Effect of boron species on carbon surface on oxidative dehydrogenation of propane
Tingcong Wang,Mingyuan Zhu
Department College of Chemistry and Chemical Engineering, Yantai University, Yantai 264005, China
ABSTRACT Carbon catalysts for propane oxidative dehydrogenation(PODH)can potentially replace metal oxide catalysts due to their environmental friendliness (greenness) and excellent catalytic performance.Biomass carbon materials have the advantages of being abundant in variety,inexpensive,and easily available,but their catalytic selectivity is relatively poor in PODH.Therefore,we report here on a boron-doped sisal fiber carbon catalyst,which showed excellent selectivity of propylene in PODH,excluding the effect of surface-covered B2O3 on the catalytic performance by hot water washing.The carbon material exhibited the best catalytic performance with a load of 2% (mass) and a calcination temperature of 1100 °C.At a reaction temperature of 400 °C,the conversion rate of propane was 2.0%,and the selectivity toward propylene reached 88.6%.The new chemical bonds formed by boron on the surface of the carbon materials had an important effect on the catalytic performance,as determined by XPS characterization.The B-O groups affected the catalytic activity by inhibiting the generation of electrophilic oxygen species,while the B-C content improved the selectivity toward propylene by changing the electron cloud density.
1.Introduction
Propylene resources have attracted extensive attention from researchers due to their wide application in the energy field [1].At present,their preparation process mainly relies on hightemperature catalytic cracking,steam cracking,the coal-toolefins process,and other methods [2].As an emerging process,the catalytic dehydrogenation of propane to propylene has received considerable interest from scientists due to its low energy consumption and high selectivity compared with other processes.However,direct propane dehydrogenation(PDH)suffers from high reaction temperatures and the easy deactivation of the catalyst due to carbon deposition [3].Therefore,the propane oxidative dehydrogenation (PODH) process presents a good alternative to the PDH reaction.PODH is an exothermic reaction that is not bound by thermodynamic considerations.Compared with PDH,PODH involves a lower reaction temperature,and the catalyst is less prone to coking and deactivation [4].However,the overoxidation of propylene (which affects the catalytic selectivity),the safety of the process,and other issues limit the industrial application of PODH;thus,the preparation of efficient,safe,and green catalysts is a hot research topic.
Currently,two main types of catalysts exist for PODH reactions:metal-and non-metal-based ones.Metal-based catalysts mainly study vanadium [5],chromium [6],molybdenum [7],and other metals and their metal oxides [8,9],but such catalysts are highly toxic,environmentally unfriendly,and not conducive to the concept of sustainable development.Nonmetallic catalysts mostly comprise carbon-based [10,11] and molecular sieve [12,13] catalysts.Among them,carbon materials have been extensively studied in PODH reactions and exhibit excellent catalytic performance due to their stable physical and chemical properties,large specific surface areas,abundant surface oxygen-containing functional groups,and pore structures [14].However,their greatest disadvantage is their poor catalytic selectivity in the PODH reaction[15,16].Therefore,the modification of carbon materials to improve their catalytic performance has also become a hot research topic.
Heteroatom doping constitutes one of the methods to improve the performance of carbon catalysts in PODH.Non-metallic atoms such as nitrogen[17],phosphorus[18],and boron[10,11]are often used as heteroatoms for doping carbon materials.Boron species,especially B2O3,have formed the most popular dopants for inhibiting side reactions and enhancing the selectivity of carbon catalysts.Marcoet al.[15] used boric acid as a precursor to obtainboron-modified carbon nanofibersviahigh-temperature calcination,with different calcination temperatures resulting in the formation of different boron species on the surface of the carbon material.The catalytic performance was affected because boron doping generated new oxygenated surface groups,with B2O3clusters covering the pore walls and individually improving the conversion and the catalytic selectivity.Appropriate amounts of boric acid became adsorbed onto the catalytic sites of side reactions to inhibit the generation of by-products,such as COx,and improve the reaction selectivity.However,Franket al.[16] proposed a different viewpoint and concluded that B2O3clusters could inhibit the generation of surface electrophilic oxygen species,which were significant causes of overoxidation in PODH.The optimal amount of boron on the surface of the carbon catalysts has also been under debate;excessive B2O3clusters would cover a large number of catalytic sites,resulting in poor catalytic stability at high reaction temperatures [15,19].
