Preparation of sodium alginate gel microspheres catalysts and its high catalytic performance for treatment of ciprofloxacin wastewater
Gaoyan Shao, Jianjie Chen, Yuming Tu, Feng Liu, Zhiyong Zhou, Shichao Tian, Zhongqi Ren
State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Keywords: Sodium alginate gel microspheres Heterogeneous Fenton-like catalysts Dual-metal ions Hydroxyl radical Ciprofloxacin wastewater
ABSTRACT The discharge of the antibiotic wastewater has increased dramatically in our country with the development of medical science and wide application of antibiotic,resulting in serious harm to human body and ecological environment. In this work, ciprofloxacin (CIP) was selected as one of typical antibiotics and heterogeneous Fenton-like catalysts were prepared for the treatment of ciprofloxacin wastewater. The sodium alginate (SA) gel microspheres catalysts were prepared by polymerization method using double metal ions of Fe3+and Mn2+as cross-linking agents.Preparation conditions such as metal ions concentration, mass fraction of SA, polymerization temperature and dual-metal ions as crosslinking agent were optimized. Moreover, the effects of operating conditions such as initial concentration of CIP, pH value and catalyst dosage on CIP removal were studied. The kinetic equation showed that the effect of the initial concentration of CIP on the degradation rate was in line with second-order kinetics,and the effects of catalyst dosage and pH value on the degradation rate of CIP were in line with first-order kinetics.The SA gel microspheres catalysts prepared by dual-metal ions exhibited a high CIP removal and showed a good reusability after six recycles. The SA gel microspheres catalysts with an easy recovery performance provided an economical and efficient method for the removal of antibiotics in the future.
1. Introduction
Antibiotics are widely used in the control of infectious diseases[1–3], however, a large amount of antibiotic wastewater was produced in the preparation and synthesis of antibiotics, resulting in serious harm to human body and ecological environment [4].Ciprofloxacin (CIP) is a typical antibiotic with the characteristics of complex composition,large content of dissolved organic matter,high toxicity and high value of COD. In recent years, due to the drug abuse of antibiotics, the negative effects of CIP are becoming more and more serious [5]. Some of these antibiotics are metabolized in the body and more than 50%of the drug will enter the natural environment in the form of active drugs and eventually becoming persistent pollutants in water and soil when ingested by humans and animals [6,7]. The harm on the environment will be permanent if the antibiotics cannot be removed in time, therefore,it is very necessary to control antibiotics at a reasonable concentration level before discharging into the natural environment.
Recently, methods for antibiotic wastewater treatment mainly included physical method, biological method and chemical method. Physical methods commonly include adsorption, Yilmazet al.[8]synthesized Fe3O4-ACLM for adsorption of CIP with a good adsorption capacity. Although physical method could quickly remove antibiotics in wastewater [9,10], it only separated antibiotics from wastewater and did not thoroughly treat them into non-toxic substances. So it was still existing in the environment in the form of antibiotics or their derivatives, and there was still harmful to the environment. Biological method technology shows the characteristics of low cost and convenient operation, but it is difficult to achieve advanced treatment of refractory antibiotic wastewater by a single biological method[11].Advanced oxidation technologies (AOPs) such as Fenton oxidation have received much attention in antibiotic wastewater treatment because of their high oxidation, high activity and easy operation [12,13]. Among them,Fenton oxidation is an effective antibiotic wastewater treatment technology that uses the reaction of ferrous ions(Fe2+)with hydrogen peroxide(H2O2) to generate hydroxyl radicals(•OH)with high oxidizing properties.
Hydroxyl radicals with strong oxidation is generated by the H2O2decomposing under the catalytic action of Fe2+,which is used to degrade organic matter into water and carbon dioxide [14].Fenton oxidation method shows an economic and effective advanced treatment technology for antibiotic wastewater treatment. The Fenton technology could efficiently treat a variety of wastewater such as textile industrial wastewater,dinitrodiazophenol wastewater and domestic wastewater [15]. Zero valent iron(ZVI) was used to activate Fenton process and remove antibiotics,the results indicated that the application of the ZVI-Fenton technique could restrain the development of resistance in bacteria[16].Fe2+is easily oxidized to Fe3+and the reduction of Fe3+is a difficult process during the Fenton process. So adding inorganic heterogeneous cocatalysts into the reaction and establishing heterogeneous system could resolve above problem [17].
Although the Fenton technology shows some advantages and widely used in wastewater treatment,the treated wastewater containing a large amount of residual iron ions causes the color of the solution and produces a large amount of iron sludge, producing secondary pollution and increasing the treatment cost [18,19].Besides, the utilization rate of•OH in the reaction is very low,which seriously affects the oxidation performance and degradation effect of contaminant [20–22]. Based on the above problems,heterogeneous Fenton catalysts (HEFC) on the basis of the traditional Fenton oxidation technology are prepared. The principle of Fenton-like oxidation technology is utilizing H2O2to generate•OH under some conditions or some other ions [23]. The advantages of HEFC application are shown as following: Firstly, it expands the application range of the pH value during the Fenton oxidation process, so it reduces costs and avoids secondary water pollution [24]. Secondly, the HEFC greatly reduces the concentration of residual iron ions in the water and improves the utilization rate of active metal elements,without the generation of large iron sludge [25,26].
Organic carrier catalysts are made of organic matter polymerization and used in Fenton-like oxidation technology, among which, there are more ion exchange membrane catalysts and sodium alginate gel catalysts [27]. Sodium alginate works as an organic carrier because it is easy to obtain from algae and crosslink with divalent metal ions, and the carboxyl group in sodium alginate interacts with divalent ions to form gel [28]. The strong interfacial interaction is conducive to strengthening the metalcarrier bonding and effectively reduces the agglomeration of metal[29],which is great significance for improving the activity and stability of catalysts.Donget al.[30]used sodium alginate to prepare heterogeneous catalyst containing iron and degrade dye wastewater.The application range of the pH value for Fenton catalysts was successfully increased and the maximum pH value used in the system was 8.0 after the SA modification. Alginate films were prepared to fix iron ions (Fe2+and Fe3+) and improve their treatment performanceviaheterogeneous Fenton and Fenton-like processes[31].
In this work, SA gel microspheres catalysts were prepared by polymerization method using double metal ions Fe3+and Mn2+as cross-linking agents. Effects of preparation conditions such as metal ions concentration,mass fraction of SA,polymerization temperature and dual-metal ions as crosslinking agent on the catalytic performance of prepared catalysts were analyzed.The morphology of prepared SA gel microspheres catalysts were characterized by scanning electron microscope (SEM), X-ray photoelectron spectrum (XPS), X-ray diffractometer (XRD) and transmission electron microscopy (TEM). Besides, the specific surface area, pore volume,and pore diameter of the prepared SA gel microspheres catalysts were analyzed. Moreover, the optimum operating conditions such as initial concentration of CIP,pH value and catalyst dosage on CIP removal were analyzed. Thermal stability of prepared SA gel microspheres catalysts were characterized by TGA. Cycling experiments were carried out to analyze the stability and reusability of the prepared catalysts. Finally, the kinetic characteristics of CIP degradation were deeply summarized based on the analysis results.
