Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
Zhihao Zhang,Danyang Song,Hengxing Bao,Xiang Ling,Xiaogang Jin
School of Mechanical and Power Engineering, Nanjing Tech University, Nanjing 211816, China
Keywords:Thermochemical energy storage Reactor Ca(OH)2/CaO Dehydration Experiment research Numerical simulation
ABSTRACT The Ca(OH)2/CaO thermochemical energy storage (TCES) system based on calcium looping has received extensive attention owing to its high energy storage density,prolonged energy storage time,and environmental friendliness.The heat storage process of the Ca(OH)2/CaO TCES system in a mixed heating reactor was evaluated in this study,by employing a combination of direct and indirect heating modes.The dehydration process was studied experimentally,and a numerical model was established and verified based on the experimental results.The dehydration behavior of 500 g of Ca(OH)2 powder was investigated in a fixed-bed reactor with mixed heating.The experimental and simulation results indicated that mixed heating causes combined centripetal and horizontal propulsion.Heat input is the main limiting factor in the heat storage process,because the radial advance of the reaction is hindered by the low thermal conductivity of the solid reactant particles.Heat transmission partitions were added to enhance the performance of the reactor.The performance of the modified reactor was compared with that of a conventional reactor.The radial heat transmission partitions in the modified reactor effectively enhance the energy storage rate and reduce the reaction time by 59.5%compared with the reactor without partitions.
1.Introduction
In recent years,renewable energy has been increasingly utilized as a viable source for reducing carbon dioxide emissions [1].The shift towards renewable energy sources necessitates reliable energy storage systems to compensate for their fluctuating energy supply[2].Thermal energy storage(TES)is applicable for this purpose,partly because a large proportion of energy consumption involves heat utilization [3].Additionally,the energy sources for TES are extensive and economical,leading to a wide range of applications at low costs [4].
Common TES methods include sensible energy storage,latent energy storage,and thermochemical energy storage (TCES) [5].Compared with the developed technologies of sensible and latent energy storage,TCES has the advantages of a higher energy density and longer storage time with little heat at ambient temperatures[6].It is a promising technology for a society aiming to function entirely on renewable energy[7].In several chemical reaction systems of TCES,the group of hydroxides used is environmentally friendly [8],inexpensive [9],and encompasses a wide range of reaction temperatures [10].Two main hydroxide systems are considered for TCES applications: MgO/Mg(OH)2and CaO/Ca(OH)2.The CaO/Ca(OH)2TCES system based on calcium looping (CaL)has received extensive attention owing to its high energy storage density (693 kW∙h∙m-3[11]).The dehydration/hydration process of Ca(OH)2/CaO is described by the reversible reaction shown in Eq.(1).The reaction temperature of the Ca(OH)2/CaO TCES system is typically in the range of 400–600 °C [12].
Ervin [13] first used the CaO/Ca(OH)2reaction in a TES plant.Schaubeet al.[8]measured the reaction enthalpy(104.4 kJ∙mol-1)at 505°C and the partial pressure of H2O up to 0.1 MPa.Criadoet al.[14] investigated the reaction mechanism of CaO/Ca(OH)2and found the experimental results to be in good agreement with the shrinking core model.These studies indicated that the poor mechanical properties of the CaO/Ca(OH)2TCES system,owing to particle attrition,reduced the overall chemical activity and agglomeration.
