Chemical looping conversion of methane via Fe2O3-LaFeO3 calcined from LaFe-MOF precursor
Jitong Deng,Yongjun Zhang,,Xiaopeng Wang,Wei Zhang,Hongjing Han,,Haiying Wang,Huimin Yuan,Yanan Zhang,Yanguang Chen,
1 College of Chemistry &Chemical Engineering, Northeast Petroleum University, Daqing 163318, China
2 PetroChina Daqing Petrochemical Research Center, Daqing 163714, China
3 CNPC Safety and Environmental Technology Research Institute Co.LTD., Beijing 102206, China
Keywords:Composite Chemical looping conversion Carbon dioxide Metal organic frameworks Lattice oxygen Methane
ABSTRACT The effective utilization of natural gas resources is a promising option for the implementation of the‘‘dual carbon” strategy.However,the capture of carbon dioxide with relatively lower concentration after the combustion of natural gas is the crucial step.Fortunately,the lattice oxygen is used for chemical cycle conversion of methane to overcome the shortcomings mentioned above.A method was proposed to synthesize perovskite for methane cycle conversion using metal organic framework as a precursor.Morphology and pore structure of Fe2O3-LaFeO3 composite oxides were regulated by precursor synthesis conditions and calcination process.Moreover,the chemical looping conversion performance of methane was evaluated.The results showed that the pure phase precursor of La[Fe(CN)6]∙5H2O was synthesized with the specific surface area of 23.91 m2∙g-1 under the crystallization of 10 h and the pH value of 10.5.Fe2O3-LaFeO3 was obtained by controlled calcination of La[Fe(CN)6]∙5H2O and Fe2O3 with variable mass ratio.The selectivity of CO2 can reach more than 99% under the optimal parameters of methane chemical looping conversion: m(Fe2O3):m(LaFeO3)=2:1,the reaction temperature is 900°C,the lattice oxygen conversion is less than 40%.Fe2O3-LaFeO3 still has good phase and structure stability after five redox reaction and regeneration cycles.
1.Introduction
With the exploitation of gas fields and combustible ice,there will be more and more natural gas resources.How to efficiently utilize natural gas is of great significance to the implementation of the carbon dioxide peaking and neutral strategies[1].At present,the major methods of methane utilization included combustion,compression (LNG),and chemical conversion,etc.[2–4].However,the traditional combustion of natural gas generated CO2with relative low concentration (10%–15% (vol)),which caused the atmospheric greenhouse effect [5–7].For carbon capture,utilization and storage(CCUS),it is necessary to separate out other gases such as nitrogen and residual oxygen,resulting in poor economy [8].Under pure oxygen atmosphere,the combustion of methane produces high purity of CO2[9],while the instruments are easy to burn out due to the high temperature.In contrast,chemical looping combustion (CLC) of methane can obtain high-purity CO2at lower temperature [10],providing a promising option for CCUS[11,12].
The key to the CLC conversion process is focused on oxygen carriers [13].Transition metal oxides easily transfer lattice oxygen to reactant molecules for oxidation reaction[14,15],and regulate the selectivity and distribution of gas products [16–18].Furthermore,transition metal Fe-based oxides were beneficial to promote the production of CO2with high concentration in the early stage for the oxidation reaction of methane [19–22].Generally,oxygen carriers are prepared by hydrothermal method,sol–gel method,etc.,under the low temperature or in the presence of the complexing agent [23–25].When they underwent reduction reaction with methane and regeneration with air under high temperature,sintering inevitably occurred,which greatly impaired the reactivity of the oxygen carriers [26].Therefore,in CLC process,perovskite is used as the carrier of oxygen carrier to form composite oxides[27–30].Even though it cannot provide sufficient lattice oxygen to achieve complete oxidation of methane,perovskite can be the‘‘conductor” for transporting lattice oxygen in oxygen carrier due to its high thermal stability and good lattice oxygen transport performance.Meanwhile,the oxygen ion transfer rate of oxygen carrier can be improved by adjusting the composition of metal ions in perovskite [29,31].