2. Experimental
2.1. Materials
Ciprofloxacin (CIP, 99%), ferric chloride (FeCl3, AR), hydrogen peroxide(H2O2,30%),calcium chloride(CaCl2,AR),phenanthroline(C12H8N2, AR), phosphoric acidwere (H3PO4, AR), methyl alcohol(CH3OH, chromatographically pure) and manganese sulfate (Mn(SO4)2, AR) were purchased from Sinopharm Group Chemical Reagent Co., Ltd. (Beijing, China). Sulfuric acid (H2SO4, AR) and sodium acetate trihydrate (CH3COONa∙7H2O, AR) were purchased from the Beijing Chemical Plant(Beijing,China).Sodium hydroxide(NaOH, AR), potassium titanium oxalate (K2TIO(C2O4)2, AR), manganese chloride tetrahydrate (MnCl2∙4H2O, AR), sodium alginate(SA,90%)were purchased from Aladdin Chemistry Co.,Ltd.(Shanghai, China). Ferrous sulfate heptahydrate (FeSO4∙7H2O, AR), ferric nitrate(Fe(NO3)3,AR)and manganese nitrate(Mn(NO3)3,AR)were purchased from Xilong Chemical Co., Ltd. (Guangdong, China).
2.2. Catalysts preparation
SA gel microspheres catalysts were prepared by ion crosslinking method. Firstly, sodium alginate was dissolved in deionized water and placed in a water bath, heating and stirring until sodium alginate was completely dissolved. Then the sodium alginate solution was added to the crosslinking agent(metal ions,such as Ca2+,Fe3+,and Mn2+)with a basic buret,and the dripping process was kept as uniform as possible to ensure that the prepared SA gel microspheres were at the same size. Finally, the prepared SA gel microspheres were immersed in crosslinking agent for several hours to solidify. Cleaned the SA gel microspheres catalysts with deionized water for several times, and stored in sealed sample bottles for future use. In this wok, SA gel microsphere catalysts prepared by single metal ion of Fe3+was recorded as SA-1 and SA gel microsphere catalysts prepared by dual-metal ions of Fe3+and Mn2+was recorded as SA-2.
2.3. Treatment of CIP wastewater
In this experiment,degradation efficiency of CIP was used as the index to evaluate the catalyst performance.Oxidation treatment of antibiotic wastewater was carried out in a reactor containing 50 ml wastewater at room temperature ((25 ± 2) °C). The concentration of COD for antibiotic wastewater was 50–100 mg∙L–1, and the amount of catalyst was 20–100 beads. Hydrogen peroxide as the oxidizing agents were added into the reactor. The pH value of solution(3.0–8.0)was adjusted with H2SO4solution or NaOH solution. The concentration of CIP was measured with high performance liquid chromatography (HPLC, Shimadzu LC-20A), and the mobile phases were 0.1% formic acid solution and acetonitrile(Vformicacidaqueoussolution:Vacetonitrile= 80:20). The detection wavelength was 278 nm, and the column temperature was 40 °C. The degradation efficiency of CIP(D)was calculated with the following Eq. (1):
where,C0was the initial concentration of CIP (mg∙L–1) andCwas the final concentration of CIP (mg∙L–1) after treatment.
2.4. Analysis
Surface of prepared SA gel microspheres catalysts was analyzed by scanning electron microscopy (Hitachi SU8010, Japan). Surface elements and valence of the prepared catalysts were analyzed by an X-ray photoelectron spectrum (ESCALAB Xi+, America). The crystal structure of prepared catalysts was analyzed by X-ray diffractometer (Rigaku, Japan). Brunauer-Emmett-Teller surface areas for SA gel microspheres catalysts were analyzed with the N2adsorption–desorption method (ASAP 2460, America). Morphology of prepared SA gel microspheres catalysts was analyzed by transmission electron microscopy (Talos F200X G2, Czech Republic). The thermal stability of the SA gel microspheres catalysts was analyzed by thermogravimetric analysis (TGA) (Seiko Exstar 6300, Japan).
3. Results and Discussion
3.1.Effect of preparation conditions on catalytic performance of SA gel microspheres catalysts for CIP removal
3.1.1. Effect of metal ions concentration
Fe3+ions solution as the crosslinking agent is used for preparation of SA gel microspheres catalysts,so the concentration of ferric chloride is an important parameter for preparation of the catalyst and it mainly affected the crosslinking degree of sodium alginate[32].Effect of metal ions concentration on CIP removal was shown in Fig.1(a),the removal efficiency of CIP was increased from 70%to 77%with the increase of iron ion concentration,indicating that the higher concentration of Fe3+ions for catalysts preparation was benefit for CIP removal.The reasons for this phenomenon was that the higher concentration of Fe3+ions promoted the Fenton reaction and generated more ∙OH,which could degrade more CIP molecules and eventually decompose CIP into water and carbon dioxide[33,34]. Besides, the increased concentration of iron ion accelerated the polymerization degree of SA. Round sheet rather than standard microspheres shape were formed when the sodium alginate was dripped into the iron ion solution (the concentration of iron ion was increased to 0.20 mol∙L–1), reducing the specific surface area of the catalysts and influencing the catalytic performance of the SA gel microspheres catalysts. In addition, the higher concentration of iron ion resulted in the loss of iron ion during the process of CIP degradation. Therefore, the optimal concentration of iron ion was 0.10 mol∙L–1for catalyst preparation.
3.1.2. Effect of mass fraction of SA
The main material for the preparation of SA gel microspheres catalysts is SA solution, so the effect of SA concentration is an important parameter for the preparation of SA gel microspheres catalysts [35]. Therefore, concentration of SA mainly affected the catalytic performance, water holding capacity and mechanical properties of SA gel microspheres catalysts.Effect of SA concentration on CIP removal was shown in Fig. 1(b), the catalytic performance of SA gel microspheres catalysts was improved gradually at first and then decreased as the mass fraction of SA increasing.The oxygen-containing groups in sodium alginate could crosslinked with more iron ions, so the higher mass fraction of sodium alginate could produce more active sites.The increased concentration of iron ion participated in the Fenton-like oxidation reaction and generated more ∙OH[36],as a result,the catalytic performance of SA gel microspheres catalysts was increased gradually when the mass fraction of SA was increased from 1.0%to 2.0%.However,the catalytic performance of SA gel microspheres catalysts was reduced when the mass fraction of SA was 2.5%. The viscosity of SA was very large when the mass fraction of SA was higher than 2.0%, it was different to drip the SA from the buret. The dripped SA was not in the form of standard microspheres and existence in the teardrop-shaped,and the change of catalysts shape reduced the specific surface area and the active sites of SA gel microspheres catalysts. So the optimal mass fraction of SA was 2.0% for catalyst preparation.
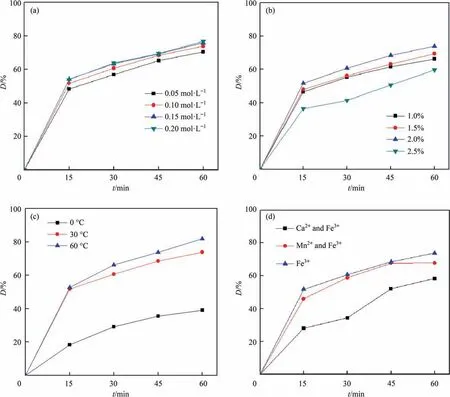
Fig. 1. Effects of preparation conditions on catalytic performance of SA gel microspheres catalysts for CIP removal: (a) concentration of metal ions; (b) mass fraction of SA;(c) polymerization temperature; and (d) dual-metal ions.
