A simple hydroxypyridine ionic liquids for conversion of CO2 into quinazoline-2,4(1H,3H)-diones under atmospheric conditions
Bowen Jiang,Meiling Weng,Jigang An,Yuewei Fan,Jia Liu,Ying Liu,Ting Yu,Leizhi zheng,Guoqiang Yang,,Zhibing Zhang,
1 School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
2 School of Materials Science and Engineering, Suzhou University of Science and Technology, Suzhou 215009, China
Keywords:CO2 conversion Homogeneous catalyst Ionic liquids Reaction kinetic
ABSTRACT The transformation of CO2 into high value-added product is a promising pathway for utilizing CO2.However,the process tends to require harsh reaction conditions owing to CO2 chemical inertness.Designing a high efficiency catalytic system with environmentally benign characteristic are important determinants.In this work,protic ionic liquids [TMG][2-OPy] were prepared via one-step neutralization between 1,1,3,3-tetramethylguanidine and 2-hydroxypyridine,applying to the domain of synthesizing quinzoline-2,4(1H,3H)-diones from CO2 and 2-aminobenzontiles without any solvent or metal,achieving the yield of 97% at 90 °C for 8 h under atmospheric.A series of substrates with good to acceptable yield were detected,revealing the generality and universality of the catalyst.Furthermore,the system could be facilely reused for at least six runs,retaining the yield of 94%.A preliminary kinetic equation is calculated with the activation energy of 68 kJ∙mol-1,and a plausible reaction mechanism was put forward.This study highlights that the [TMG][2-OPy] enables to activate CO2 carboxylation efficiently.
1.Introduction
The excessive accumulation of carbon dioxide(CO2)has caused serious environmental issues such as global warming and EI Nino phenomenon in the past few decades [1].Realizing carbon peak and carbon neutralization have become a human consensus [2].As CO2is a sort of nontoxic,abundant and economical C1chemical feedstock,the CO2capturing and utilization (CCU) technology are receiving concerns in both academic and industry domains.
Many valuable and fine chemicals such as cyclic carbonates[3–8],methyl phenyl carbonate [9],benzimidazolones [10],dimethyl carbonate [11–16],formic acid [17,18] and polycarbonate [19]are synthesized originated from CO2gaseous.Among them,the synthesis of quinazoline-2,4(1H,3H)-diones between CO2and 2-ambrobenzontrile is an effective pathway to meet the requirement of atomic economic conception.The products involve a wide range of application in physiological and pharmacological fields,which are the important pharmaceutical intermediates for synthesizing bunazosin,doxazosin,prazosin,and zenarestat [20].Therefore,CO2carboxylation is the research hot spot over the last few decades and a series of heterogeneous and homogeneous catalysts are explored [21–25].In order to boost the catalytic effect of the heterogeneous catalysts,metal component such as Ln/Eu and MgO/ZrO2[26,27] are generally introduced into the reaction system which disposals could pollute water and ground resources[28].Further,as the reactants and the products are solid components in the heterogeneous reaction systems,the organic solvent such as DMF,DMSO,THF or toluene are inevitably added into the system,which brings tedious post-treatment and energy consumption to separate the product from solvent and catalyst.Hence,it is significative to develop an environmentally benign catalytic system with high activity for synthesizing quinazoline-2,4(1H,3H)-doines.
Ionic liquids,combing the merits of cations and anions characters,exhibiting superiority for CO2capture and transformation owing to the inherent advantages such as good thermal stability,switchable properties,flexible assemble of cations and anions[29–33].To date,various ionic liquids are applied into the quinazoline-2,4(1H,3H)-diones synthesis.Wenget al.[34] delivered the preparation of the products by using [HTMG][Lae] as the candidate,good to excellent were achieved towards various substrates under 70 °C,1.0 MPa for 12 h.Pinget al.[35] prepared the[HTMG][m-AP],which was able to catalyze CO2simulated flue gases and 2-ambrobenzontrile for 12 h under atmospheric condition at 80 °C using DMF solvent.Shiet al.[36] reported that [Ch][Im] could obtain the yield about 98% at 80 °C for 20 h with a CO2balloon.Liuet al.[37] synthesized [HTMG][Suc] and it was capable of achieving the yield of 97%at 60°C,6 h,2 MPa.Although tremendous progress has been made,the catalytic activity still needs to be enhanced.Thus,it is urgent to design a catalyst that enable to conduct this reaction under atmospheric with high efficiency without any additives.