Several novel composite materials have been developed to address this.Sakellariouset al.[15] prepared a mixed calcium oxide–alumina composition to improve the hydration/dehydration performance by increasing the surface area of the materials.Criadoet al.[16] synthesized a new composite material and showed that the mechanical and reactivity properties achieved peak values when the molar Ca/Si was 4.8–6.2 at a particle size of 36–63 μm.However,follow-up studies indicated that the hydration capacity of the composite material was significantly reduced after 500 cycles owing to the formation of hydrated silicates [17].Kariyaet al.[18] prepared a mixed calcium-hydroxide–vermiculite composition to improve the cycling stability.The cycling stability of this composite material was superior to that of pure Ca(OH)2over 15 cycles;however,its hydration rate (0.62 × 10-2s-1) was reduced during the first cycle.Yanet al.[19] studied the effect of Li or Mg doping on the thermochemical properties of CaO/Ca(OH)2materials.Mg doping did not affect the heat storage process,whereas the charging process of CaO/Ca(OH)2doped with Li required a shorter time than that of the undoped sample.Density functional theory analysis[20]was consistent with the experimental results.Afflerbachet al.[21]developed semipermeable ceramic materials wrapped around CaO/Ca(OH)2to achieve more stable reversibility.The transfer of steamviaa porous ceramic shell improved the cycle stability and reduced the quality and volume of the material.Roβkopfet al.[22] investigated the effect of SiO2addition on the reaction performance in a lab-scale reactor.The addition of small amounts of SiO2prevented agglomeration,thereby shortening the time required for both hydration and dehydration.Subsequent studies showed that adding up to 30% (mass)SiO2formed (Ca5(SiO4)2(OH)2) as a side product of the reaction[23].This resulted in capacity loss in the thermochemical reactor,although the composite materials stabilized the surface structure.
The reactor,as an important vessel,determines the heat storage performance of the CaO/Ca(OH)2TCES system.Thus,the cycling behavior of CaO/Ca(OH)2TCES systems at the reactor level has been examined.Three main types of reactors are available for CaO/Ca(OH)2systems: indirect,direct,and continuous [24].Schaubeet al.[25] developed a reactor with direct heat transfer.The experimental results showed that the heat output and particle reaction rate were the dominant limiting factors for thermal flux.Schmidtet al.[26] designed a reactor with indirect heat transfer and experimentally calculated its peak power (approximately 7.5 kW of thermal power).Subsequent studies showed that the CaO/Ca(OH)2reaction rate was highly sensitive to small changes in reaction conditions [27].A storage efficiency of 87% was obtained by using a CaO/Ca(OH)2TCES system in a concentrated solar power plant [28].Yanet al.[29] experimentally analyzed the reaction characteristics of CaO/Ca(OH)2in a fixed-bed reactor.During the heat storage process,a higher dehydration temperature increased the heat storage rate and the heat storage efficiency (47% and 65% at 510 and 540 °C,respectively).Azpiazuet al.[30] analyzed the thermal behavior of hydration/dehydration cycles in a fixedbed reactor equipped with fins.The experimental results indicated that the heat efficiency in the modified reactor was improved by 72.5%.Schaubleet al.[31] reported that a fluidized bed was more suitable as a reactor than a fixed-bed reactor with low thermal conductivity.Pardoet al.[32]investigated CaO/Ca(OH)2with inert particles(SiO2,Al2O3,and SiC)in a fluidized bed reactor.The reactants were not fluidized easily;thus,the inert particles considerably influenced powder fluidization and sensible heat recovery.Criadoet al.[33]proposed a conceptual process design for applying fluidized bed reactors to a CaO/Ca(OH)2TCES system.Rougeet al.[34]established a standard Kunii–Levenspiel(KL)bubbling reactor model to accurately describe average hydration conversion in a fluidized bed,wherein the molar mass of the outlet steam was in agreement with that of experimental results.Criadoet al.[35]demonstrated the validity of the KL bubbling reactor model and investigated the reaction behavior of hydration/dehydration cycles.Rapid dehydration kinetics resulted in considerable emulsion–bubble mass-transfer resistances.
In reactor design,simultaneously ensuring sufficient heat and mass transfer is challenging because solid reactants are characterized by either low thermal conductivity when used as loose granules/powders or by low permeability [36].However,fixed-bed systems continue to be prevalent in many industrial applications,owing to their simple geometry and symmetry [37].Typically,the fixed-bed reactor is most commonly used to study the CaO/Ca(OH)2TCES system.Schaubeet al.[38] compared the indirect and direct types of CaO/Ca(OH)2TCES fixed-bed reactors and found the thermal performance of the reactor with direct heat transfer to be superior to that of the reactor with indirect heat transfer.In the direct dehydration process,the flow of the heat transfer fluid removes the water vapor generated by the decomposition reaction,thereby accelerating the mass transfer of the reaction.However,the specific heat of the gas is considerably lower than that of the solid reactant.Heating the reactants with gas to provide sufficient heat input is not advantageous.Thus,the dehydration temperature of the reaction must be increased to prevent carbonation and reduce the cycle stability of the reaction [30].However,high temperatures increase expansion and grain agglomeration [39].In industrial applications,the overall efficiency is decreased with the loss of each additional unit of temperature owing to the internal temperature gradient during storage[40].Therefore,a uniform temperature of the reactants is required.Increasing the aspect ratio of the reactor is conducive to the energy input/output [41].However,an increase in the reactor height limits the steam flow owing to the pressure drop,and an increase in the width further reduces heat transfer in the reactor.