In this paper,we used boron-doped sisal fiber carbon as the model catalyst in the PODH reaction.To eliminate their influence on the reaction,the B2O3clusters covering the catalyst surface were removed by hot water washing.In addition,we changed the contents of the B-C and B-O groups using different boron loading amounts to investigate their impact on the reaction.We found that the B-O groups mainly affected the conversion of propane,while the B-C groups mainly influenced the selectivity toward propylene.These conclusions were justified by characterizing the fresh catalyst and the catalysts used in different reaction time.
2.Experimental
2.1.Materials
The sisal fiber was purchased from Longzhou Qiangli Hemp Industry Co.,Ltd.H3BO3solid (99.5%) was purchased from Sinopharm.Quartz sands (AR) were purchased from Guangfu Technology Development Co.,Ltd.The propane (99.5%),O2(99.999%),Ar(99.999%) and N2(99.999%) were purchase from Yantai Feiyuan special gases Co.,Ltd.H2(99.999%) and air were obtained by H2and air generator,separately.
2.2.Catalyst preparation
The sisal fiber carbon material was prepared by alkali activation,with reference to a method described in Ref.[20].The sisal fibers were cut into small pieces and calcined at 550 °C for 3 h in a nitrogen atmosphere to obtain pre-carbonized sisal fiber carbon.2.0 g pre-carbonized material and 2.0 g KOH were mixed with 10 ml water,and the mixture was stirred for 1 h.The resulting solution was left to stand for 1 h and then dried at 120 °C overnight.The obtained solid was calcined at 900°C for 3 h under nitrogen,and then washed until neutral with 2 mol∙L-1hydrochloric acid and deionized water.After drying,the alkali-activated sisal fiber carbon material was obtained,denoted as SFC.
A series of boron-doped sisal fiber carbons were prepared by the isometric impregnation method,using H3BO3as the source of boron.2.0 g SFC and 0.23 g H3BO3particles were weighed according to different B: C mass ratios (Eq.(1)) and dissolved in 5 ml deionized water using ultrasound treatment,and the surface of the SFC was impregnated with the resulting solution.After drying at 120°C for 12 h,the obtained solid was calcined at 1100°C for 2 h in an argon atmosphere.To remove the B2O3crystals covering the catalyst surface,the solid was washed with a large amount of hot deionized water and dried at 120 °C for 12 h to obtainx% B–SFC catalyst wherexrepresents the mass percentage of boron atoms and calculated by Eq.(1),wheren(Batom)represents the mole of the B atoms,M(Batom)represents the relative atomic mass of B,m(Carbon)represents the mass of the SFC.
2.3.Catalyst characterization
X-ray powder diffraction (XRD) was performed by SmartLab(XRD6000)using 45 kV and a 2000 mA instrument with Cu Kα radiation.Nitrogen adsorption–desorption isotherms and specific surface area were characterized by the ASAP-2460 physical adsorption apparatus at -196 °C.The samples were required to be degassed pretreatment in a vacuum at 150 °C for 4 h.The specific surface area (SBET) was calculated by the Brunauer-Emmett-Teller (BET)method.Pore volume (Vpore) was calculated by Barrett-Joyner-Halenda (BJH) method,and pore diameter (Dpore) was adsorption average pore diameter.Scanning electron microscopy (SEM)images were obtained with TESCAN MIRA4 instrument at 20 kV.Transmission electron microscopy (TEM) images of the materials were recorded on an FEI Talos F200s instrument (operated at 200 kV).Raman spectra were obtained using an inVia Raman spectrometer with λ=532 nm.Thermogravimetric analysis (TGA) was performed on a Netzsch TG 209 F3 Tarsus instrument in steps of 10 °C∙min-1from room temperature to 800 °C under nitrogen and air atmospheres,respectively.X-ray photoelectron spectroscopy (XPS) was obtained from the Thermo Scientific K-Alpha spectrometer.The X-ray source was from Al Kα with 1486.6 eV,and all the spectra were corrected using the C 1s peak at 284.8 eV.
