Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
Hemayat Shekaari,Fariba Ghaffari,Masumeh Mokhtarpour
Department of Physical Chemistry, University of Tabriz, Tabriz, Iran
Keywords:Deep eutectic solvents Thiamine hydrochloride Density Speed of sound Redlich-Meyer equation
ABSTRACT Important efforts have been made over the past years to improve the drug acts,which leads to the discovery of novel drug preparations and delivery systems.The optimal design of such processes requires a molecular-level understanding of the interactions between drug molecules and biological membranes.The thermodynamic investigation provides deep and complete knowledge of interactions and the choice of appropriate and suitable production compounds in pharmaceutical fields.Particularly,the analysis of drugs+co-solvents in aqueous media is the central issue in many types of research because they exert their impact by interacting with biological membranes.This work is aimed to measure the density and speed of sound for the thiamine hydrochloride in water+deep eutectic solvents(DESs)mixtures(choline chloride/urea,choline chloride/ethylene glycol and choline chloride/glycerol) at temperature range(293.15–308.15) K.By correlation of the evaluated parameters in some standard relations,the partial molar parameters i.e.apparent molar volumes, Vφ,m,and apparent molar isentropic compression,κs,φ,m,are calculated.In addition,apparent molar isobaric expansion, ,and Hepler’s constant are computed from the density and speed of sound data.For fitting the experimental Vφ,m and κs,φ,m the Redlich-Meyer equation was employed that the important quantities;standard partial molar volume, and partial molar isentropic compression, were obtained.The thermodynamic analysis of the studied system also plays a crucial role in the pharmaceutical industry.
1.Introduction
Vitamins are one of the various organic materials applied by cells and organs to sustain their functions and developments.They take an important role in enzymic processes and genetic regulation processes [1].Physicochemical interactions between the vitamins and chief biomolecules including amino acids,proteins,carbohydrates and lipids are key parameters to understand the pharmacodynamics and pharmacokinetics of these compounds and to develop the drug formulation in the pharmaceutical industry[2,3].A comprehensive investigation of various thermophysical properties of the vitamins in the water and aqueous media of essential biomolecules can be applied to elucidate the representation of the different molecular interactions of the co-solutes with hydrophilic and hydrophobic moieties of the vitamins and to recognize the conformational stability of biomolecules in the biological system [4,5].
Vitamin B1[VB1](or thiamine/thiamin)is considered an essential vitamin and has been the subject of decades,and perhaps centuries,of research.The VB1 is a water-soluble vitamin and behaves as coenzyme for a variety of enzymes like transketolase,aketoglutarate dehydrogenase,pyruvate dehydrogenase,etc.It is engaged in the synthesis of proteins,metabolism of carbohydrates and fatty acids as well as in breakdown of polyhydroxy compounds inside biological systems [6].
Ionic liquids(ILs)have been employed as one of the appropriate alternatives for organic solvents in recent decades due to their low toxicity,non-flammability,insignificant vapor pressure,and low melting point [7,8].However,concerns about environmental acceptability,sophisticated synthesis methods,high cost,and particular toxicity have limited their usage [9,10].Deep eutectic solvents (DESs) are a neoteric generation of environmentally friendly solvents employed in different industries.These compounds frequently become liquid at room temperature and exhibit a lower melting point in comparison with the individual components [11].DESs have been manufactured by combining a high melting point salt (e.g.,quaternary ammonium salt as a hydrogen bond acceptor (HBA) such as choline chloride (ChCl)) and the appropriate mole fraction of a hydrogen bond donor (HBD) compounds such as urea (U),glycerol (G),or ethylene glycol (EG)[12].Generally,ILs and DESs are neoteric generation of green solvents in various fields but DESs have additional benefits including biodegradability and low cost [13].Owing to these remarkable merits,DESs have been applied to liquid–liquid extraction,catalysis,electrochemistry,and material chemistry.Todays,these kinds of solvents also have many applications in the pharmaceutical industries [13–15].Some investigations indicated that poorly soluble medicines were soluble in various DESs [16].The knowledge of volumetric and acoustic of thiamine hydrochloride with DESs is valuable in elucidating the assorted interactions prevailing in their solutions and for improving the design of various processes.