3.1.3. Effect of polymerization temperature
Temperature is one of the important parameters in the process of SA polymerization,so the temperature is also the main parameter for preparing the SA gel microspheres catalysts and influencing the degree of SA polymerization[37].During the process of SA dissolution,2.0%SA solution could not be completely dissolved within 24 h if it was only stirred without heating, while it could be completely dissolved if it was only heated for 6 h in a water bath at 60°C.Besides,temperature also has a great influence on the viscosity of SA. Effect of polymerization temperature on CIP removal was shown in Fig. 1(c), the catalytic performance of the prepared SA gel microspheres catalysts was increased with the temperature increasing. The degradation efficiency of CIP was increased from 39% to 73% when the polymerization temperature was increased from 0°C to 30°C,while,the degradation efficiency of CIP was only increased by 8% when the polymerization temperature was increased from 30 °C to 60 °C. The viscosity of SA was low at high temperature,while,the viscosity of SA was increased rapidly when the temperature was decreasing.The higher temperature was benefit for the generation of uniform SA gel microspheres catalysts during the polymerization process. Considering the energy consumption and preparation process,30°C was selected as the polymerization temperature for catalyst preparation.
3.1.4. Effect of dual-metal ions as crosslinking agent
Most of the divalent or trivalent metal ions could crosslink SA to form a polymer with egg-box structure [38,39]. In addition, the hydroxyl and carboxyl groups in SA molecule interacted with metal ions to form a complex complex [38], so the prepared SA gel microspheres showed an ‘‘egg-box” structure. The prepared SA gel microspheres catalysts using ferric chloride as the crosslinking agent had the property of poor mechanical strength [40], and almost all of the divalent and trivalent metal ions solution could be used as the crosslinking agent. The SA gel microspheres catalysts prepared using calcium chloride as crosslinking agent had a high mechanical strength, but the water holding capacity was so poor. So the influence of dual-metal ions as crosslinking agent on the catalytic performance of gel microspheres was investigated[41]. As shown in Fig. 1(d), dual-metal ions of Fe3+and Ca2+as the crosslinking agent reacted with SA had the worst effect on the catalytic performance of SA gel microspheres. The catalysts prepared with the dual-metal ions of Mn2+and Fe3+had a similar catalytic performance to that of the prepared catalysts only using Fe3+as the crosslinking agent. Mn2+ions could decompose H2O2to produce ∙OH, but Ca2+did not have this catalytic property. So the catalytic performance of SA gel microspheres catalysts prepared by dual-metal ions of Mn2+and Fe3+was far greater than that of SA gel microspheres catalysts prepared by dual-metal ions of Ca2+and Fe3+. Due to Mn2+ions showed a lower catalytic performance on H2O2than Fe3+, so the catalytic performance of SA gel microspheres catalysts prepared by dual-metal ions of Mn2+and Fe3+was inferior to that SA gel microspheres prepared by Fe3+ions alone. But the introduction of Mn2+ions in the preparation of catalysts could change the range of pH application.So the dual-metal ions of Mn2+and Fe3+were selected as the crosslinking agent for catalyst preparation.
The optimal conditions for the preparation of SA gel microspheres catalyst were summarized as following: concentration of iron ion was 0.10 mol∙L–1, mass fraction of SA was 2.0%,polymerization temperature was 30°C,and the dual-metal ions of Mn2+and Fe3+as the crosslinking agent (molar ratio of the two metal ions was 1:1).
3.2. Characterization of prepared SA gel microspheres catalysts
3.2.1. SEM analysis
The morphology of prepared SA gel microspheres catalysts under the optimal conditions were analyzed by SEM technology and shown in Fig. 2. The spherical structure of catalysts crosslinked with different metal ions were observed (Fig. 2(a) and Fig. 2(b)). Besides, it was clearly observed that the surface of gel microspheres crosslinked with single ions was smooth and wrinkle-free surfaces (Fig. 2(c)), while the surface of gel microspheres crosslinked with dual-metal ions was wrinkled (Fig. 2(d)). The catalysts prepared with Fe3+ion could only hold water,while the catalysts prepared by Mn2+ion with a high mechanical strength [42]. Therefore, the prepared SA gel microspheres with dual-metal ions of Mn2+and Fe3+as crosslinking agent were suitable for wide application.
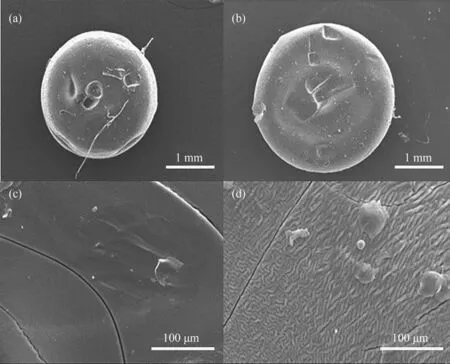
Fig. 2. SEM analysis of SA gel microspheres catalysts: (a, c) SA-1 catalysts; and (b, d) SA-2 catalysts.
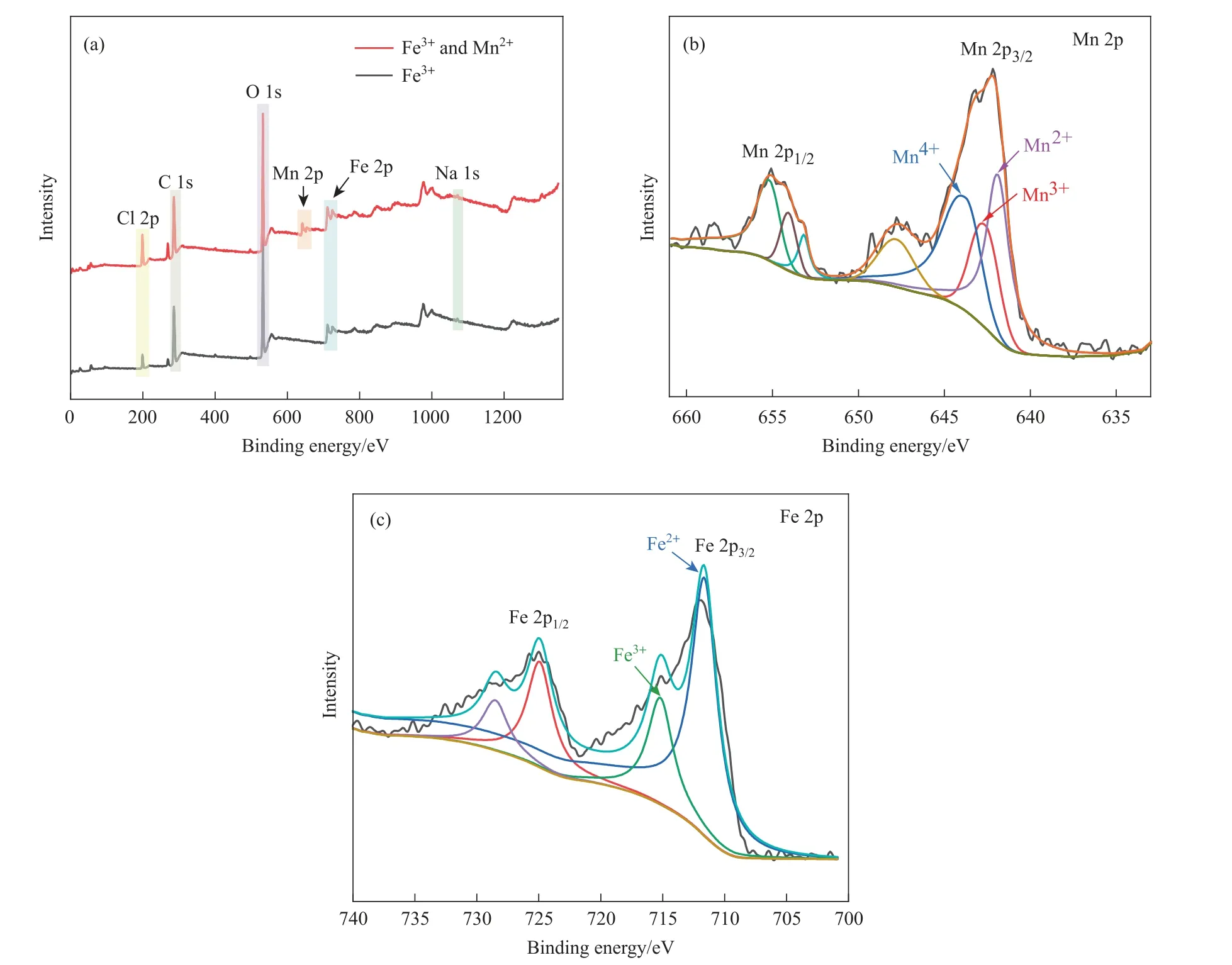
Fig.3. XPS analysis of SA gel microspheres catalysts:(a)XPS survey spectra of SA-1 catalysts and SA-2 catalysts;(b)high resolution XPS spectra of Mn 2p obtained from SA-2 catalysts; and (c) high resolution XPS spectra of Fe 2p obtained from SA-2 catalysts.