2.4.Catalyst test
All the catalysts were tested in an atmospheric pressure fixedbed reactor.The reactor was a stainless-steel tube with an inner diameter of 10 mm and a length of 600 mm containing 2 ml of catalyst diluted with quartz sand with a particle size of 0.25 mm to 0.38 mm at 400 °C.The gaseous reactants included C3H8,O2and Ar(C3H8:O2:Ar molar ratio=2:1:37,total flow rate=40 ml∙min-1).The exhaust gas was analyzed with a gas chromatograph(GC,9790Ⅱ).CH4,C2H6,C2H4,C3H8,and C3H6were detected using a flame ionization detector with a Kromat Al2O3/Na2SO4chromatographic column.The permanent gases,such as O2,CO,and CO2,were analyzed by a thermal conductivity detector(TCD)with Porapak Q and TDX-01-packed columns.All gases were quantified using the external standard method.The propane conversion(Eq.(2))and propylene selectivity (Eq.(3)) were calculated as follows:
where(nC3H8)inrepresents the molarity of C3H8entering the reactor,and (nC3H8)outrepresents the molarity of C3H8in the exhaust gas.ThenC3H6indicates the molarity of C3H6in the reactor outlet.
The specific activity (SA) was calculated as in Eq.(4):
whereXC3H8represents the C3H8conversion,FC3H8represents the molar flow rate of C3H8in the feed gas;Wcatis the mass of the catalyst,and SSA denotes the specific surface area of the catalyst.
2.5.DFT computational methods
The density functional theory(DFT)was simulated by Guassian 09 software [21].The nonlocal correlation functional described by literature[22]with the 6-31G**basis,and all structures geometric optimization without symmetry constraints was applied to C,O,H and B atoms.
3.Results and Discussion
The catalytic performances were evaluated at a reaction temperature of 400 °C,a gas hourly space velocity (GHSV=60 h-1),and the gas flow rate of 40 ml∙min-1(C3H8:O2:Ar=2:1:37).Figs.S1(a) and (b) (in Supplementary Material) showed that the catalysts with lower calcination temperatures exhibited similar catalytic performances to the undoped catalysts.As the calcination temperature increased,the conversion of propane decreased,and the selectivity toward propylene increased in the PODH reaction.At a calcination temperature of 1100°C,the initial conversion rate of the reaction was 3.0%,and the selectivity reached 68.1%.However,with increasing the calcination temperature,the selectivity toward propylene did not improve further;furthermore,high calcination temperature lead to greater energy consumption,which was unfavorable for subsequent applications.Therefore,the optimal calcination temperature was 1100 °C.Fig.1(a) and (b) displayed the catalytic performance at different B loadings in the PODH reaction at the same calcination temperature of 1100 °C.The conversion of propane gradually decreased while the propylene selectivity gradually increased with the enhancement in the B loading.At a B loading of 2% (mass),the selectivity of propylene reached 85.1%at an initial propane conversion of 2.3%;this was the highest propylene selectivity achieved in PODH among the different B loadings used.When the B loading was further increased from 2% (mass) to 5% (mass),the selectivity was decreased.Table S1 displayed the different catalytic performance,2% B-SFC showed the highest propylene selectivity and showed the lowest propylene yield,and the 1% B-SFC(500) catalyst showed the highest yield.
XRD was used to characterize the crystal structures of the B-SFC catalysts at different B loadings,as shown in Fig.S2.From the literature,boric acid would decompose on the catalyst surface under high-temperature conditions to form B2O3crystals,and a characteristic diffraction peak of B2O3would appear at 2θ=27.7°in the XRD patterns [12,23].From Fig.S2.only two broad diffraction peaks at 2θ=24.0° and 43.6° are observed,corresponding to the (0 0 2) and (1 1 0) crystallographic planes of carbon,respectively [20].Moreover,no obvious characteristic diffraction peaks of B2O3were observed in any of the B-SFC catalysts,indicating that the B2O3crystals formed on the catalyst surface were completely removed by hot water washing in the catalyst preparation process.