The physical properties of perovskites such as specific surface area (SSA),crystal phase,and morphology are difficult to regulate by traditional methods [32–35].Fortunately,the above shortcomings can overcome by the synthesis of perovskites using precursors.Metal-organic frameworks (MOFs) are ordered network structures composed of organic bridging ligands and inorganic metal ions through self-assembly.By changing the types of metal ions,the coordination modes between them and organic ligands are controlled,which resulted in the diversity of topological structures of MOFs[36–38].Due to the crystal phase and shape memory function,MOFs precursors can be calcined to obtain perovskites with relatively uniform crystal size and large specific surface area[39–42].
In this work,the adjustable morphology of MOFs precursor of La[Fe(CN)6]∙5H2O was prepared by adjusting pH value using La(CH3COO)3∙1.5H2O and K4Fe(CN)6∙3H2O in HCl solvent.After the regulation of calcination,LaFeO3with a large specific surface area and adjustable morphology was obtained.The lattice oxygen conversion in Fe2O3,Fe2O3-LaFeO3with different mass ratios and gas product distribution during 5 cycles CLC processes were explored.In addition,the possible reaction mechanism of CLC with CH4viaFe2O3-LaFeO3was discussed.
2.Experimental
2.1.Experimental reagents
FeCl3∙6H2O and Na2CO3were provided from Tianjin Damao Chemical Reagent Factory,China.La(CH3COO)3∙1.5H2O,K4[Fe(CN)6]∙3H2O were of analytical grade and obtained from Aladdin Biochemical Technology Co.,Ltd,China.CH4(3% (vol),the rest is argon) was supplied by Daqing Xuelong Petrochemical Co.,Ltd,China.
2.2.Synthesis of Fe2O3
Fe2O3was synthesized by hydrothermal synthesis method.Weigh 1.37 g of FeCl3∙6H2O to dissolve in 50 ml deionized water(D.I.H2O),then add 2 mol∙L-1Na2CO3solution dropwise to make pH=9.The product sample was carried out at 200 °C for 12 h,and dried after centrifugation to obtain Fe2O3samples.
2.3.Synthesis of LaFeO3
0.54 g of La(CH3COO)3∙1.5H2O was dissolved in 1 mol∙L-1diluted hydrochloric acid and 65 ml D.I.H2O,then 0.165 g of K4[Fe(CN)6] was added,and the pH value adjusted by ammonia water was added to form mixed solution A.The crystallization time was controlled within 2–24 h.When the reaction is done,the precipitate was filtered and washed 3–5 times with D.I.H2O and ethanol,and finally dried to form the precursor of La[Fe(CN)6]∙5H2O.
The precursor of La[Fe(CN)6]∙5H2O(La-Fe MOF)was placed in a muffle furnace,heated to 800–1000°C at 2 °C∙min-1,and calcined for 4–6 h to obtain LaFeO3.The schematic diagram of synthesis process was illustrated in Fig.1 [43].
2.4.Synthesis of composite Fe2O3-LaFeO3
0.12 g of the as-synthesized Fe2O3was taken and sonicated for 30 min in ethanol aqueous mixture withV(ethanol):V(water)=1:1.The upper clarifier was poured out after standing for 6 h,and then the pretreated Fe2O3and mixed solution A were ultrasonically dispersed.After dispersing them uniformly,the precursor of Fe2O3-La[Fe(CN)6]∙5H2O was obtained by hydrothermal synthesis of the mixed solution,and then calcined under the same condition as in Section 2.3 to obtain the Fe2O3-LaFeO3.Among them,the precursors at different pH values with corresponding perovskite are named as follows: for example,the precursor at pH 2.5 is defined as P2.5,and the corresponding perovskite obtained after calcination is named S2.5,and other names of the rest samples at different pH values are similar to the above.The names of the composite oxides with different mass ratios are abbreviated as LF-x,wherex=m(Fe2O3):m(LaFeO3).