3.2.2. XPS analysis
The chemical states of the SA gel microspheres prepared using single metal ion of Fe3+and dual-metal ions of Fe3+and Mn2+were further analyzed with XPS characterization.As shown in the Fig. 3(a),peaks observed in the range of 705 to 730 eV were corresponding to Fe, which confirmed the presence of Fe element on the surface of both catalysts. Besides, peaks observed in the range of 640 to 656 eV were corresponding to Mn, which confirmed the presence of Mn element only on the surface of SA gel microsphere prepared by dual-metal ions of Fe3+and Mn2+.The element content of these two kinds of prepared SA gel microspheres was shown in the Table 1.The element content of Mn for SA-2 was 1.65%comparing with SA-1 (without Mn element), indicating the presence of dualmetal ions of Fe3+and Mn2+.
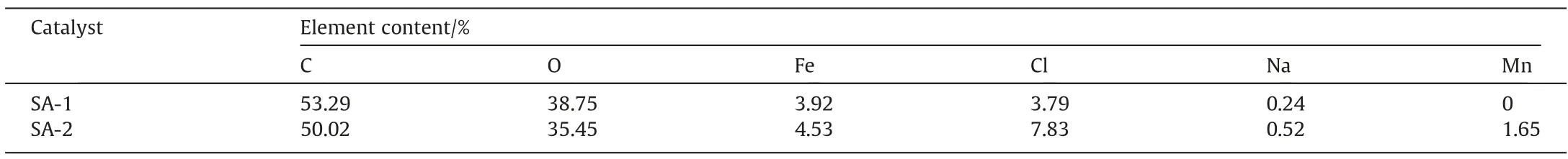
Table 1Elemental contents of SA-1 and SA-2
The high-resolution XPS spectra of Mn 2p were shown in Fig. 3(b). The characteristic peaks of Mn 2p3/2and Mn 2p1/2were observed at 641 eV and 655 eV. Three typical peaks of Mn 2p3/2are 641.98, 642.88 and 644.08 eV, corresponding to Mn2+, Mn3+and Mn4+respectively [43]. The high-resolution XPS spectra of Fe 2p were shown in Fig. 3(c). The characteristic peaks of Fe 2p3/2and Fe 2p1/2were observed at 712.08 eV and 710.58 eV. Two typical peaks of Fe 2p3/2were 708.63 and 710.32 eV,corresponding to Fe2+and Fe3+respectively [44]. The presence of dual-metal ions and the multiple valence states of the elements was conducive to the decomposition of H2O2to generate active free radicals, and then promoted the degradation of organic matter [45].
3.2.3. BET analysis
Morphology of prepared SA gel microspheres catalysts were characterized.BET technology was used to analyze the specific surface area, pore volume, and pore diameter of the prepared SA gel microspheres catalysts. As shown in Fig. 4, a typical type-V isotherm with H3-type hysteresis loop at a high relative pressure(P/P0= 0.7–0.97) was observed, indicating the presence of mesoporous structure in the both catalysts [46]. The specific surface area of different catalysts was shown in the Table 2, the specific surface area and pore volume of SA-2 were slightly reduced.Although Mn ions distributed in some pores reduced the specific surface area and pore volume of the catalyst, the mesoporous structure is beneficial to the chemisorption of CIP molecules on the catalyst surface.
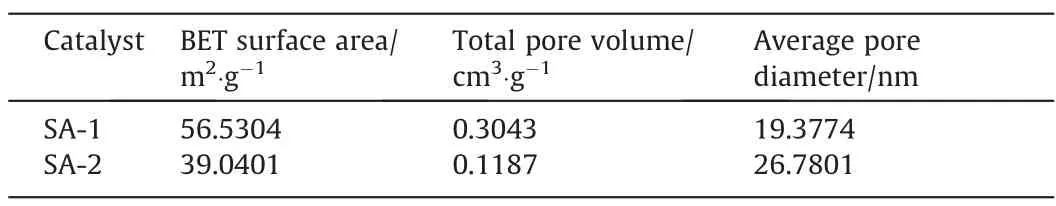
Table 2Specific surface area of different catalysts
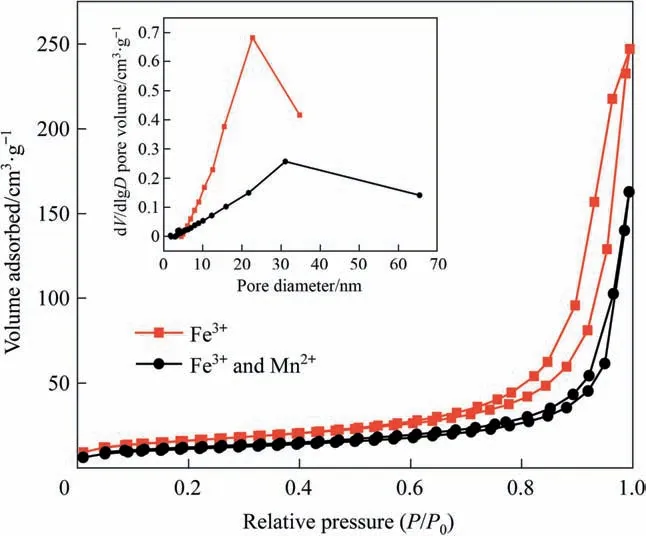
Fig. 4. BET analysis of SA gel microspheres catalysts.
3.2.4. TEM analysis
TEM characterization showed the three-dimensional network pore structure of the catalyst, which was consistent with BET results that the prepared catalysts with a mesoporous structure(Fig.5(a)).The mesoporous structure is conducive to strengthening the mass transfer and diffusion process in the oxidation process,and increasing the good contact between organic molecules and the active sites [47]. The elements of C, O, Fe and Mn were well distributed on the catalysts without obvious agglomeration(Fig. 5(b)–(e)). In addition, the elemental content distribution of SA gel microspheres catalysts was observed using EDS (Fig. 5(f)),which consisted of C, O, Fe and Mn. It was demonstrated that the SA gel microsphere catalysts were successfully prepared.