Raman spectra were used to characterize the defects in the obtained B-SFC catalysts,as shown in Fig.S3.The D band(1350 cm-1) represents amorphous carbon and the G band(1590 cm-1) represents the graphitic structure.TheID/IGratio has generally been used to reflect the defect degree of the catalysts[3,24].Fig.S3 showed that the B-SFC catalysts all had higherID/IGratios than pure SFC,which indicated that B doping improved the carbon defect degree in the catalysts [25].However,when the B loading was 5%(mass),theID/IGratio was similar to 2%B-SFC,indicating that excessive B doping did not further increase the defect degree of the B-SFC catalysts.
The physical N2adsorption and desorption isothermal curves revealed the type of pore channels on the surface of the materials,and the specific surface areas were obtained using the BET method.Fig.S4 presented the N2adsorption isotherms for thex%B-SFC catalysts.It could be seen that all the catalysts showed a type IV adsorption curve,indicating their mesoporous structure[3].Table 1 presents theSBET,Vpore,andDporeof thex%B-SFC and SFC catalysts;theSBETincreased with increasing the B loading,indicating that more pores were formed on the carbon surface during the process B doping [15].However,5% B-SFC had a lowerSBETthan 2% B-SFC,indicating that excessive B loading would block the pores on the carbon surface,leading to a reduction in theSBET[19].
TGA was used to characterize the pyrolysis temperature of the SFC andx% B-SFC catalysts.Fig.2 showed the change in the mass of catalysts with temperature under a nitrogen atmosphere,and Table 2 presented the relative change in their mass at each temperature from 300 °C to 800 °C.From Fig.2,one could note that the quality of the B-doped catalysts did not change as much as that of SFC with temperature,from room temperature to 800 °C.In addition,Table 2 showed that the mass loss inx%B-SFC were smaller than those in the SFC catalyst at the N2atmosphere.In an inert atmosphere,the loss of carbon material mass had generally been attributed to the pyrolysis of surface functional groups [3],and the catalytic mass loss increased with increased in the B loadings when the temperature exceeded 400 °C.Furthermore,Fig.S5 depicted the TGA curves in the air atmosphere,where one could observe thatx%B-SFC appeared a significant weight loss at almost 550 °C,which higher than SFC catalyst about 50 °C.Figs.2 and S5 indicated that B doping could improve the thermal stability of the resultantx% B-SFC catalysts as compared to that of pure SFC[26,27].
XPS was used to analyze the oxygen-containing functional groups and elemental species on the surface of the catalysts.Fig.S6 showed the complete XPS spectra of the different catalysts,and Table 3 displayed their elemental and functional group content.In Table 3,it could be found that the oxygen and boron contents of the catalysts increased with the increased in the B loading,indicating that increasing the B content in the carbon framework could increase the content of oxygen-containing functional groups on the surface of the carbon materials.

Fig.1. The(a)propane conversion of the x%B-SFC,(b)propylene selectivity of the x%B-SFC.All the catalysts were tested at 400°C,GHSV=60 h-1,C3H8:O2:Ar=2:1:37,total gas flow rate=40 ml∙min-1.