Thus,for dehydration,the direct-type reactor improves the mass transfer in the reaction;however,the low specific heat of the gas is not conducive to the energy input for the energy storage reaction.The indirect-type reactor can effectively provide the energy input required for the reaction;however,it is limited by the low thermal conductivity of the solid reactant particles,and the heat transfer performance of the reactor needs to be optimized.In this study,an experimental platform was developed for indirect and direct mixed-heat-transfer fixed-bed reactors.A typical dehydration experiment was conducted,and a numerical model of a mixed-heating fixed-bed reactor was established.The results of this study provide solutions for improving the reactor design for the CaO/Ca(OH)2TCES system.
2.Experimental
2.1.Materials and apparatus
Calcium hydroxide powder (Ca(OH)2;>95%pure) was obtained from Tianjin Zhiyuan Chemical Reagent Co.,Ltd.,China.The measured bulk density was 517.65 kg∙m-3in contrast to its theoretical density of 2200 kg∙m-3[8].
Fig.1 shows the main experimental apparatus of the test bench.The setup included an air compressor,a steam generator,a gas heater,a buffer tank,a cooler,and a reactor.The air compressor(BLT-10A/8 (1.25 m3∙min-1,0.8 MPa)) that was obtained from Bolaite (Shanghai) Compressor Co.,Ltd.provided compressed air for the heat and mass transfer of fluids.The steam generator(LDR3-0.4 (4 kg∙h-1,0.7 MPa))was obtained from Shanghai Guanshen Machinery Equipment Co.,Ltd.,and it provided steam to react with CaO in the reactor.It was not used in the current study because only the energy storage process was investigated.The gas heater (15 kW),obtained from Nanjing Baose Co.,Ltd.,was used to increase the temperature of the gas.The buffer tank,which was made of stainless steel 304,was obtained from Nanjing Xinchuang Gear Co.,Ltd.It mainly stabilized the gas flow rate and was employed to measure the gas properties,including humidity,pressure,and temperature.The cooler (stainless steel 304) was obtained from Nanjing TeamWork Energy Conversation and Environment Protection Technology Co.,Ltd.It was primarily utilized to condense steam,using water as the cooling medium.The reactor(stainless steel 304;inner diameter: 125 mm;wall thickness:5 mm) was provided by Yixing Kangda Co.,Ltd.
The experimental data collection system included an NI data collector,a hygrometer,a pressure meter,a pressure difference transmitter,a thermocouple,and a vortex street flowmeter.The primary details are listed in Table 1.
2.2.Experimental procedure
The heat storage process was evaluated in this study.The reactor provided heat for heat storage through direct and indirect mixed heating.Hot air entered the reactor after being dispersed from the bottom through the gas distributor.The air produced by the compressor was heated to the reaction temperature using a gas heater,and the reactor wall was heated using a ceramic heating belt.The reactor was filled with 500 g of Ca(OH)2.
The experiment was divided into two stages: drying and reaction.The reactants were initially heated to 530 K to dry the experimental system and the reactants.When the inlet and outlet humidities were equal,the air and reactor wall temperatures were increased to 823 K and maintained until the completion of the reaction.Ca(OH)2was gradually decomposed during the heating process,and the conversion was calculated as follows:
whereXis the conversion andnis the number of moles of the sample.
nH2Ois calculated as follows:
whereQis the flow rate of air,tis the reaction time,MH2Ois the molar mass of H2O,anddis the humidity ratio,which can be calculated as follows:
whereBis the pressure of the gas mixture,φ is the relative humidity,which can be measured by the hygrometer,andpsis the water vapor saturation pressure at the corresponding temperature.
ps(0–200 °C) is calculated as follows [42]:
whereTis the temperature of the mixture gas,c0=-5.8002206× 103,c1=1.3914993,c2=-4.8640239 × 10-2,c3=4.1764768 ×10-5,c4=-1.4452093 × 10-8,andc5=6.5459673.