To the best of our knowledge,we initially introduced 2-hydroxypydrine based protic ionic liquid [TMG][2-OPy] into the area of synthesizing quinazoline-2,4(1H,3H)-diones from CO2gaseous through one-step neutralization.The catalyst structures were analyzed by FT-IR,TGA and NMR spectrum.The CO2gaseous is specially separated from the flue gas generating from the coal burning.The impact factors such as various ion combination and catalytic conditions were thoroughly discussed.It was concluded that[TMG][2-OPy]exhibited optimum catalytic performance and could act as both catalyst and solvent in synthesizing inazoline-2,4(1H,3H)-diones.Moreover,the promising reusability and broad substrate applications have predicted its potential serviceability in industrial field.
2.Experimental
2.1.Materials
Raw materials were used as received without further clarification.2Aminobenzonitrile and relative substrates(Bide Pharmatech Ltd.).1,8-Diazabicyclo [5.4.0] undec-7-ene (DBU),choline hydroxide (Ch) (Aladdin Pharmatech Ltd.).1,1,3,3-Tetramethylguanidine(TMG),pyrrole (Py),2-hydroxypyridine (2-OPy),methyltertbutyl ether (MTBE) (Meryer Shanghai Co.,Ltd.).Ethanol (Nanjing Nanshi Chemical Reagent Co.,Ltd.).CO2gaseous (Nanjing Tianze Gas Co.,Ltd.).Distilled water was supplied from laboratory.
2.2.Characterization and measurements
Fourier transform infrared (FT-IR) spectrum was tested on a NICOLET is 10 equipment in the range of 400–4000 cm-1.Thermo-gravimetric analysis (TGA) was conducted on a Perkin Elmer Pyris 1 DSC instrument at the heating rate of 5 °C∙min-1from 25 °C to 600 °C under N2atmosphere.The1H NMR and13C NMR spectrum were tested in DMSO-d6or CDCl3solvent on a Bruker AVANCE III 400 MHz instrument.The CO2absorption equipment was performed in a double pot equipment.
2.3.Synthesis of various ionic liquids
The preparation of the ILs wasviaone-pot synthesis method.Taking [TMG][2-OPy] as the typical example,10 mmol TMG(1.15 g) and 10 mmol 2-OPy(0.95 g)was mixed with 20 ml anhydrous ethanol in a 100 ml round bottom Schlenk flask,and the batch was stirred at 45°C for 4 h under nitrogen atmosphere.Faint yellow viscous liquid was obtained and washed with MTBE three times to separate the un-reactant.The ILs was dried at 50°C under vacuum for 24 h after removing the solvent by rotary evaporator.The [DBU][2-OPy],[Ch][2-OPy],[TMG][Py],[DBU][Py] and [Ch][Py] were literally collected by the similar procedure.
2.4.Synthesis of quinazoline-2,4(1H,3H)-diones between 2-aminobenzonitrile and CO2
A certain ratio of catalyst and 2-aminobenzonitrile was added into a 10 ml Schlenk tube with a magnetic stir.The reaction was carried out in an oil batch from 1 h to 8 h after exchanging with CO2three times,and CO2gaseous was constantly injected into the tube through CO2balloons.The white solid production was washed with distilled water and MTBE three times after the reaction was completed,drying under vacuum at 50 °C for 12 h.The isolated yield of quinazoline-2,4(1H,3H)-diones was calculated by weighting the mass with an analytical balance (±0.1 g).The pressure testing experiment was conducted in a 25 ml stainlesssteel reactor,equipping with a Teflon lining.
2.5.The recovery of catalyst
After each cycle,deionized water was used to separate the ILs from the reaction system,and washing solution was subsequently removed by rotary evaporator.The recycled catalyst was using for the next run after drying under vacuum at 50 °C for 24 h.