2.5.Physicochemical characterization
Crystal phases of fresh and redox composite oxides determined by X-ray diffraction (Max 2200,Rigaku,Japan) using Cu Kα radiation under the setting conditions of 40 kV and 40 mA in the range of 5°–80°with step size of 10(°)∙min-1.The surface area of samples were characterized by BET (NOVA-2000e,Quantachrome,USA),besides the pore size and pore volume were calculated from the Barrett-Joyner-Halenda method through equipment of adsorptiondesorption property measurements using N2adsorbent at-196 °C.The characteristic vibration peaks were obtained in the wavelength scanning range of 400–4000 cm-1viaFourier infrared spectrometry (FT-IR-6200 Jasco,Japan).The composite oxide and dried KBr powder are mixed,and then pressed into tablets for detection.The morphology and state of composite oxides were observed on scanning electron microscopy (SEM,JSM-6510LV,JEOL,Japan)and energy dispersive spectroscopy(EDS),an accelerating voltage of 20 kV was conducted for image capture.Thermogravimetric analyses (TG),differential scanning calorimetry (DSC)and derivative thermogravimetric analysis (DTG) were used on a thermogravimetric analyzer(HTG-1,Beijing Hengjiu Experimental Equipment Co.,Ltd,China) to detect the phase transition of composite oxide and the redox reaction during CLC with methane.In the article,the mass loss rate at timetis the ratio of mass lost at timetto initial mass.The valence state of elements and binding energy of the sample was measured using X-ray photoelectron spectroscopy (XPS,ESCALAB MKII,Thermo Fisher Scientific Co.,USA) with a Mg Kα X-ray radiation operated at 15 kV.Gas chromatographic analyzer (GC,9790 plus,Zhejiang Fuli Analytical Instrument Co.,Ltd,China) was used for gas product analysis,the CO2and CO contents in the product were detected with the FID detector.
2.6.CLC performance evaluation of methane
The oxidation reaction of methane was carried out in a thermogravimetric analyzer,and the gas product was on-line detected by a gas chromatographic analyzer.The thermogravimetric analyzer was evacuated with N2and ramped up to 900 °C at a rate of 20°C∙min-1.Methane(3%(vol))was carried to the reactor at a flow rate of 25 ml∙min-1for 15 min of oxidation,then air with a flow rate of 50 ml∙min-1was switched for 30 min regeneration of the composite oxide to achieve 5 cycles of the CLC process.
3.Results and Discussion
3.1.Crystal phase and structure of Fe2O3
By adding Na2CO3precipitant,CO32–was hydrolyzed to produce OH–,while FeCl3was hydrolyzed to produce Fe3+.The specific formation process [44] of Fe2O3shown in Eqs.(1) and (2):.
The nucleation,aggregation and growth of crystals are affected by nucleation rate,crystal growth rate and other factors [45].According to Fig.2(a),at the early stage of the reaction,the nucleation time is about 2.5 h from the growth curve of Fe2O3,combined with the XRD spectra in Fig.2(b),all diffraction peaks are consistent with the standard card,with sharp peaks and no other diffraction peaks.It can be found that the crystalline phase of Fe2O3was found.Fe2O3nanocrystals were obtained by the synergistic effect of orientation and maturation mechanism [46].These small nanocrystals tend to diffuse and aggregate to form larger Fe2O3structures.While with the same mass,spherical surface area is large,which will make the reaction easier.Therefore,spherical Fe2O3was prepared.Combined with Fig.2(c),the SEM image of Fe2O3was shown.It can be seen that the particles are uniform and spherical with the particle size of 20–50 nm.This is conducive to reducing mass transfer resistance and easier diffusion.Meanwhile,the higher specific surface area could provide more active sites for the reaction,which was beneficial to the reaction.The N2adsorption–desorption curve and pore size distribution curve shown in Fig.2(d) can also be proved,the adsorption–desorption curve of the Fe2O3belonged to IV-type.It showed a hysteresis loop in the high-pressure region,which illustrated Fe2O3synthesized is a mesoporous material with a relatively regular and ordered pore structure [47].The specific surface area of Fe2O3sample is 33.50 m2∙g-1,and the average pore size is 27.77 nm.It could be seen that porous structure provides more pores and contact areas for the reaction [48].