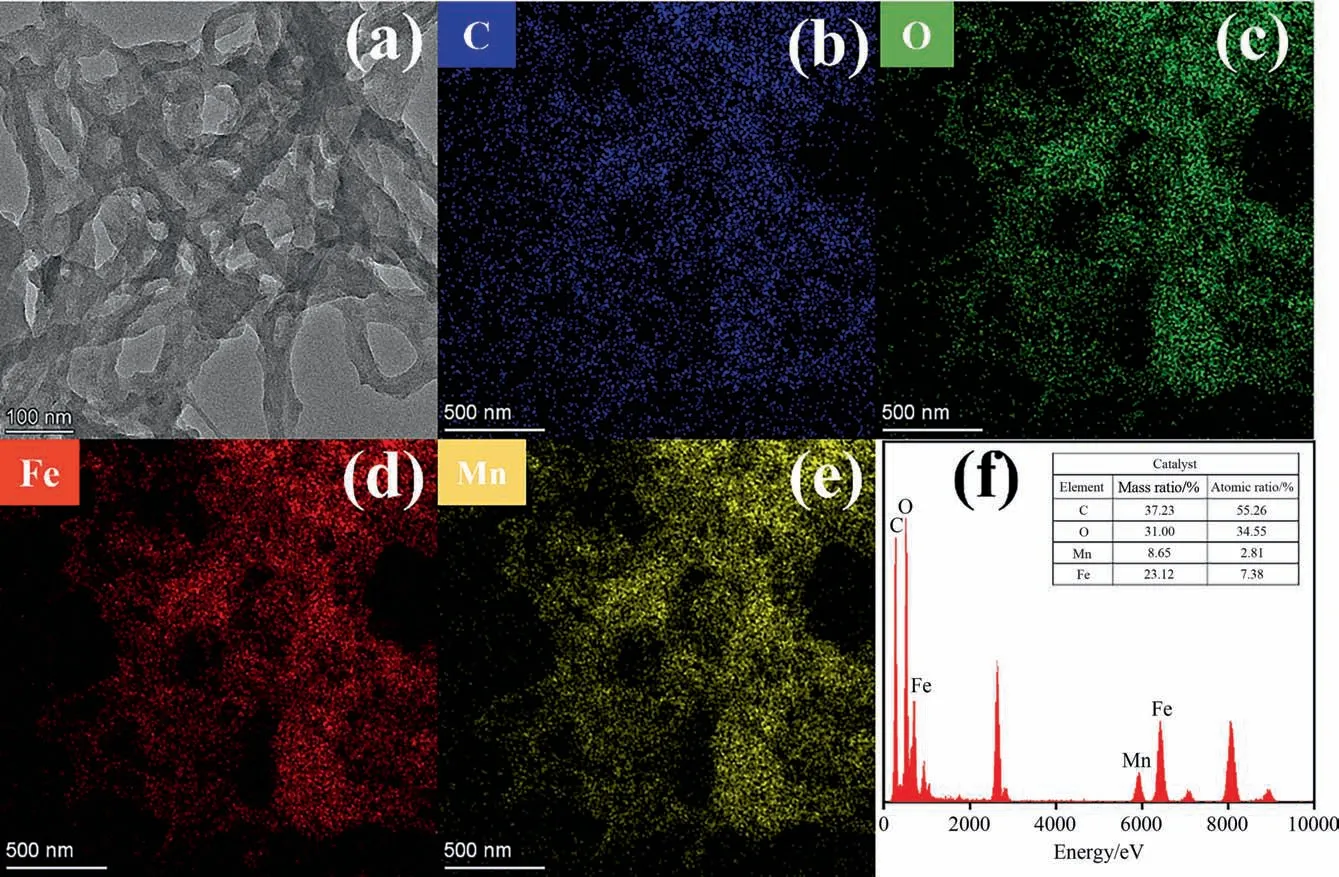
Fig. 5. (a) HRTEM images of SA-2 catalysts; Elemental mapping images of SA-2 catalysts: (b) C, (c) O, (d) Fe, (e) Mn, and (f) EDS analysis of SA-2 catalysts.
3.3. Effect of operating conditions on catalytic performance of SA gel microspheres for CIP removal
3.3.1. Effect of initial concentration of CIP
As the initial concentration of CIP was one of the important parameters for the investigation of Fenton oxidation process, it mainly affected the utilization of Fenton reagent during the oxidation process. So the effect of initial concentration of CIP on CIP removal was shown in Fig. 6(a). The degradation efficiency of CIP was lower as the initial concentration of CIP increasing,the degradation efficiency of CIP was 76% at the initial concentration of 50 mg∙L–1and it was 49% at the initial concentration of 200 mg∙L–1. The breaking degree of chemical bond for CIP molecules was varied at different initial concentrations.Low concentration of CIP wastewater was benefit for CIP molecules decomposing into products such as water and carbon dioxide. The degradation efficiencies of CIP at the initial concentrations of 50 mg∙L–1and 100 mg∙L–1were almost similar,therefore, the optimal initial concentration of CIP in the system was selected as 100 mg∙L–1.
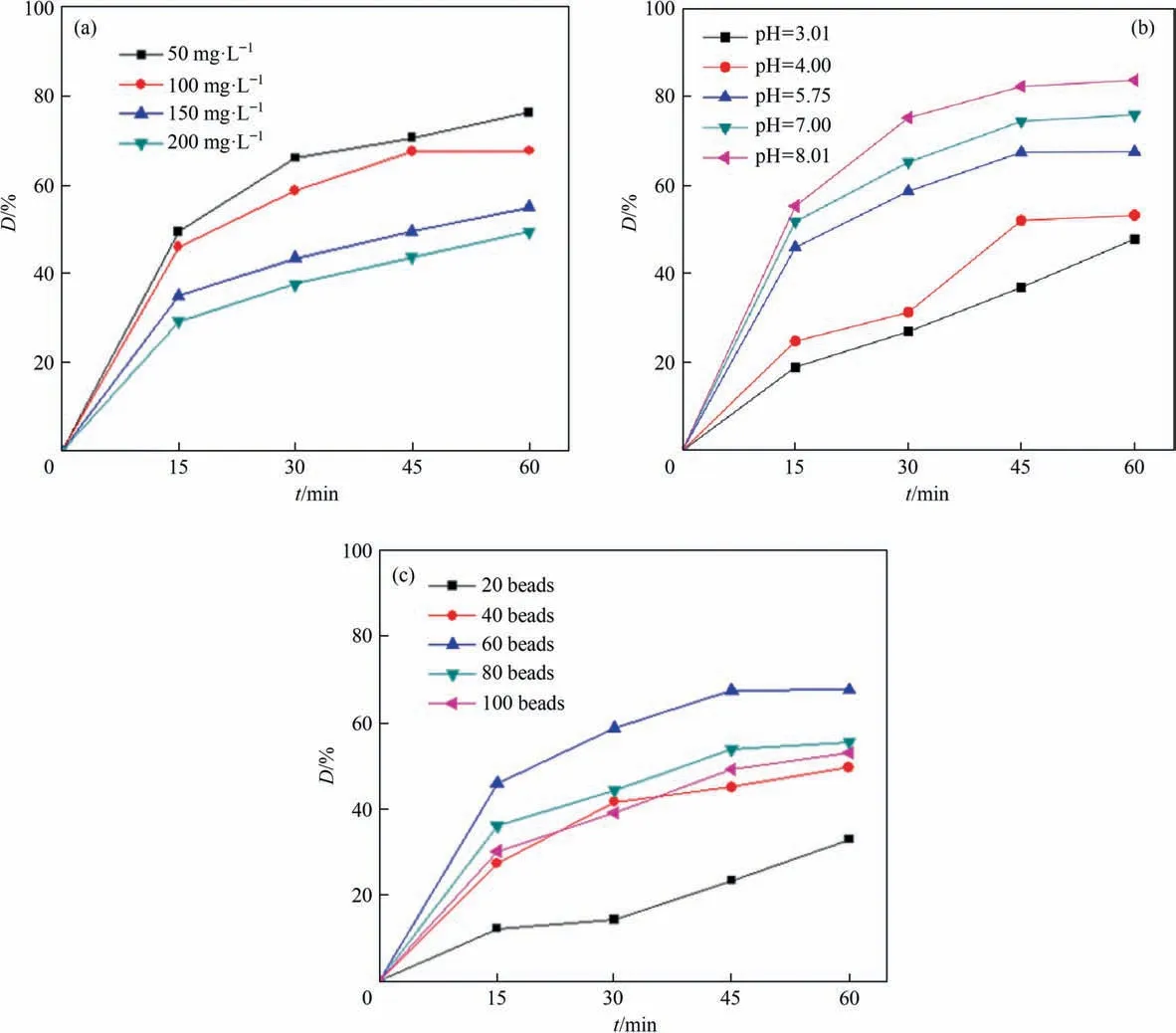
Fig. 6. Effects of operating conditions on CIP removal: (a) initial concentration of CIP; (b) initial pH value; and (c) catalyst dosage.