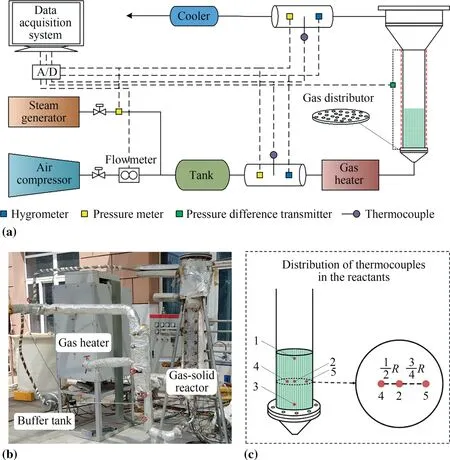
Fig.1. Experimental platform: (a) flow chart;(b) apparatus;(c) schematic of temperature measuring points.

Table1 Details of the measuring instruments
2.3.Uncertainty analysis
The accuracies of the experimental measuring instruments are listed in Table 1.The uncertainty can be calculated by using Eqs.(6) and (7) [37].The uncertainty of the experimental results was estimated to be less than 1.2%.
whereaiis the error bound of the measured valuexiandu(xi)is the uncertainty of the measured value,xi.ucis the uncertainty of the calculated value,andfis the relationship between the measured valuexiand the calculated value.
3.Numerical
3.1.Computational domain and assumptions
To reduce the calculation time,only the section filled with reactants was considered in the computational domain of the numerical model characterizing the dehydration reaction in the fixed-bed reactor.Fig.2(a) shows that the diameterDand heightHof the reactor filled with Ca(OH)2are 125 and 78.7 mm,respectively.Hot air entered from the bottom of the reactor;it served as a heating gas that partially provided the heat required for the reaction.Moreover,it removed the water vapor generated by the reaction.Five observation points were set corresponding to the temperature collection points of the experiment to analyze the heat and mass transfer inside the reactor compared with the experimental results.Their positions are shown in Fig.2(b).Initially,the reactor was filled with Ca(OH)2powder.To establish the physicochemical model,the following assumptions were made [31,43]:
(1) The reactants were regarded as porous media,and the porous bed was treated as a continuum.
(2) The particle diameter and porosity of the reactants would not change as the reaction proceeded.
(3) The effective thermal conductivity of the bed was constant.
(4) The specific heat of the solids would change with changes in the temperature and bed density during the reaction.
(5) The solid reactant and gas were in thermal equilibrium.
3.2.Governing equations
During the dehydration process,the reactant Ca(OH)2is consumed;CaO and H2O are generated in the same molar amount.The reaction rate is calculated as follows:
wherecCa(OH)2,cCaO,cH2Oare the molar concentrations of Ca(OH)2,CaO,and H2O,respectively.
The reaction rate coefficient,K,was determined by using the Arrhenius equation,and the effect of pressure was considered[41].
whereAis the pre-exponential factor of dehydration andEis the activation energy of dehydration.Both parameters are constant for a certain reaction and can be experimentally obtained.In this study,the values obtained in Refs.[8,41]were considered,as listed in Table 2.
The equilibrium temperatureTeqis a function of pressure,and the equilibrium equation is described as follows [8].
Eq.(12) is plotted in Fig.3.When the temperature exceeds the equilibrium temperature,Teq,at the related pressure,the reaction proceeds in the positive direction,and endothermic dehydration occurs.Below the equilibrium temperature,dehydration stops,and exothermic hydration starts.Thus,the reaction rate coefficient,K,calculated using Eq.(11),is affected by both the pressure and temperature.The reaction rate coefficient increases when the temperature is increased under the same pressure.
The mass conservation equations of the gas and solid are given as
where u is the superficial velocity of the gas;ε is the porosity of the reactant;ρgasand ρsolidare the densities of the gas and solid,respectively.According to the measured bulk density and theoretical density,the porosity of Ca(OH)2was calculated to be approximately 0.76.