The complex nature and the types of molecular interactions that occur in mixtures can be studiedviaphysicochemical and thermodynamic investigations[17,18].Thermodynamic properties are very useful for the understanding of the ionic,hydrophilic and hydrophobic interactions in different solutions media as they provide information elucidating the solute–solute and solute–solvent interactions in the solution phase.The volume and compressibility are two fundamental thermophysical properties that allow the deep consideration of interactions between the solute and solvent molecules in the mixtures[19,20].The contributions of structurally similar vital DESs that affect the interactions of the thiamin and the around environments concerning temperature are limited.Consequently,in this research,the values of densities (d) and speeds of sound (u) of the thiamin hydrochloride (as vitamin B1 derivative)in water and DESs(ChCl/U,ChCl/G and ChCl/EG)aqueous solutions atT=(293.15–308.15) K in an interval of 5 K as a function of concentration are measured and reported.The obtained data has been applied for the calculation of numerous derived thermodynamic parameters.The derived thermophysical parameters including the apparent molar volume,Vφ,m,standard partial molar volume,apparent molar isentropic compression,κs,φ,m,and partial molar isentropic compression,These findings can be helpful to predict the performance and behavior of solvents through the drug manufacturing processes.
2.Materials and Methods
2.1.Chemicals
The detailed descriptions of the used chemicals are specified in Table 1.To preparation of solutions double distilled deionized water was applied at 298.15 K.
2.2.Preparation of DESs
All of the DESs were prepared using an electronic balance with a precision of ±10–7kg.The current uncertainty for the DES composition approaches,i.e.the mole ratio of the DESs’ ingredient,was within ±4 × 10–3.The DESs can be made with the specific mole ratio by stirring at 373.15 K and 0.1 MPa.A homogenous liquid was obtained after stirring for 60 min.Finally,the mixture was allowed to cool down to room temperature naturally for further use [21].The water content of used DESs was analyzed by Karl-Fischer titration.
2.3.Apparatus and procedure
An analytical balance(AND,GF202,Japan)was used to weigh all the solutions with an uncertainty ±10–7kg and then were kept inside the glass vials which were closed stoutly with parafilm.A vibrating tube densimeter (Anton Para,DSA 5000 densimeter and speed of sound analyzer)was used to measure densitydand speed of sounduof prepared solutions.Also,dry air at atmospheric pressure and degassed and double distilled deionized water were used to calibration of apparatus.The temperature was kept within±10–3K.Uncertainty of density and speed of sound measurements were 0.15 kg∙m-3and 0.5 m∙s-1,respectively.
3.Results and Discussion
3.1.Volumetric properties
The experimental densities for thiamin in water and aqueous solutions of DESs(ChCl/EG,ChCl/G,and ChCl/U with concentration of (0.1,0.3,and 0.5) mol∙kg-1) atT=(288.15,298.15,308.15 and 318.15 K) are listed in Table 2.By analyzing Table 2,it is noticed that values of densities increase monotonously with increase in concentration of DES,and at a fixed DES concentration these values decrease with increasing in temperature.
Apparent molar volumes (Vφ) could be calculated from the experimental values of density by using Eq.(1) [22,23]:
wheremandMare the thiamin molalities and molecular mass in the studied solutions,respectively.Also,d0andd,are the densities of the solvent and solutions.For the ternary systems the(DES+water)is considered as the solvent.The experimental values of apparent molar volume for thiamin in water and aqueous solutions of each DES at different temperatures are reported in Table 3.The plot of apparent molar volumes for thiamin in a fixed molality of each DES(0.3 mol∙kg-1)and at 298.15 K are shown in Fig.1.From Table 3,it is implied that the apparent molar volumes increase as the concentration of DES increases.
Standard partial molar volume,is obtained by using the following Redlich-Mayer equation [24]:
whereis equal to the partial molar volume at infinite dilution which is called standard partial molar volume,andSvandbvare empirical parameters.Since solute–solute interactions at infinitedilution are negligible,important information on solute–solvent interactions is provided by standard partial molar volumes.The values ofSvandbvtogether with its standards deviation of thevalues are reported in Table 4.It is noteworthy that the all values ofas a criterion of the solute–solvent interactions are positive and increase with an increase in each DES concentrations and temperature.This is due to the low electrostriction of water and strong interactions between solute and solvent molecules.The larger valuesat high temperature and in presence of DES are probably refers to the release of the solvent molecules to the bulk.The positivebvvalues are indicative of dominance of hydrophilic hydrogenbonding interactions over hydrophobic interactions [25–28].

