higher than that calculated in the above reference. It may be due to the difference in the mass ratio of C/H while our higher mass ratio of C/H is considered to be beneficial to the formation of acetylene.
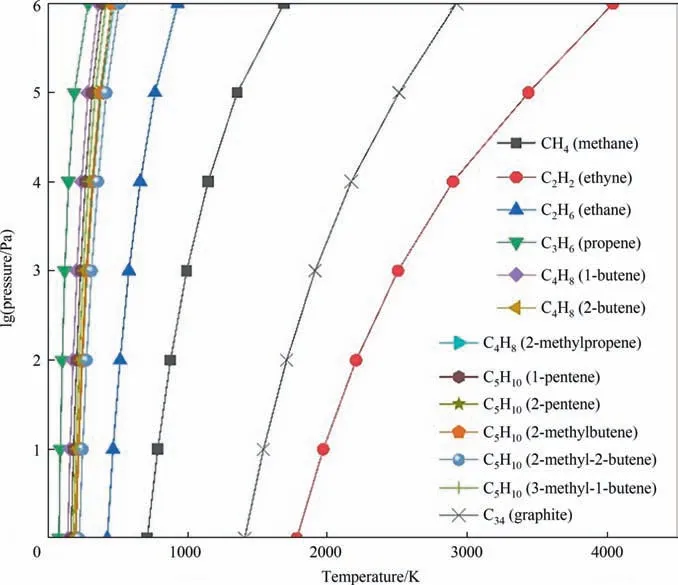
Fig. 3. Phase diagram of coal cracking reaction.
3.3. Co-pyrolysis of methane and coal
Previous researches have calculated the thermodynamic equilibrium to evaluate the effects of additional CH4in the systems coal pyrolysis. Wuet al.[7] explored the effects of additional methane in coal pyrolysis, and it could be regarded as the co-pyrolysis of methane and coal. They showed the mole fraction of C2H2increased as the increased mass flow rate of introduced CH4,which proved the co-pyrolysis of coal with CH4could be helpful to increase energy efficiency. Besides, the thermodynamic equilibrium diagrams calculated by them shows that the content of byproducts is the least at 3000 K under different methane flow rates.
The plotted phase diagram for the pyrolysis of methane and coal is shown in Fig. 4. Our calculation results show that the ΔGof most pyrolysis reactions increase as temperature rises.However,the ΔGof coal pyrolysis to acetylene and benzene decrease as temperature rises.It means that as temperature rises,the co-pyrolysis tends to produce benzene and acetylene. The red part of the Fig. 4 is considered to be the most favorable area for the production of acetylene. It was reported that the maximum mole fraction of C2H2was obtained and the least by-products were produced at around 3000 K with different mass flows of CH4as the carrier gas [7]. In our calculation, from the view of atomic economy, the C/H ratio of 1:1 is the most favorable for the formation of acetylene, which corresponds the molecule ratio of coal to methane is 1:2. From Fig. 5, the temperature at which the ΔGequals 0 decreases as the ratio of coal to methane decreases. It means that more methane in the feed favors the production of acetylene. By comparing the co-pyrolysis of coal and methane to the pyrolysis of coal and methane alone, it can be seen that the temperature of acetylene production during co-pyrolysis is lower than that of coal pyrolysis alone, and the byproducts during co-pyrolysis are less than that the pyrolysis of coal and methane alone.
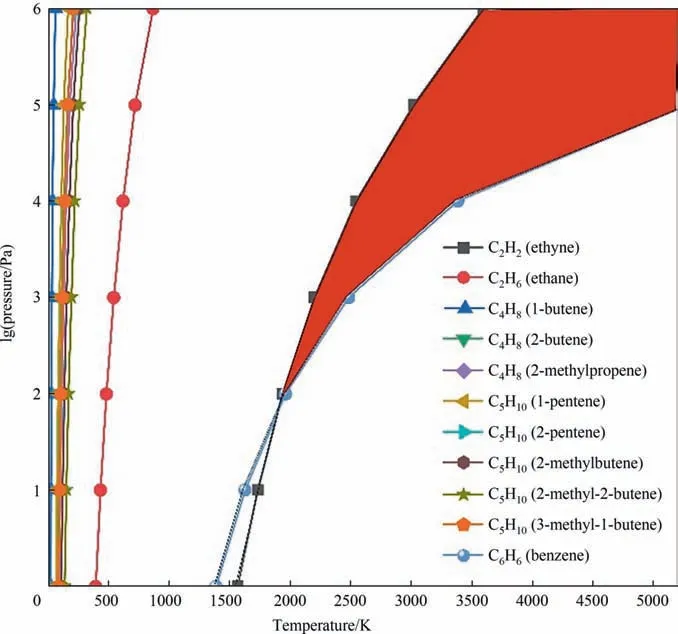
Fig. 4. Phase diagram of co-pyrolysis of methane and coal.