3.3.2. Effect of pH
Effect of pH on CIP removal was shown in Fig.6(b),the degradation efficiency of CIP was increased as the pH of the system gradually increasing, which was different from the traditional Fenton reaction. The introduction of Mn ions for the prepared SA gel microspheres catalysts would change the range of pH application.Mn ions were benefit for H2O2transforming into, and further generating ∙OH andunder alkaline condition. Both ∙OH andhad a strong oxidation capacity to degrade CIP more thoroughly[48]. Although the degradation efficiency of CIP was not the highest under the pH of 5.75, it showed the higher degradation rate.The wide application of pH for SA gel microspheres catalysts avoided the adjustment of pH during the treatment of CIP wastewater, saving lots of cost and avoiding introduction of other ions.
3.3.3. Effect of catalyst dosage
Dosage of SA gel microspheres catalysts was an extremely important factor in Fenton reaction.The different ratios of catalyst dosage and hydrogen peroxide would directly affect the utilization efficiency of hydrogen peroxide reagent. Effect of catalyst dosage on CIP removal was shown in Fig. 6(c), the degradation efficiency of CIP was first increased and then decreased with the increase of catalyst dosage, and the degradation efficiency of CIP was 67%when the catalyst dosage was 60 beads.As shown in the following Eqs. (2)–(4), the increased amount of SA gel microspheres could produce more ∙OH [49], so the degradation efficiency of CIP could increase gradually.However,Fe2+ions would react with ∙OH when the amount of catalyst was too much, it was not beneficial for CIP removal. Therefore, the dosage of catalysts was selected as 60 beads in this study.
3.4. TGA analysis
In order to confirm the thermostability of the SA gel microspheres catalysts, the relationship between mass and temperature was analyzed by TGA analysis. As shown in Fig. 7, the mass of SA was decreased slowly when temperature was below 220°C,which was attributed to the existence of water molecules [50]. The mass of SA was decreased fast when temperature was between 220–360°C, and the molecular skeleton of SA was broken into some relatively stable intermediates.The mass of SA decreased slowly when temperature was above 360 °C. For SA gel microspheres catalysts,it showed a better thermal stability when the temperature was lower than 170 °C. And the thermal stability of the catalysts was also significantly higher than that of SA molecules when the temperature was between 250–360 °C. It proved that iron ion crosslinked with hydroxyl groups could improve the thermal stability of SA gel microspheres catalysts.
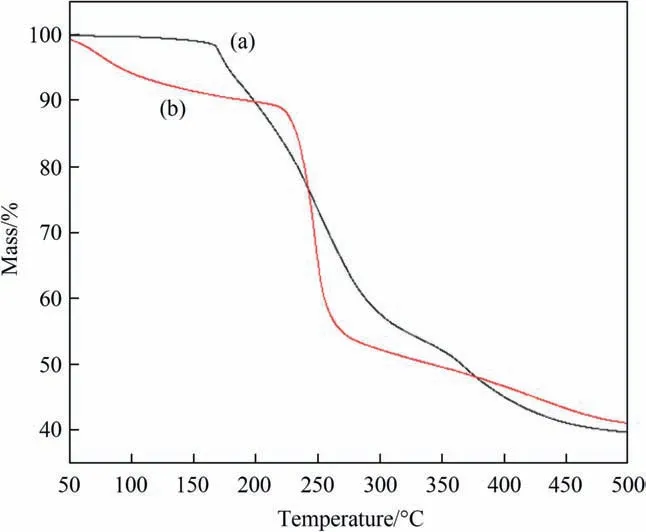
Fig. 7. TGA analysis: (a) SA-2 catalysts; and (b) SA molecule.
3.5. Stability analysis of the SA gel microspheres catalysts
The stability performance was an important index to determine the application of catalysts. As shown in Fig. 8(a), the degradation efficiency of CIP was above 65% and remained unchanged among sixth recycle, which indicated that the structure and active site of SA gel microspheres catalysts remained unchanged after repeated recycles and the catalytic performance was still maintained. Besides, SA was a biodegradable polymers, and it caused very little pollution to the water environment.
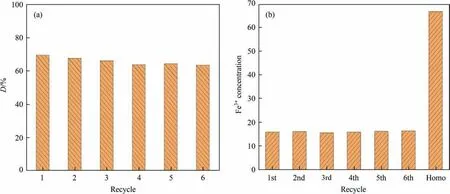
Fig. 8. (a) Stability analysis of the heterogeneous catalysts among six recycles; and (b) concentration of Fe3+ in the heterogeneous and homogeneous system.
The concentrations of dissolved iron ions in the heterogeneous and homogeneous system were measured to further analyze the stability of SA gel microspheres catalysts. As shown in Fig. 8(b),the concentration of iron ions dissolved from SA gel microspheres catalysts was about 15–16 mg∙L–1in the heterogeneous system. A comparison experiment was carried out to treat CIP wastewater using the traditionally homogeneous Fenton oxidation process.The concentration of dissolved iron ion was 66.8 mg∙L–1, which was much higher than that in the heterogeneous system. Besides,the results also confirmed that the mechanism of the heterogeneous catalyst was not entirely dependent on the dissolved iron ions. Therefore, the heterogeneous catalysts could effectively reduce the concentration of iron ions, reducing large amount of iron sludge and saving cost of post-treatment.
XRD, SEM and TEM were used to further investigate the structural morphology of the catalysts before and after the reaction.The crystal structure of the reacted catalyst did not change, indicating that the reaction sites of the catalysts were still retained after reuse(Fig.S1).The catalysts had a network-like porous structure before and after the reaction (Fig. 9(a)–(d)), and the catalyst surface was smooth after the reaction, indicating that the organic pollutants were effectively degraded and did not accumulate on the catalyst surface. The internal structure of the catalyst before and after the reaction was further investigated using TEM, and the results showed that the recycled catalysts retained the network-like structure (Fig. 9(e)–(f)). In summary, the SA gel sphere microsphere catalysts had an excellent stability.