3.2.Crystallization optimization of MOFs precursor
The crystal phase regulation schematic diagram of MOFs precursor was shown in Fig.3(a).It could be found from Fig.3(b)that the main crystalline phase of the precursor is La[Fe(CN)6]∙5H2O(JCPDS no.84-1954),with the extension of the crystallization time,the peak intensity of the main phase gradually increased,however,the intensity of the main phase weakened and the impurity phase enhanced after the extension of the crystallization time to 10 h.By standard card comparison,the impurity crystal belonged Fe4[Fe(CN6)]3(JCPDS no.72-0968),and the peak intensity of Fe4[Fe(CN6)]3gradually increased when the time continued to extend.Therefore,the single crystal phase of MOFs precursor can be regulated by changing the crystallization time.To obtain the pure phase MOF precursor of La[Fe(CN)6]∙5H2O,the optimal crystallization time is 10 h.Fig.3(c) showed the XRD spectra of LaFeO3(JCPDS no.74-2203)obtained by the calcination of the precursor prepared at different crystallization times.The time conditions of precursor synthesis were marked in the figure,and the corresponding crystallinity was shown on the right.There is little difference because the peaks were squeezed,but the crystallinity was different.When the crystallization time was 10 h,the diffraction peak of LaFeO3is high and narrow,and the relative crystallinity is the highest,reaching 98.73%.
3.3.Morphology regulation of LaFeO3
Ammonia was used to control the pH value of the precursor reaction system.The increase of the pH value of the precursor was beneficial to the stability of the perovskite structure [49].The XRD diffraction patterns of different LaFeO3samples were illustrated in Fig.4(a).It could be seen that there is no other impurity phase.At the same time,the diffraction peak intensity of S10.5 was the strongest.It was found that S10.5 has better crystallinity,which is 98.58%.The N2adsorption and desorption curves and pore size distribution curves were shown in Fig.4(b) and (c),and the specific surface areas and pore structure parameters of S2.5,S4.5,S7.5 and S10.5 were shown in Table 1.
It could be found that LaFeO3obtained by calcination of MOFs precursor has larger specific surface area than that prepared by other traditional method,pore volume and pore size are the same as above [32–35],as shown in Table 2.For example,the specific surface area of the S10.5 sample reached 23.91 m2∙g-1[42],which was 2–3 times higher than that of the perovskite synthesized by the sol–gel method.This was because the selection and collocation of metal nodes and organic ligands in precursor MOF affect the structure of perovskite.A higher specific surface area could provide more adsorption sites and active sites for the catalytic reaction of CH4and facilitate mass transfer.