Smis the mass source term related to the dehydration reaction and is calculated by
whereis the molar mass of H2O.
The density of the gas and the solid can be calculated by
where ξH2O,X,ρH2O,ρair,ρCaO,and ρCa(OH)2are the mass fraction of H2O,the conversion,which can be calculated by using Eq.(2),and the densities of H2O,air,CaO,and Ca(OH)2,respectively.
The porosity of the reactant is assumed to remain constant during the dehydration process.Hence,the momentum equation for the gas can be described by
where μgasis the dynamic viscosity of gas,and F is the force term due to porous media,which can be calculated as [31]:

Fig.2. Schematic of the computational domain: (a) boundaries of the reactants;(b) observation points on the profile.
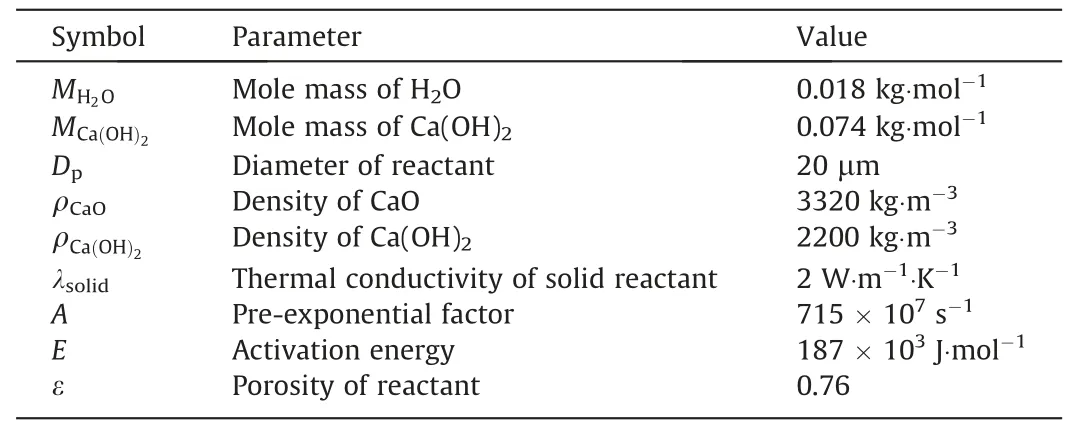
Table2 Values of parameters considered in the simulations
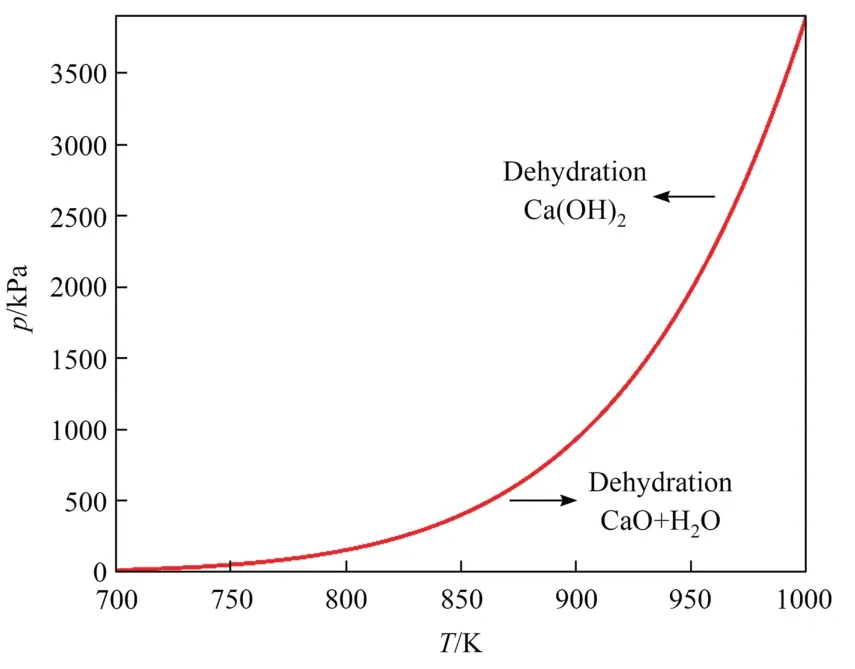
Fig.3. Relationship between equilibrium temperature and pressure.
wherekis the permeability calculated by using Kozeny–Carman equation [41]:
whereDpis the average diameter of the reactant particles.