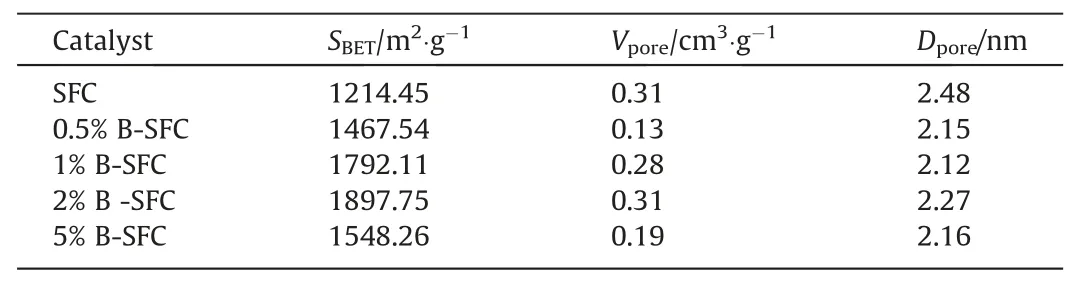
Table1 The pore structure parameters of x% B-SFC

Fig.2. The TG curves in N2 of the SFC and x% B-SFC.
The high-resolution B 1s and O 1s XPS spectra of the catalysts at different loadings were shown in Fig.3(a) and (b),respectively.In Fig.3(a),the B species could be divided into two forms: the B-O bond (BE=192.7 eV) and the B-C bond (BE=190.7 eV) [1,26].Besides,with the increasing of the loadings,the content of the B-C bond showed an increasing trend,and the B 1s peak shape shifted to the direction of lower binding energy,which indicated that the density of the electron cloud around the B atoms increased.However,when the loading was 5% (mass),the peak shape did not continue to shift.It was due to the loadings increasing,and it did not further increase the content of the B-C bond,but significantly increased the content of B-O bond.The oxygen species in the O 1s spectra in Fig.3(b)could be decided into five peaks,corresponding to -C=O groups (BE=531.4 eV),-COOH groups(BE=532.6 eV),the B-O bond (BE=533 eV),C-O groups (BE=533.5 eV),and adsorbed water(BE=535.8 eV) [3,15].Table 3 presented specific data on the absolute content of the functional groups in the B-doped catalysts: the B-O and C=O content increased with increased in the B loading,and the B-C content was similar for 2% B-SFC and 5% B-SFC.This indicated that excessive B loadings in the carbon catalysts mainly resulted in the formation of B-O groups.
To eliminate the influence of the specific surface area and catalyst quality on the catalytic performance,the SA was used to represent the catalytic ability of the catalyst and elucidate the effect of the specific surface areas and mass ofx% B-SFC on the catalytic activity for PODH.Detailed experimental data for the calculation of the SA was given in Table S2.Fig.4(a)displayed a good positive linear relationship between the B-O and C=O content in the oxygencontaining functional groups and the SA.It could be found that SA increased with increasing B-O and C=O content,implying that it was mainly influenced by the content of these functional groups[28–30].By considering the catalyst performance,the reaction selectivity toward propylene was observed to improve after B doping;further,B was present in the catalyst in the form of B-O and B-C groups[15].For this reason,as shown in Fig.4(b),the relationship between the relative content of B-C in B and the propylene selectivity in PODH,indicated that the latter improved with increasing in the former.The improvement in the propylene selectivity may be due to alterations to the electron cloud distribution on the surface of the carbon catalyst,with the electron cloud of the electrophilic groups shifting to the vicinity of B or the B-O groups,inhibiting the occurrence of electrophilic reactions and promoting the nucleophilic reactions[19,31,32].The side reactions and by-products arose due to the electrophilic reactions[15,16].
Boron-doped sisal fiber carbon nanocatalysts could effectively improve the catalytic performance of PODH.Doping with B atoms could effectively improve the surface defects of carbon materials.In addition,incorporating B atoms into the carbon skeleton to form new chemical bonds could enhance the thermal stability of carbon materials.Furthermore,these new chemical bonds greatly impacted the catalytic performance.Figs.S7 showed the surfaceelectrostatic potential of SFC and B-SFC catalysts.Blue represents the positive charge,and red represents the negative charge.It could be seen from the results that the electron cloud at the oxygen-containing functional group on the surface of pure SFC catalyst gathered and had a large intensity,which indicated that the oxygen-containing functional group had a strong ability to absorb electrons.After doping B to form the B-C bond,the density and intensity of the surface electron cloud had changed significantly,which weakened the ability of the carbon surface functional group to absorb electrons,thus improving the nucleophilicity.Therefore,the B-C content mainly affected the selectivity toward propylene by changing the electron cloud density and strengthening the nucleophilic effect [16,19].On the other hand,the B-O and C=O content mainly affected the conversion of propane,and the existence of the B-O groups could also have inhibited the side reactions by preventing the formation of electrophilic oxygen species [1,15,33].