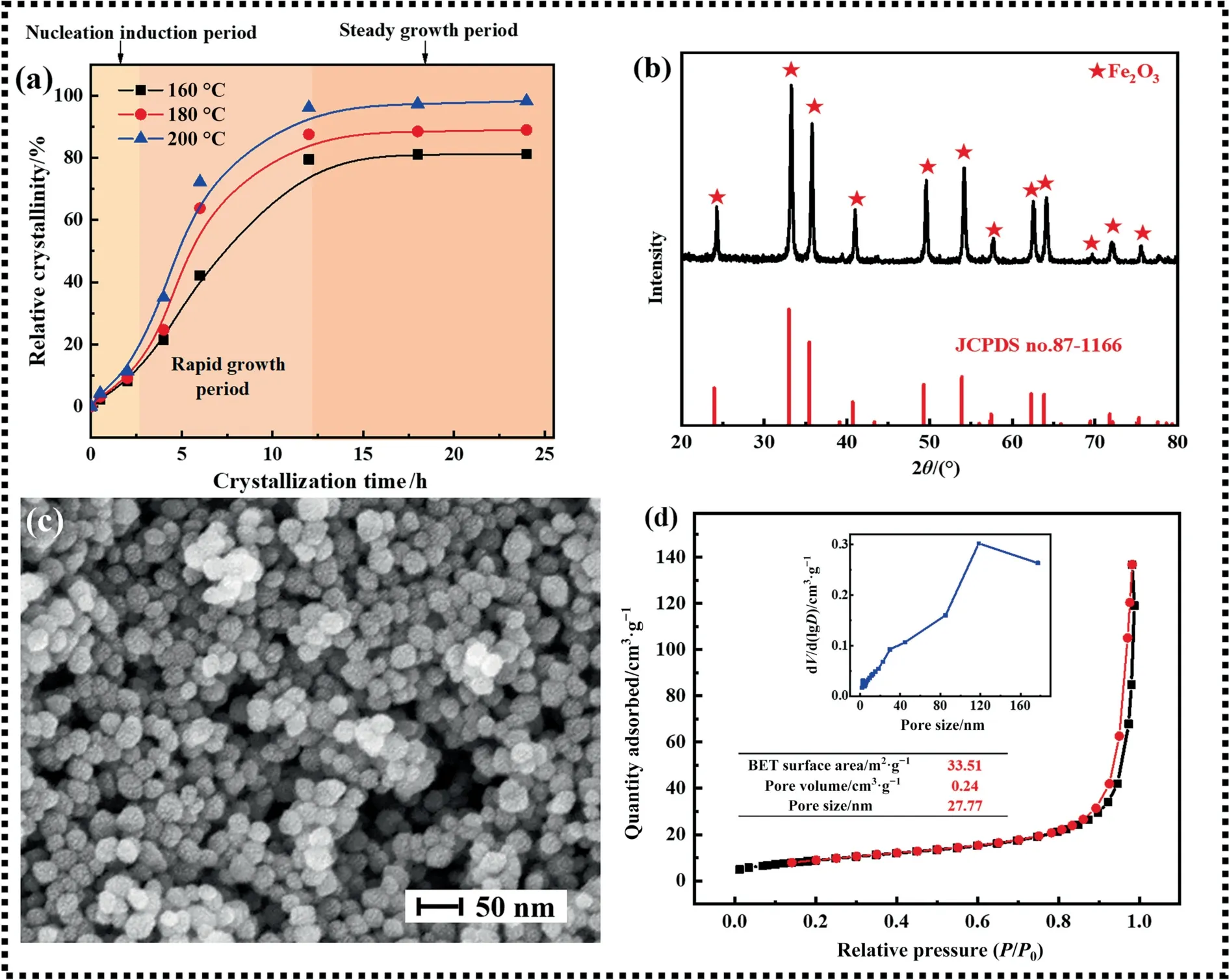
Fig.2. Crystallization growth curve (a),XRD spectra (b),SEM image (c),and N2 adsorption–desorption curve and pore size distribution (d) of Fe2O3.
Fig.4(d)showed the mass loss rate of S2.5,S4.5,S7.5 and S10.5 in the process of redox reaction with methane (3% (vol)).It was found that pH value adjustment of MOF precursor has a great influence on the stability of LaFeO3.The mass loss rate degree of LaFeO3was S2.5 >S7.5 ≈S4.5 >S10.5.The mass loss rate and stability of S2.5,S4.5,S7.5 and S10.5 samples for 5 cycles were shown in Table 3.Where,the mass loss rate at timetis the ratio of mass lost at timetto initial mass.As the mass loss rate of S10.5 is 6.20%,which is the lowest among the samples,so it is the most suitable support for the combination with Fe2O3.The mass loss rate of the sol–gel method in the literature was approximately 7% [50].The mass loss of the perovskite prepared with MOF as the precursor was 6%–9%.It was attributed to the following causes: (1) the LaFeO3originated from MOFs contained a fraction of lattice oxygen;(2) the LaFeO3originated from MOFs had hollow structure,which is beneficial to methane adsorption and oxidation reaction[41].Compared to the mass loss rate of the four LaFeO3types,the mass loss rate of S10.5 was relatively stable after five cycles of redox reaction.
It can be seen from Fig.4(e1) that P2.5 presents hollow structure.Not only the surface of LaFeO3was porous,but also the morphology was consistent with that before calcination in Fig.4(e1)and (e2),which was attributed to the regulation and shape memory function of MOFs precursor.Fig.4(f1) and (f2) presented that P4.5 shows a hexagonal crystal with grain accumulation on the surface and uneven pores.The calcined S4.5 sample appeared a cluster of spherical structural unit and has abundant pore structure.According to Fig.4(g1)and(g2),the surface of P7.5 appeared petal-like,and the unit structure was oblate spheroid.After calcination,the structure of S7.5 was exactly the same as P7.5.At the same time,a large number of irregular pore structures were generated on the surface.In Fig.4(h1) and (h2),the P10.5 showed a petal-shaped surface morphology with a wide upper part and a narrow lower part.After calcination,S10.5 presented hexagonal crystal system structure with two narrower ends and a wider middle part,simultaneously there were abundant pores on the surface.