Based on the local thermal equilibrium assumption,the energy equation is given as
whereSis the source term,which is caused by the endothermic reaction and can be calculated as
where (ρcp)effand λeffare the effective physical parameters of the reactants.Considering the different characteristics of Ca(OH)2and CaO,the effective physical parameters are defined as follows.
The heat capacity of solid reactants is calculated by
wherecp,CaO,are the specific heat capacities of CaO and Ca(OH)2.These are functions of temperature and are described as follows [43].
Details of the aforementioned parameters are listed in Table 2.For comparison with the experiment,a base case simulation was performed with the same boundary conditions as that of the experimental reaction stage.The inlet pressure was 0.15 MPa,and the heating rates of the inlet and wall were identical to those in the experiment.The change in the inlet and wall temperatures with time can be expressed as
whereTin,Twallare the temperatures at the inlet and wall,respectively.
4.Results and Discussion
The typical heat storage process of the Ca(OH)2/CaO TCES system with direct and indirect mixed heating was experimentally studied,and a corresponding mathematical model of the dehydration process was established.The numerical results were obtained by simulating the model under the same working conditions of the reaction stage.The conversion rates in the simulation and experiments were compared to verify the reliability of the numerical model.Accordingly,possible improvements to the fixed-bed reactor were proposed.Thus,the performances of the conventional fixed-bed reactor and the improved reactor under the same working conditions were compared using simulations.

Fig.4. Experimental results: (a) trends of average temperature and humidity;(b) temperature variation during heat storage.

Fig.5. Average conversion obtained in the experimental and numerical studies.
4.1.Base case
For the base case,the inlet gas flow was approximately 2.5 m3∙h-1,and the experiment was divided into two stages:heating to 530 K for drying and heating to 823 K for the reaction.Fig.4(a)shows the relationship between the average temperature of the reactants at each measuring point and the inlet and outlet humidities.To eliminate the influence of the original H2O in the test system on the experimental results,a prolonged period of drying was implemented below the reaction temperature until the inlet and outlet humidities were equal.In the reaction stage,the inlet and wall temperatures were gradually increased to the reaction temperature.Fig.4(b) shows the changing curve of the inlet,reactor wall,and reactant temperatures during the experiment.After the temperature of the reactants close to the wall and inlet rapidly increased to the equilibrium temperature under the local H2O pressure,the dehydration reaction occurred,and the heat was stored.Additional heat was transmitted to the reactants inside the reactor only after this dehydration reaction was complete.Therefore,the reactants far from the wall and inlet ascended slowly.
The amount of H2O removed during the experimental process was calculated by applying Eq.(3),and the conversion rate of Ca(OH)2during the reaction process was calculated by using Eq.(2).The reaction conversion in the numerical simulation and the experimental calculation is compared in Fig.5.In the initial stage of heating,the reaction did not occur in the simulation process,whereas it began to occur in the experiment.Uniform temperature distribution at the reactor wall was considered during heating,in the simulation.However,in the experiment,the local temperature increased rapidly because the reactor wall was heated by ceramic heating.Therefore,in the initial stage of heating,the local temperature of the wall exceeded the equilibrium temperature of the reaction,and a portion of the Ca(OH)2near the wall was decomposed.The conversion rate of the simulated results was higher than that of the experimental results,mainly because the effect of carbon dioxide in the air was not considered in the simulation process.The maximum error in the conversion rate between the simulated and experimental results was 9.91%.Typically,CO2reacts with CaO/Ca(OH)2under dry conditions,generating a small amount of CaCO3[44].Eliminating the negative effects of CO2in air is one of the objectives of our future studies.