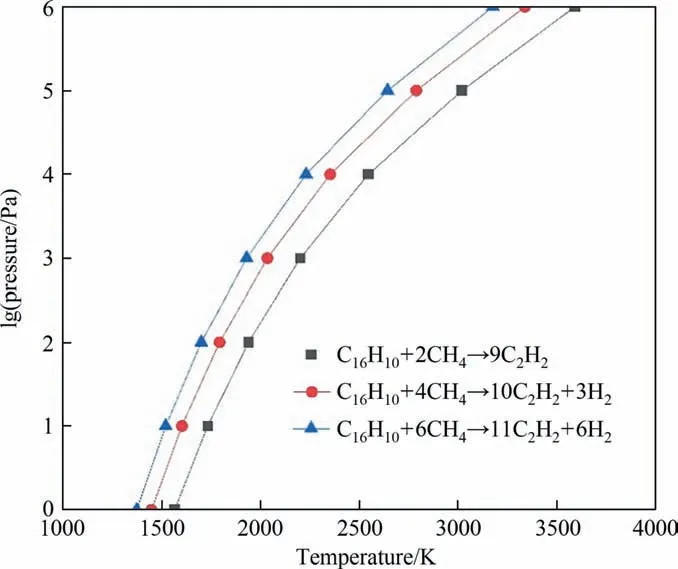
Fig. 5. Phase diagram of co-pyrolysis in various ratio of coal to methane.
4. Conclusions
The Gibbs free energy and enthalpy change(ΔGand ΔH)for the co-pyrolysis of methane and coal are calculated on the basis of density functional theory.Series of possible products and different C/H ratio are considered in our calculation. The results are presented in the form of phase diagrams showing the favorable conditions for the acetylene field.The results of methane pyrolysis alone shows that temperature higher than 1750 K and pressure at 0.1 MPa are favorable for the production of acetylene. Pyrolysis of coal alone shows temperature higher than 3400 K and pressure at 0.1 MPa are favorable for the production of acetylene. Copyrolysis of methane and coal shows that the C/H ratio of 1:1,temperature around 3000 K and pressure at 0.1 MPa are most favorable for the production of acetylene. The condition of co-pyrolysis is milder than pyrolysis of coal alone. By calculating the enthalpy change of methane cracking reaction and coal cracking reaction in 298 K and 0.1 MPa, it is found that the reactions of methane pyrolysis to acetylene, ethylene and other low carbon hydrocarbons are endothermic. The reactions of coal pyrolysis to acetylene and ethylene are endothermic. However, coal pyrolysis to byproducts is exothermic. Though the reactions of coal pyrolysis to by-products should be limited,the heat can be used as the energy absorbed by methane cracking. In addition, the coal pyrolysis,without hydrogenation reaction, is an endothermic process in a whole with the products of charred coal and coal tar.The methane pyrolysis is also an endothermic process with the products of hydrocarbon and hydrogen.However,it should be mentioned that the hydrogen produced by methane pyrolysis could be hydrogenated to the coal pyrolysis products, which is exothermic, in the co-pyrolysis reaction of coal and methane. As shown in Tables 2 and 3,the hydrogenation reactions are exothermic.It proves that there exists the energy coupling in the co-pyrolysis of methane and coal, which can improve energy utilization and lower the reaction temperature. The extra methane in the co-pyrolysis reaction is beneficial to the formation of acetylene. The calculation results could provide useful basic thermodynamics data for industrial pro-cess for the production of acetyleneviaco-pyrolysis of coal and natural gas.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This paper is dedicated to Prof.Haijun Jiao on the occasion of his 60th birthday. This work is supported by the National Natural Science Foundation of China (21875096), and the Natural Science Foundation of Jiangxi Province, China (20181BCD40004, No.20224BAB213015).State Key Laboratory of Clean and Efficient Coal Utilization of Taiyuan University of Technology (SKL2022007).Gang Feng thanks Prof. Ruo-Yu Hong, Jiyu Zhang (Fuzhou University) and Jian Zhou (SRIPT-SINOPEC) for helpful disscussions.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.05.007.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