Table1 Descriptions of the used chemicals

Table2 Density (d) and speed of sound (u) of thiamine hydrochloride in the aqueous solutions of deep eutectic solvents at different temperatures and pressure (P=86.6 kPa)

Table2 (continued)

Table3 The apparent molar volumes ( Vφ) and apparent molar isentropic compressibility ( kφ) of thiamine hydrochloride in aqueous solutions of DESs at different temperatures and pressure (P=86.6 kPa).

Table3 (continued)
Temperature dependence ofvalues can be defined by following equation:
Table4 Standard partial molar volumes( ),adjustable parameters of Eq.2( Sv and Bv),transfer volume(Δtr),and standard deviations(σ())for thiamine in aqueous solutions of DESs at different temperatures
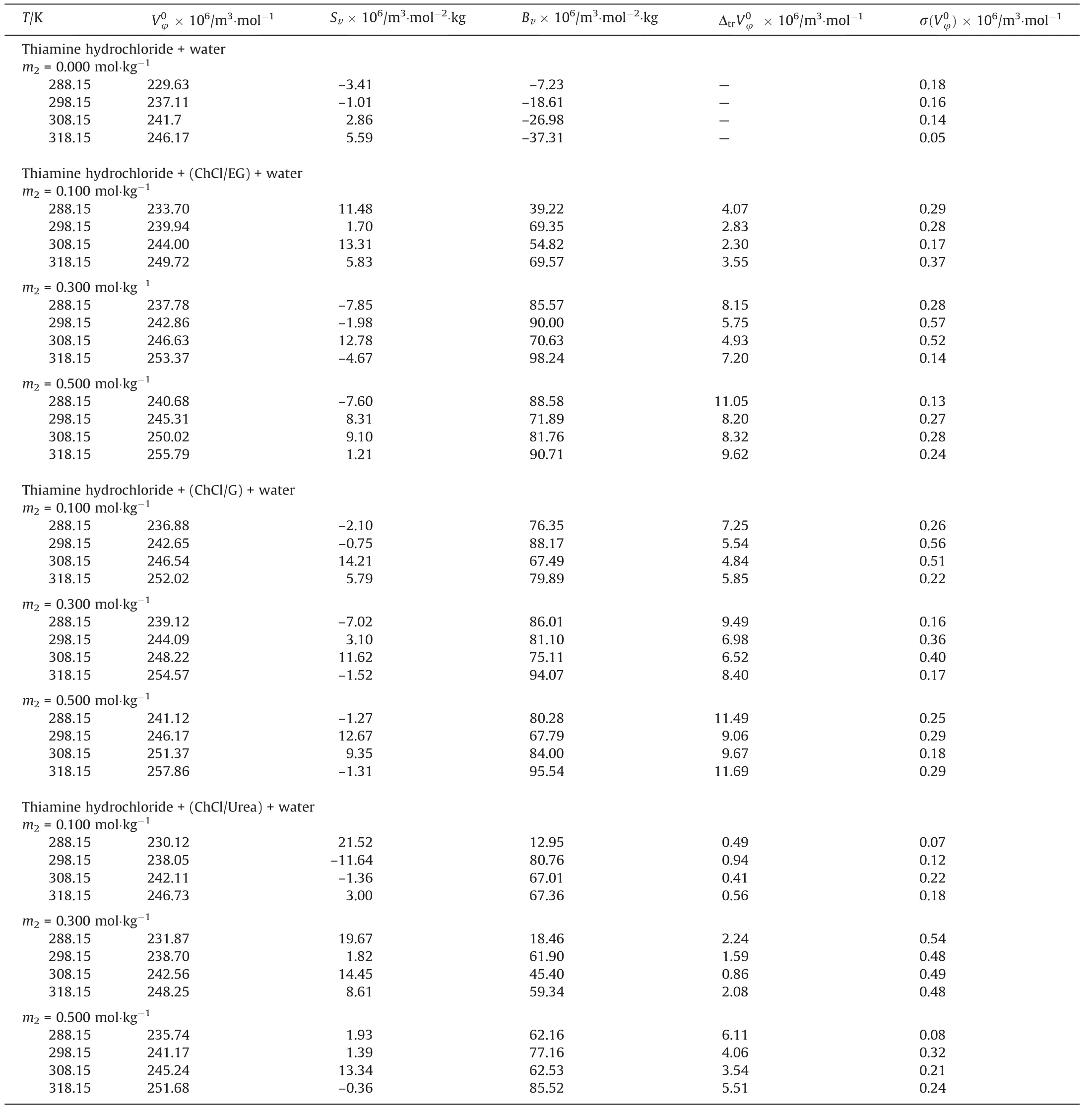
Table4 Standard partial molar volumes( ),adjustable parameters of Eq.2( Sv and Bv),transfer volume(Δtr),and standard deviations(σ())for thiamine in aqueous solutions of DESs at different temperatures
whereA,BandCare empirical constants which are calculated by the least-square fitting of standard partial molar volume at studied temperatures [29,30]:
On the basis of the derivative of standard partial molar volumefrom Eq.(3) as function of temperature and at constant pressure,the standard apparent molar expansibilitieswere computed which are given in Table 5.The values ofare positive for the studied drug in aqueous DESs solutions.Positive expansibility is a characteristic property of aqueous solutions of hydrophobic hydration.This would increase the solution volume a little more rapidly than that of the pure water and sowould be positive.
Thevalues are positive and increase with increasing in concentration of DES and temperature.This may indicate that systems are sensitive to temperature and molecular motilities are increased at higher temperature [31,32].
The thermal expansion coefficient,α,was calculated by the values of the standard partial molar volume,using Eq.(4) [33]:
Table5 Standard apparent molar expansibility, coefficient of thermal expansion,α,Hepler’s constant expansion,(∂2/∂T2)p of thiamine in water and in the aqueous solutions of DESs at T=(288.15–318.15) K

Table5 Standard apparent molar expansibility, coefficient of thermal expansion,α,Hepler’s constant expansion,(∂2/∂T2)p of thiamine in water and in the aqueous solutions of DESs at T=(288.15–318.15) K