In Fig.5,the slope of conversion denotes the reaction rate,which increases gradually and subsequently slows down.This can be explained by the reaction kinetic equation (Eq.(11)).Initially,the Arrhenius term in Eq.(11) increases with an increasing temperature.Moreover,the difference between the current temperature and the equilibrium temperature under local pressure increases with increasing temperature,which promotes the reaction.Therefore,the reaction rate increases with an increase in temperature at this stage.When the reaction progresses to a certain extent,the decrease in the concentration of Ca(OH)2significantly influences the reaction rate,and the reaction slows gradually with a decrease in the concentration of Ca(OH)2.

Fig.6. Numerical results:(a)temperature trend at each monitoring point;(b)conversion trend at each monitoring point;(c)temperature distribution profile;(d)conversion distribution profile.

Fig.7. Schematic of the computational domain with thermal partitions.
Fig.6(a)shows the temperature variation curve at each measuring point during the reaction process.The heat transferred from the wall and provided by the direct contact of hot air increases the local temperature,whereas the dehydration process of Ca(OH)2absorbs a large amount of reaction heat and reduces the local temperature.Therefore,the temperature curve at each point is produced by the combination of these factors.The fastest increase in temperature was observed at monitoring points 3 and 5.This is consistent with the experimental observations,because these points are in close proximity to the entrance and wall,thereby facilitating rapid heat input.Accordingly,the changing trend of the conversion is shown in Fig.6(b).Moreover,the slope of the conversion curve at measuring points 3 and 5 is the largest,indicating that a fast heat input can maintain a fast reaction.
The curves depicted in Fig.6(a) and (b) reveal that in the axial direction,the heating rates at points 1,2,and 3 decrease in turn,and the slope of the conversion curve(the reaction rate)decreases.In the radial direction,the heating rates of points 2,4,and 5 decrease in turn,and the reaction rate at these three points simultaneously decreases.The reaction with mixed heating proceedsviacombined centripetal and layer-by-layer.This is indicated by the temperature and conversion distribution profiles in Fig.6(c) and(d).In addition,the distribution characteristics of temperature and conversion are consistent.Therefore,the heat input is the main factor limiting the dehydration reaction for the energy storage process in the mixed heating reactor.
For heat transfer by hot air directly passed through the reactants,we typically expect a fast rate of heating and conversion.However,the volumetric heat capacity of air (1.17 kJ∙m-3∙K-1(300 K))is considerably less than that of Ca(OH)2(2934 kJ∙m-3∙K-1(300 K)).Thus,heating Ca(OH)2to the same temperature as that of air is difficult.Moreover,a significant amount of the reaction heat has to be provided by the sensible heat of hot air.The conversion distribution profiles show that the reaction moves forward faster in the axial direction than in the radial direction.One reason is that the low thermal conductivity of the reactants limits heat transfer from the wall to the interior of the reactants.However,the flow of hot air removes the H2O generated by the dehydration reaction,thereby avoiding the accumulation of local H2O partial pressure.Furthermore,the axial flow of gas weakens the radial diffusion of water vapor produced by dehydration,subsequently avoiding the suppression of the local H2O partial pressure in the same horizontal layer of the reactants.Thus,the complexity of the reaction is reduced from the perspective of mass transfer.
4.2.Reactor improvement
During the study described earlier,the heat input was the primary factor influencing the heat storage of the Ca(OH)2system in the fixed bed.Indirect wall heating ensures sufficient power input;however,the low thermal conductivity of the solid reactants prevents heat from transferring into the reactor center easily.Heating the reactantsviadirect contact heat transfer with hot air supplies heat directly into the reactor.However,the sensible heat of gas is considerably lower than the specific heat of the solid reactants and the enthalpy of the reaction.Therefore,sufficient heat input cannot be provided by using hot air alone.In particular,the heat input power provided by the direct gas heating is inadequate.Additionally,the enhancement of mass transfer caused by gas flow is significant;hence,the mixed heating method,which combines the characteristics of both direct and indirect heating,is advantageous.