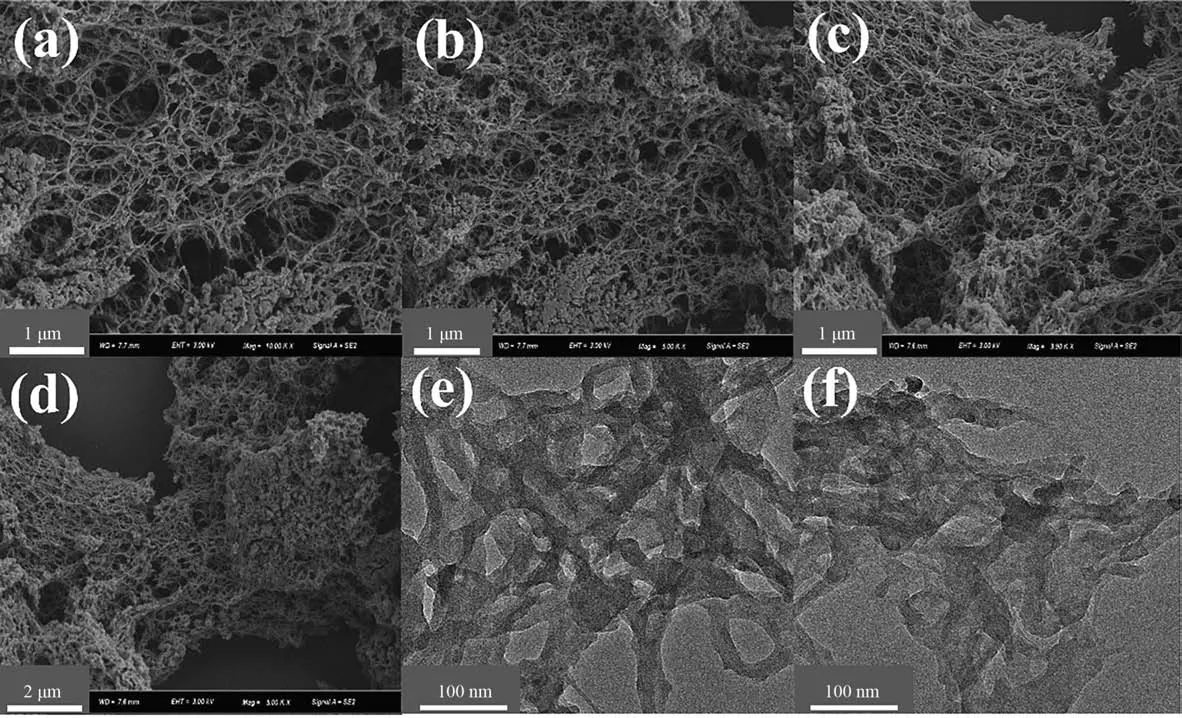
Fig. 9. (a, b) SEM images of catalysts before use; (c, d) SEM images of catalysts after use; (e) TEM images of catalysts before use; (f) TEM image of catalysts after use.
3.6. Kinetics analysis of degradation of CIP with SA gel microspheres catalysts
3.6.1. Influence of initial concentration of CIP on degradation rate of CIP
In order to investigate the degradation rate of CIP in different concentration of CIP, the obtained data was analyzed with zeroorder, first-order and second-order reaction equation dynamics,respectively [51]. As shown in Table 3, the degradation rate of CIP wastewater at different initial concentrations of CIP were more in line with second-order reaction kinetics. According to the Fig. 10(a), the higher the initial concentration of CIP, the lower the degradation rate of CIP. To verify the above results, the initial concentration of CIP and rate constantKwere fitted. As shown in the Fig. 10(b), the relationship between initial concentration and degradation rateK, and kinetic equation of second-order reaction were shown as following(Crepresented the concentration of CIP):
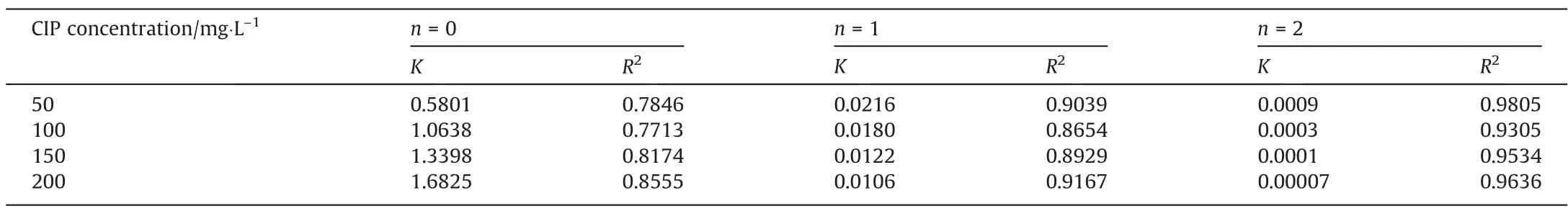
Table 3Influence of initial concentration of CIP on degradation rate constant under different reaction orders
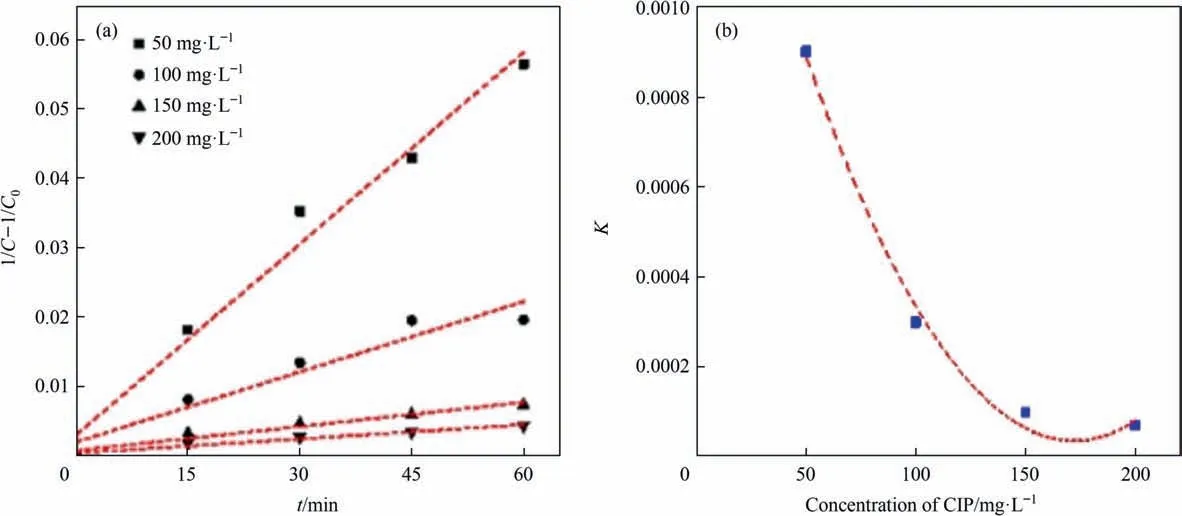
Fig. 10. (a) Second-order kinetic rate under different initial concentrations of CIP; and (b) relationship between K and initial concentrations of CIP.