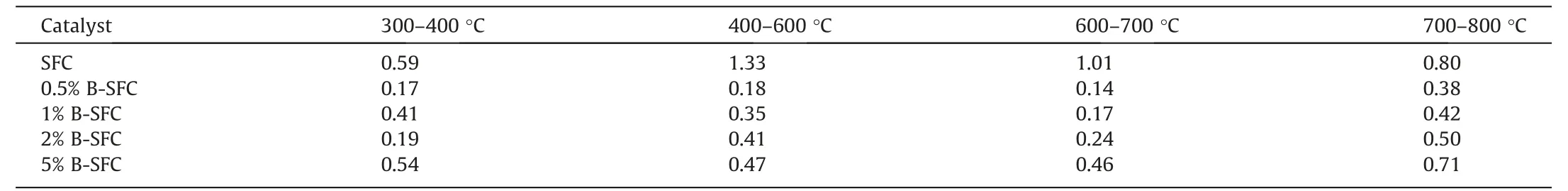
Table2 The mass loss (%) of the catalysts with different B loadings at different temperature stages in N2 atmosphere

Table3 Absolute contents (%) of the elements and functional groups of the catalysts with different B loadings
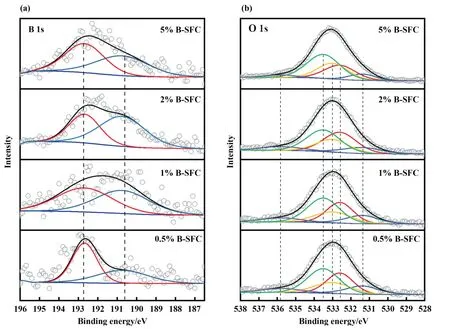
Fig.3. (a) B 1s high-resolution XPS spectra of the catalysts with different B loadings,and (b) O 1s high-resolution XPS spectra of the catalysts with different B loadings.

Fig.4. (a)Relationship between the SA and the proportion of B-O and C=O in the oxygen content,and(b)relationship between the selectivity toward propylene and the B-C content.
In Fig.5,the performances of 2% B-SFC and the SFC catalyst were compared using the optimized catalytic reaction conditions.From Fig.5(a),it could be seen that the propylene selectivity in PODH exhibited a volcano-shaped relationship with temperature in the case of 2% B-SFC,which displayed the highest selectivity among the different B loadings and SFC at 400 °C.Further increasing the reaction temperature improved the propane conversion,but decreased the propylene selectivity,which was even lower than that of undoped SFC at the higher reaction temperatures.Therefore,conducting the B-SFC catalyzed PODH reaction at a lower reaction temperature was suitable.Fig.5(b) showed the catalytic performance of 2% B-SFC for different GHSVs at 400 °C;as the GHSV improved,the selectivity toward propylene also grad-ually increased.Comparing the catalytic performance at a GHSV=120 h-1and 80 h-1,minor differences in the selectivity toward propylene were observed,implying that continuing to increase the reaction space velocity might not improve the selectivity to a significant extent.Therefore,the GHSV=120 h-1was chosen as the optimal reaction space velocity.

Fig.5. (a) Performance of the SFC and 2% B-SFC catalysts at different reaction temperatures,with the GHSV=60 h-1 and total gas flow rate=40 ml∙min-1;(b) performance of 2% B-SFC at different GHSVs at 400 °C,with the total gas flow rate=40 ml∙min-1.

Fig.6. (a) Catalytic stability of 2% B-SFC,(b) SEM image of fresh 2% B -SFC,(c) the SEM image of 2% B -SFC used 55 h,(d) high-resolution B1s XPS spectra of 2% B-SFC for different reaction times,and (e) high-resolution O1s XPS spectra of 2% B-SFC for different reaction time.