The values of thermal expansion coefficients,α,for the investigated systems are listed in Table 5.This parameter is basically a balance to evaluate the reaction of the solutions to temperature variations.
The sign of the second derivative ofwith respect to temperature leads to obtaining the qualitative information about solute ability as a structure maker or structure breaker in solutions by using the following expression [34]:

Fig.2. Apparent molar isentropic compressibility of thiamine hydrochloride, Kφ versus its molality, m1,in aqueous DESs solutions at m2=0.3 mol∙kg-1 at T=298.15 K: ●,(Thiamine+water);■,(Thiamine+(ChCl/Urea)+water);▲,(Thiamine+(ChCl/EG)+water);◆,(Thiamine+(ChCl/G)+water).
The values of (∂2/∂T2)pfor studied systems are given in Table 5.Negative value seems to associate with a structurebreaking solute and positive one or closer to zero is associated with a structure-making solute [35].It is noticed that the values of(∂2/∂T2)pare negative and closer to zero for aqueous solutions of thiamine hydrochloride,and become positive with increasing of DES concentration which implies that drug essentially acts as a structure maker.
The partial molar volume of transfer,Δtrfor thiamine from water to the aqueous DESs solutions have been calculated as [36]:
The values of Δtrfor the present solutions are given in Table 4.The Δtrvalues are positive at all temperatures and increase with increasing in concentration of each DES.The type of interactions possible between solute (thiamine hydrochloride) and co-solute(DES)in ternary solutions(thiamine+DES+water)are:(i),ion–hydrophilic and hydrophilic–hydrophilic interactions (between ions of thiamine hydrochloride with DESs polar groups)(ii)hydrophilic interactions between ions of thiamine hydrochloride and polar groups of DESs (iii) hydrophobic-hydrophobic interactions (between alkyl groups of drug and hydrophobic groups of DESs) (iv)hydrophilic–hydrophobic interactions between the cation and anion parts of the thiamine hydrochloride and hydrophilic groups of DESs.Considering co-sphere overlap model[37,38]two types of interactions including ion–hydrophilic and hydrophilic–hydrophilic have positive effects on Δtrvalues whereas,other types result in negative transfer volumes.The positive values of Δtrexpress that hydrophilic interactions overcome to the other types of interactions and this trend becomes stronger by increasing in concentration of each DES [39,40].