3.4.Characterization of composite oxides of Fe2O3-LaFeO3
Fig.5(a) was the TG-DSC curve of La[Fe(CN)6]∙5H2O calcinated under air condition,it showed that the TG curve can be divided into two mass loss stages in the range of 25–800°C.The first mass loss stage was in the temperature range of 25–264°C,the mass loss rate was 5.13%,which was attributed to the adsorbed water and bound water.Another 18.38% mass loss rate in the range of 264–800 °C was attributed to the decomposition of the precursor La[Fe(CN)6]∙5H2O and La combination of Fe ions with O [31].At the same time,it can be seen from the DSC curve that there is an obvious exothermic peak at 253–583 °C,which also confirms the decomposition of the La[Fe(CN)6]∙5H2O precursor.At 800 °C,it

Wtis the mass loss rate at timet,Nlatis the amount of lattice oxygen involved in the reaction andXlatis the conversion rate of lattice oxygen.

Fig.4. XRD spectra (a),N2 adsorption–desorption curve of (b) and pore size distribution (c) of S2.5,S4.5,S7.5 and S10.5,mass loss rate of S2.5,S4.5,S7.5 and S10.5 during redox reactions with methane (d),SEM images of P2.5(e1),S2.5(e2),P4.5(f1),S4.5(f2),P7.5(g1),S7.5(g2),P10.5(h1),S10.5(h2).

Table1 Specific surface areas and pore structure parameters of LaFeO3 calcinated by La(Fe(CN6)∙5H2O

Table2 Specific surface areas of perovskites synthesized by different methods
When the lattice oxygen conversion reached 22.33%,theoretically Fe2O3was completely converted into Fe3O4,and when the lattice oxygen conversion rate reached 67.60%,Fe3O4was completely converted into FeO.Simultaneous lattice oxygen conversion was calculated as Eq.(5):

Table3 Mass loss rate of different LaFeO3 samples for 5 cycles reduction

Fig.5. TG-DSC curves of the precursor La[Fe(CN)6]∙5H2O calcined under air condition (a),FT-IR spectra of La[Fe(CN)6]∙5H2O before and after calcination (b) at the crystallization time of 10 h.

Fig.6. XRD spectra of Fe2O3-LaFeO3 with different ratios of m(Fe2O3):m(LaFeO3) (a),SEM image of LF-2 (b).
whereXlatis the average conversion rate of lattice oxygen,Δmtis the content of lattice oxygen participating in the reaction at the time oft(s),minis the initial lattice oxygen content of the reaction,andmtewas the achievable final residual lattice oxygen content of the reaction.
3.5.1.Activity and stability of Fe2O3-LaFeO3 for the oxidation of methane
Fig.8(a)was the result of the 1st cycle reaction of composite oxide with methane.WhenXlat=22.33%,it was the process of converting Fe2O3to Fe3O4.Since Fe2O3has a high ability to provide lattice oxygen,when it reacted with methane alone at almost the same rate as the composite oxide reacts with methane.The conversion time of Fe2O3to Fe3O4or FeO was listed in Fig.8(a),and when the increase rate of Fe2O3conversion curve slowed down,Xlatonly reached 76%,the time required weret(Fe2O3)=5.83 s,t(LF-1)=2.96 s,t(LF-2)=1.86 s,t(LF-3)=2.183 s.It could be found that the addition of a certain amount of perovskite cause a synergistic effect between LaFeO3and Fe2O3,which could not only shorten the time of methane conversion but also increase the conversion of lattice oxygen in Fe2O3.
It could be seen from Table 4 that the actual lattice oxygen conversion of the composite oxides was greater than the theoreticalvalue,which was due to the synergistic effect between LaFeO3and Fe2O3,LaFeO3promoted the reactivity of lattice oxygen in Fe2O3,leading to the increase of lattice oxygen conversion,and providing more lattice oxygen for the methane oxidation reaction.Whenm(Fe2O3):m(LaFeO3)=2:1,the actual lattice value of lattice oxygen was 35.34% higher than the theoretical value.Compared with the other two composite oxides,the actual lattice oxygen conversion of LF-2 was the highest,so it was the optimal choice to participate in the CLC reaction.