3.6.2. Influence of pH on degradation rate of CIP
In order to investigate the degradation efficiency of CIP in different pH values, the obtained data was analyzed with zero-order,first-order and second-order reaction equation dynamics, respectively. As shown in Table 4, the degradation rate of CIP wastewater under different initial pH values were more in line with first-order reaction kinetics. According to the Fig. 11(a), the lower the pH value,the lower the degradation efficiency of CIP.To verify the above results, the initial pH value and rate constantKwere fitted, as shown in the Fig. 11(b). The relationship between initial pH value and degradation rateK, and kinetic equation of first-order reaction were shown as following(Xrepresented initial pH value):
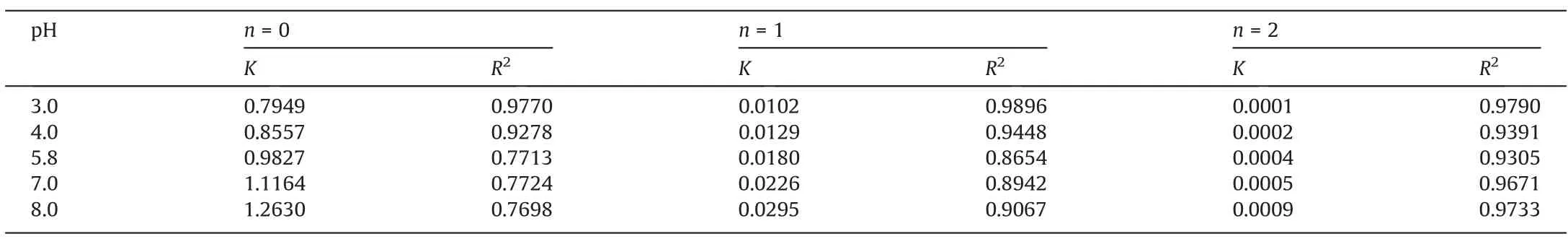
Table 4Influence of initial pH on degradation rate constant under different reaction orders
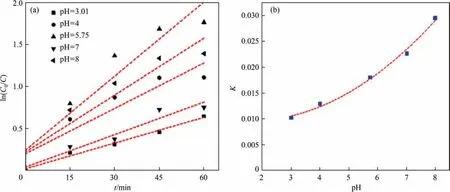
Fig. 11. (a) First-order kinetic rate under different initial pH; and (b) relationship between K and initial pH.
3.6.3. Influence of HEFC dosage on degradation rate of CIP
In order to investigate the degradation efficiency of CIP, the obtained data was analyzed with zero-order, first-order and second-order reaction equation dynamics, respectively. As shown in Table 5, the degradation rate of CIP wastewater with different HEFC dosages were more in line with first-order reaction kinetics.According to the Fig. 12(a), the degradation rate of CIP was first increased and then decreased with the increase of HEFC dosage,and the degradation rate of CIP reached the maximum when the HEFC dosage was 60 grains. To verify the above results, the HEFC dosage and rate constantKwere fitted, as shown in the Fig. 12(b). The relationship between HEFC dosage and degradation rateK, and kinetic equation of first-order reaction were shown as following (Xrepresented dosage of catalysts):
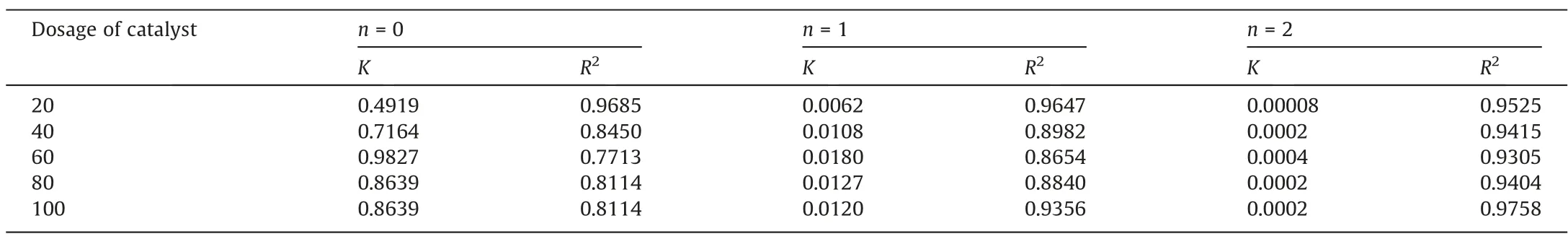
Table 5Influence of catalysts dosage on degradation rate constant under different reaction orders
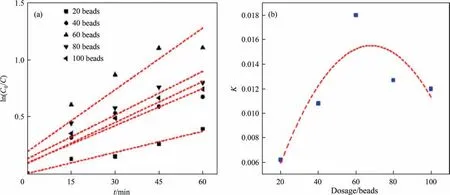
Fig. 12. (a) First-order kinetic rate under different catalyst dosage; and (b) relationship between K and catalyst dosages.
To evaluate the catalytic performance of the SA gel microsphere catalyst,catalytic performances of the current catalyst in this work with some recently reported catalysts have been compared. Asshown in Table 6, the degradation efficiency of CIP for SA gel microsphere catalyst was higher than the results reported in the literature, indicating its excellent catalytic activity.
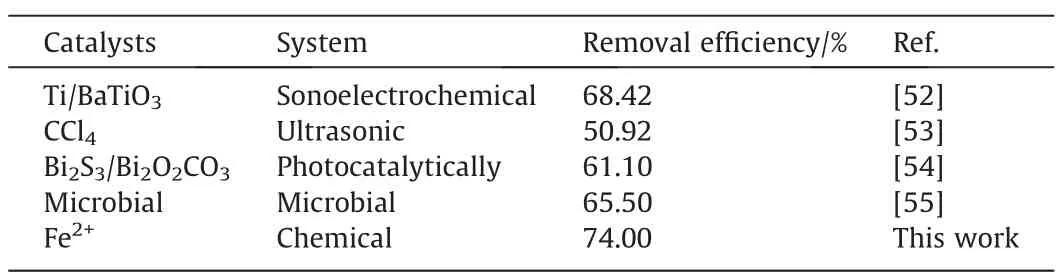
Table 6Catalytic performance of the current catalysts with some recently reported catalysts for CIP degradation
4. Conclusions
In this study, CIP was selected as one of the typical antibiotics and heterogeneous Fenton-like catalysts were prepared for the treatment of CIP wastewater. Optimal preparation conditions for SA gel microspheres catalysts were obtained: concentration of Fe3+was 0.1 mol∙L–1, mass fraction of sodium alginate was 2.0%,and polymerization temperature was 30 °C.In order to strengthen the mechanical properties of the catalysts, double metal ions of Mn2+and Fe3+were used as cross-linking agents.Besides,the optimal operating conditions in the heterogeneous system were investigated and obtained: concentration of CIP was 100 mg∙L–1, pH of the system was about 5.75, and dosage of catalysts was 60 beads.The prepared mesoporous structure was beneficial to the chemisorption of CIP molecules on the catalyst surface, and the presence of dual-metal ions and the multiple valence states of the elements was conducive to the decomposition of H2O2to generate active free radicals. The prepared catalysts showed a high catalytic activity and a good reusability after repeated use.Finally,the analysis of kinetic equation showed that the effect of initial concentration of CIP on the degradation rate of CIP was in line with second-order kinetics, and the effects of catalyst dosage and pH value on the degradation rate of CIP were in line with first-order kinetics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22125802 and 22108012), Natural Science Foundation of Beijing Municipality (2222017), and Fundamental Research Funds for the Central Universities (BUCTRC-202109).The authors gratefully acknowledge these grants.
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.05.008.
 Chinese Journal of Chemical Engineering2023年11期
Chinese Journal of Chemical Engineering2023年11期
- Chinese Journal of Chemical Engineering的其它文章
- Effects of the original state of sodium-based additives on microstructure,surface characteristics and filtration performance of SiC membranes
- Comprehensive analysis on the economy and energy demand of pressure-swing distillation and pervaporation for separating waste liquid containing multiple components
- Esterification of acetic acid with isobutanol catalyzed by ionic liquid n-sulfopropyl-3-methylpyridinium trifluoromethanesulfonate:Experimental and kinetic study
- Numerical investigation of film forming characteristics and mass transfer enhancement in horizontal polycondensation kettle
- COF-derived Co nanoparticles@N-doped carbon electrocatalysts for highperformance Zn-air batteries
- A potential-responsive ion-pump system based on nickel hexacyanoferrate film for selective extraction of cesium ions