3.2.Ultrasonic properties
Experimentally measured speeds of sound,u,for thiamine hydrochloride in water and aqueous solutions of DESs at different temperatures are listed in Table 2.The Laplace–Newton’s equation[41]was used to obtain the isentropic compressibility value,κsby following equation:
Table6 Partial molar isentropic compressibility(),adjustable parameters of Eq.(9)(Sk and Bk),transfer isentropic compressibility(Δtr),and standard deviations(σ())for thiamine in aqueous solutions of DESs at different temperatures

Table6 Partial molar isentropic compressibility(),adjustable parameters of Eq.(9)(Sk and Bk),transfer isentropic compressibility(Δtr),and standard deviations(σ())for thiamine in aqueous solutions of DESs at different temperatures
Isentropic compressibility(κs)is defined by the participation of two parts κs1(solvent intrinsic) is due to the compression of the solvent(water or aqueous solutions of DESs),and κs2(solute intrinsic)is due to the compression layer of solute molecules due to the influence of solvent molecules into the empty space of solute.At low concentration of thiamine hydrochloride,the κs1(solvent intrinsic) is the dominant contribution to the value of κs[42].The lower values of κswith increasing in DES concentration is due to the breaking the three-dimensional structure of water and hydrogen bonds form around thiamine hydrochloride molecules,this leads to reduces the compression of water molecules in bulk.
The apparent molar isentropic compressibility,(κφ)is obtained from Eq.(8):
where κs0and κsare isentropic compressibility values of the solvent and solutions,respectively.The calculated κφvalues for investigated systems are given in Table 2.Also,the apparent molar isentropic compressibility values for thiamine hydrochloride in the aqueous solutions of DESs with concentration of 0.3 mol∙kg-1of each DES at 298.15 K are represented in Fig.2.As can be seen from Table 2,the values of apparent molar isentropic compressibility increase with increasing of temperature and concentration of studied DESs.The variation of apparent molar isentropic compressibility with molality of thiamine hydrochloride can be presented by using Eq.(9):
Partial molar isentropic compressibility of transfer,Δtrfor water and the aqueous solutions of DESs have been calculated using the following equation:
The values of Δtrfor studied solutions are represented in Table 6.The sign of Δtris positive in the studied systems and these values increase with a rise in the concentration of DESs.Positive values offor thiamine hydrochloride illustrates the dominance of the head charged groups N+and COO–of DESs with the ions of thiamine hydrochloride which increase with a rise in the concentration of DESs.This behavior which observed for partial molar isentropic compressibility of transfer,are in good agreement with volumetric results and supports them.
4.Conclusions
In this study,the thermodynamic properties of thiamine hydrochloride (vitamin B1) as an essential micronutrient in the aqueous solutions of three DESs composed of choline chloride as HBA and ethylene glycol,glycerol,and urea as HBD (ChCl/EG,ChCl/G,and ChCl/U with concentrations of 0.1,0.3,and 0.5 mol∙kg-1) at different temperatures were investigated.The volumetric and ultrasonic properties indicated that the existence of strong interactions between thiamine hydrochloride and studied DESs and dominance of hydrophilic-hydrophilic types of the interactions between solute and solvent.The structure making property of thiamine hydrochloride in aqueous solutions of mentioned DESs are shown by Helper’s constant,(∂2/∂T2)p.Generally,it is concluded that with addition of DES in aqueous solutions of thiamine hydrochloride,the water molecules are released from the hydration layer of solute and finally the interactions between thiamine hydrochloride and DES become stronger.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors wish to thank financial support from the graduate council of the University of Tabriz,Tabriz,Iran.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
- Recent development of catalytic strategies for sustainable ammonia production