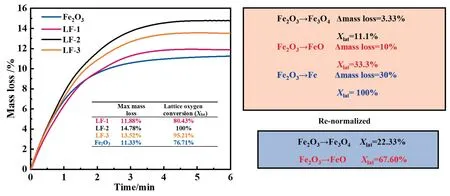
Fig.7. Mass loss rate in redox reaction of composite oxides in different ratios and Fe2O3 with methane and re-normalized to lattice oxygen conversion.

Fig.8. Effect of mass ratio of catalysts (a) and effect of reaction temperature (b) on Xlat.
The effect of temperature onXlatduring the redox reaction of LF-2 with methane was compared in Fig.8(b).WhenXlatreached 22.33%,the lattice oxygen conversion of LF-2 was relatively consistent at different temperatures.The times ofXlat=67.60% wast(700 °C)=2.183 s,t(800 °C)=1.7 s,t(900 °C)=1.45 s,t(1000 °C)=1.5 s.While at 700 °C,when theXlatreached 81%,the LF-2 curve became flat and the lattice oxygen conversion rate reached the maximum value.At the same time,it was obvious thatXlat(900°C)≈Xlat(1000°C)>Xlat(800°C)>Xlat(700°C).The evaluation results indicated that the lower temperature is not favorable to carry out the methane conversion reaction and limits the rate of lattice oxygen conversion.Low temperature could cause methane activity to be reduced.At the same time,the conversion of lattice oxygen in Fe2O3reached a maximum when the reaction temperature was 900°C,and remained essentially unchanged even the temperature went up.On the contrary,it has been studied previously that excessive high temperatures tend to cause carbon accumulation on the catalyst surface,leading to the coverage of the active center and a continuous decrease in activity [51].

Table4 Comparison of the theoretical and actual values of the lattice oxygen mass loss rate and conversion of composite oxides

Fig.9. (a) CO and (b) CO2 contents in gas products at different Xlat.
3.5.2.Gas product distribution analyses
The main oxidation reaction of methane was shown as Eqs.(6)and (7).According to Fig.9,the CO and CO2contents in gas products at differentXlatwere shown.
In the initial stage of the reaction atXlat=10%,the CO2content in the gaseous products for the reaction of all samples with methane exceeded 99%.Since Fe2O3had a higher equilibrium oxygen chemical potential at the beginning of the reaction,therefore,a higher tendency for CO2formation can be obtained [30].WhenXlat=22.33%,theoretically Fe2O3was completely converted into Fe3O4,the CO2contents for the reaction of LF-2 and LF-3 with methane were still above 98%.However,the CO2content of Fe2O3reacted with methane was lower than 90%,it was indicated that LaFeO3combined with Fe2O3to improve the reactivity of Fe2O3.WhenXlat=40%,the CO2content for the reaction of Fe2O3with methane was lower than 80%.Moreover,the CO2content for the reaction of LF-2 with methane still reached more than 99%,what’s more,it indicated that the addition of LaFeO3caused a synergistic effect to Fe2O3,which promoted the transfer of lattice oxygen and improved the lattice oxygen conversion rate.WhenXlat=70%,theoretically Fe3O4was completely converted into FeO.According to Fig.8(a) and Fig.9,it could be found that in the range ofXlatbetween 60%and 70%,the growth trend of the lattice oxygen conversion rate slowed down when Fe2O3reacts with methane,simultaneously the CO2content in the reaction also dropped sharply to 54.52%.It showed that the lattice oxygen conversion rate of Fe2O3decreased gradually with the increase ofXlatat this stage.At the same time,the CO2content in the reaction process of the other three samples decreased significantly,while the CO content increased obviously.This indicated that at this stage,the Fe2O3oxidation state decreased,causing the formation of CO2became less favorable,and the CO content gradually approached its maximum as the lattice oxygen in the oxygen carrier was depleted during the reduction process.In order to obtain high CO2content,it was necessary to ensure that Fe2O3achieves complete oxidation of methane,and the presence of perovskite not only provided an oxygen ion channel for lattice oxygen,but also assisted Fe2O3to provide more lattice oxygen,which slowed down the tendency of weak oxidation of methane.Atm(Fe2O3):m(LaFeO3)=2:1,Xlat≤40% and reaction temperature was 900 °C,the CO2selectivity of gas products in the methane conversion reaction reached more than 99%.