During the experimental and numerical studies on the mixed heating reactor,the reaction advance velocity in the axial direction was higher than that in the radial direction.Therefore,to enhance the heat storage performance of the reactor,the addition of radial heat transmission partition plates was considered.As shown in Fig.7,2-mm-thick heat transmission barriers (the same material as the reactor wall) are added to separate the reactor into eight independent zones.Because of this modification,heat was transmitted from the wall of reactor to the reactor interior through the heat transmission partition plates,which acted as thermal fins.However,the separation of reactants in the same layer weakened the inhibiting effect of H2O diffusion and accumulation in the horizontal layer.
Fig.8(a)shows the comparison of the reaction time both in the presence and absence of the heat transmission radial partition plates.The energy storage processes of the two reactors were performed under the same boundary conditions.The inlet air and reactor wall temperatures were maintained at 873 K,and the inlet flow rate was consistent with that in the above experiment.The reaction time in the modified reactor was 59.5% lower than that in the reactor without the partitions.Fig.8(b)shows the temperature distribution at different times on three horizontal sections of the reactor (Z=2,39.4,and 76.7 mm,respectively).Heat was rapidly transmitted to the interior through the reactor wall after heat transmission partitions were added.The reaction advanced centripetally in each separation chamber,in contrast to the overall centripetal advance in the horizontal direction in the absence of the heat transmission partitions.The partitions effectively increased the area of heat advance in the horizontal direction,which enhanced the reaction process.A comparison of the temperature distribution in Fig.8(c) shows that the advance velocity of the reaction is enhanced in the axial direction.This is because the partitions confined the H2O generated by the decomposition to each separation cavity,which increased the mass transfer velocity in the axial direction.The temperature of the heat transmission partitions was relatively high;therefore,the surrounding reactants were hotter than those in other parts of the reactor.The hot gas passed through these regions with relatively low heat loss;therefore,it carried more heat to the reactants in the subsequent horizontal layer.
5.Conclusions
The heat storage process of the Ca(OH)2/CaO TCES system in a fixed-bed reactor was studied both experimentally and numerically.The temperature and conversion distributions and the time evolution of Ca(OH)2dehydration in the reactor with direct and indirect mixed heating were evaluated.First,an experimental Ca(OH)2/CaO TCES system was developed,and a numerical model of the heat storage process was established.The dehydration behavior of 500 g of Ca(OH)2powder in a fixed-bed reactor with mixed heating was investigated.The reliability of the numerical model was verified by comparing its conversion curve with that obtained in the experiment;the maximum error between them was 9.91%.The error was caused by the local high temperature of the reactor wall and the negative effect of CO2in the air during the experimental heating process.The analysis of the temperature,conversion curve,and distribution showed that direct and indirect mixed heating resulted in both centripetal and horizontal propulsion.The heat storage process was primarily limited by the heat input because the low thermal conductivity of the solid reactant particles impeded the radial advance of the reaction.Heat transmission partitions were added to enhance the reactor performance.The performance of the improved reactor was compared with that of an unimproved reactor.The addition of radial heat transmission partitions effectively increased the energy storage rate.Moreover,the reaction time was reduced by 59.5% in the modified reactor.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Nomenclature
Apre-exponential factor,s-1
Bthe pressure of the gas mixture,Pa
cconcentration,mol∙m-3
cpheat capacity,J∙kg-1∙K-1
Ddiameter of the reactor filled with Ca(OH)2,m
Dpaverage diameter of the reactant particles,m
dhumidity ratio,kg∙kg-1
Eactivation energy,J∙mol-1
F the force term due to porous media
Hheight of the reactor filled with Ca(OH)2,m
Kreaction rate coefficient,s-1
kpermeability,m2
Mmole mass,kg∙mol-1
mmass,kg
nnumber of moles,mol
pswater vapor saturation pressure,Pa
Qflow rate of air,kg∙s-1
Rgmole gas constant,J∙mol-1∙K-1
Senergy source term,W∙m-3
Smmass source term,kg∙m-3∙s-1
Ttemperature,K
ttime,s
u superficial velocity,m∙s-1
ucuncertainty,%
Xconversion,%
ΔHmolar reaction enthalpy,J∙mol-1
ε porosity
λ thermal conductivity,W∙m-1∙s-1
μ dynamic viscosity,Pa∙s
ξ mass fraction
ρ density,kg∙m-3
φ relative humidity,%
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
- Recent development of catalytic strategies for sustainable ammonia production