Table4 The pore structure parameters of fresh 2% B-SFC and 2% B-SFC used 55 h

Table5 Elementals and functional groups content (%) of 2% B-SFC at different reaction time
The catalyst stability test (shown in Fig.6(a)) was conducted with 2%B-SFC at a reaction temperature of 400°C,GHSV=120 h-1,and gas flow rate of 40 ml∙min-1(C3H8:O2:Ar=2:1:37).Fig.6a showed that the selectivity toward propylene decreased from 88.6% to 55.6% after a reaction time of 10 h.Thereafter,however,the propylene selectivity remained relatively stable,with a value of 55.2% after a reaction time of 55 h.The conversion of propane increased from 2.0% to 6.3% in the first 10 h of the reaction,and the reaction maintained good catalytic stability between 10 and 35 h.After 35 h,however,the catalyst activity began to decline significantly,and the carbon balance remained within the range of 100% ± 3% throughout the stability evaluation.In Fig.6(b) and(c),the SEM images of the fresh catalyst and the used one after a reaction time of 55 h showed that the structure of the catalyst noticeable changed after 55 h,and the fragmented structure appeared,due to the oxygen in the reaction gas oxidizing the catalytic carbon skeleton during the reaction.Besides,there were rich pores on the surface of the fresh catalyst,and the used one was hard to observe the pores obviously,due to the coke covered the pores,and the pore structure collapsed.Fig.S8 showed that the fresh 2% B-SFC catalyst and the one used for a reaction time of 55 h exhibited similar type IV adsorption isotherms in the physical nitrogen adsorption and desorption curves,indicating that they had a similar pore structure.However,the adsorption capacity of the 2%B-SFC used 55 h catalyst was significantly reduced;Table 4 indicated that theSBET,Vpore,andDporeof the 2% B-SFC used 55 h were reduced compared to the values for the fresh catalyst.This indicated that the catalyst pores may have been blocked and that the catalyst structure collapsed during the reaction.Fig.6(d) and(e) showed the B 1s and O 1s XPS spectra of the fresh catalyst and the ones used for different reaction times,revealing the changes in the functional groups on the catalyst surface during the reaction,and Table 5 included specific data on the different contents of the functional groups.It could be obtained that the oxygen content on the catalyst surface increased during the PODH reaction.The B-C content decreased significantly in the first 10 h of the reaction,while the B-O content increased,indicating that the B-C groups were oxidized to B-O groups by oxygen during the reaction.This corresponded to an increase in propane conversion in the first 10 h in terms of catalytic performance,while the selectivity toward propylene decreases significantly.In other words,this proved the viewpoint that B-O groups could affect the catalytic activity,while the B-C groups influenced the catalytic selectivity toward propylene.The 2% B-SFC used 55 h catalyst showed an increase in both the B-O and C=O content compared to the content in the 2% B-SFC used 10 h catalyst,but the performance showed a significant decrease with the catalytic structure collapsing and the specific surface area decreasing.Therefore,the main reasons for the deactivation of the catalyst were the collapsing catalytic structure and the decreasing specific surface area.
4.Conclusions
In this work,boron-doped sisal fiber carbon materials were prepared using different boric acid loads,removing the B2O3clusters by hot water washing.The 2%B-SFC catalyst displayed the highest selectivity toward propylene (88.6%) in the PODH reaction,with a propane conversion of 2.0%.The B-SFC catalytic activity was mainly related to the B-O and C=O content,where the B-O bond could inhibit the formation of electrophilic oxygen species to reduce side reactions.The propylene selectivity,on the other hand,showed a positive correlation with the B-C content,and the nucleophilic effect was enhanced by changing the electron cloud density through the B-C bond to improve the selectivity toward propylene.This study provided new insights into and experimental support for the mechanism of PODH catalyzed by boron-doped carbon materials.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Taishan Scholars Program of Shandong Province (tsqn202103051).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.03.006.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