3.6.Regeneration and recycling performance of Fe2O3-LaFeO3
The stability and lattice oxygen conversion of composite oxides LF-2 were tested,andXlatof the oxygen carrier for 5 CLC cycles was

gen carrier played a major role in participating during the reaction.However,after regeneration by oxidation,Olat/Oadsincreased to 0.41 again,which was consistent with the oxygen element distribution of the fresh sample,which indicated that the sample was completed regenerated.

Fig.11. Possible reaction path diagram of the redox reaction between Fe2O3-LaFeO3 and CH4.
It could be seen from SEM images in Fig.10(e)–(g)that the particle sizes of Fe2O3-LaFeO3are all micron-sized in fresh,spent and regenerated.After the CH4oxidation reaction,shown in Fig.10(f),irregular carbon clumps were deposited on the surface of Fe2O3-LaFeO3and covered the surface interstices.Combine with the Fig.10(a)and(b),after the cyclic oxidation process it can be found that the crystal phase of Fe2O3-LaFeO3is consistent with the fresh,slight sintering occurred after the reaction,while when regenerated in air oxidation,the porous morphology was restored,as shown in Fig.10(g).At the same time,combined with Fig.10(a),the lattice oxygen conversion of Fe2O3-LaFeO3changed little during the cyclic oxidation reaction with CH4.
Fig.11 was a schematic diagram of the redox reaction of Fe2O3-LaFeO3composite oxide with CH4.The chemical looping combustion (CLC) of Fe2O3-LaFeO3composite oxides with CH4is divided into two main processes.Step 1 is the reaction of the composite oxide in the reduction reactor.When methane reacted with iron oxide,methane gas diffusely adsorbs to the surface of Fe2O3with low reactivity,while when methane reacts with Fe2O3-LaFeO3,part of methane still adsorbed to the surface of Fe2O3,and other oxygen ion were transported to the LaFeO3interface to react with methane.So LaFeO3made Fe2O3be dispersed and increased the reaction area,meanwhile improving the lattice oxygen transfer rate,as Eq.(8):
wherey<3/2.
Meanwhile the reaction process of methane is:
Where the main processes are Eqs.(10)–(16) [58–60]:
Step 2 is the regeneration of Fe2O3-LaFeO3reduced by methane in the oxidation reactor,where the oxygen carrier is replenished with gas oxygen from air while the oxygen vacancies are healed.The reaction equation was shown as Eq.(17):
Steps 1 and 2 present the complete CLC cycle.
4.Conclusions
Metal-organic frameworks of La[Fe(CN)6]∙5H2O as precursors were designed and synthesized to regulate the specific surface area and morphology of LaFeO3.The CLC performance of methaneviaFe2O3-LaFeO3was evaluated.
(1) Hexagonal crystal of LaFe-MOF precursors was synthesized by regulating the crystallization time.Moreover,the morphology of the precursor could be regulated by changing the pH value of the system.With the increase of pH value,the structure of,oblate spheroid,spherical and hexagonal prism were obtained,respectively.
(2) Different morphological LaFeO3were preparedviathe morphology memory function of the LaFe-MOF,and its specific surface area is 2–3 times larger than that of sol–gel method.The actual lattice oxygen conversions of LF-1,LF-2 and LF-3 were 80.43%,100%and 95.21%,respectively,all of them were higher than that of Fe2O3(76.71%),indicating that the addition of perovskite improved the lattice oxygen conversion of Fe2O3.
(3) The results of the chemical looping conversion performance of methaneviaFe2O3-LaFeO3showed: using Fe2O3-LaFeO3with them(Fe2O3):m(LaFeO3)ratio of 2,as long as the lattice oxygen conversion rate is controlled below 40%,the CO2concentration maintained 99%.While after 5 redox cycles of CH4,Fe2O3-LaFeO3still has good stability of crystalline phase and morphology.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (21908021),the China Petroleum Science and Technology Innovation Fund project (2021DQ02-0701),the High-Level Talent Project of Heilongjiang Province of China (2020GSP17),the New Energy and New Direction Project of Northeast Petroleum University(XNYXLY202102),and the Guiding Innovation Fund of Northeast Petroleum University(2021YDL-03).
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