Fig.1. CO2 absorption curves of [TMG][2-OPy] and [TMG][Py].

Table1 Carboxylation of CO2 with 2-aminobenzonitrile based on various catalysts
2.6.The absorption of CO2
The CO2absorption experiment of[TMG][2-OPy]and[TMG][Py]were measured with an isothermal gas–liquid equilibrium method at 25 °C,0.1 MPa.The experimental equipment and method were in accordance with previous work[38].Correspondingly,the accuracy of temperature and pressure was 0.1°C and 0.1 kPa.The absolute and relative CO2uptake capacity was evaluated based on molar ratio unite (mol CO2/mol IL).
3.Results and Discussion
3.1.Characterization of catalysts
The FT-IR spectrum of the as-prepared catalysts were exhibited in Fig.S1 (in Supplementary Material).TGA profiles in Fig.S2 revealed the TMG and DBU-based catalysts had good thermal stabilities until 200 °C.The choline hydroxide-based ILs had a relatively poor thermal stabilities and they started to decompose at around 130 °C.The experiment indicated that all the ILs could maintain steady in the testing temperature range.The1H NMR and13C NMR spectrum of the catalysts were exhibited from Fig.S3 to Fig.S14.
3.2.Absorption of CO2
The CO2absorption ability of [TMG][2-OPy] and [TMG][Py]were investigated at room temperature with a variable quantity of relative pressure.Fig.1 illustrated that their absorption quantity increased rapidly at low pressure region with similar absorption isotherms.In addition,the [TMG][2-OPy] CO2absorption figure is about 1.8 mmol∙g-1,which is practically 2.4 times higher than that of[TMG][Py],and the result is possibly owing to the strong inherent nucleophilicity of [TMG][2-OPy].Although the CO2uptake capacity of the ILs was inferior to the reported hyper-crosslinked ionic polymer materials with large specific surface area [39].The ILs still have the ability to capture and convert CO2into valuable chemicals.
3.3.Catalyst screening
It is a fact that the catalytic activity was varied according to different ion and cation combinations in Table 1.The yield was not satisfactory when choosing DBU and Ch as the cations.It is noticeable that[TMG][2-OPy]showed the highest catalytic ability with a 97% yield at 90 °C,0.1 MPa.The impacts of different anions with TMG cation on CO2carboxylation activity were subsequently examined.Taking catalyst basicity into consideration (Table S1),it was concluded that the catalytic ability of the ILs with TMG cation were generally followed by the order of basicity.When the ILs were incorporated with various cations,there is no regular pattern between catalytic performance and basicity.Based on these tests,it was certificated that the catalytic performance of ILs was depended on both catalyst basicity and multiple synergistic activation in catalyzing CO2and 2-aminobenzonitrile.

Fig.2. Effects of the reaction parameters: (a) reaction time,2-aminobenzontrile 1 mmol,[TMG][2-OPy] 2 mmol,90 °C,CO2 0.1 MPa; (b) reaction temperature,2-aminobenzontrile 1 mmol,[TMG][2-OPy]2 mmol,CO2 0.1 MPa,8 h;(c)n(catalyst)/n(substrate),90°C,CO2 0.1 MPa,8 h;(d)CO2 pressure,2-aminobenzontrile 1 mmol,[TMG][2-OPy] 2 mmol,30 °C,8 h.
Taking [TMG][2-OPy] as the typical example,the carboxylation reaction was carried out without any solvents or metal.The reaction parameters such as reaction time,temperature,catalyst/substrate mole ratio and pressure were explored.The change of yield based on reaction time was initially exhibited in Fig.2(a),it showed a sustainable growth from 46% to 97% by extending time from 1 h to 8 h at 90 °C.As is represented in Fig.2(b),the yield increased with the rising temperature and reached the optimum activity at 90 °C.There was no significant growth with a further raise of the reaction temperature to 100 °C.Fig.2(c) revealed the relationship between the yield of product and catalyst loadings.When the molar ratio of [TMG][2-OPy] to 2-aminobenzonitrile was 0.25:1,barely 22% yield was achieved due to the low catalyst concentration and lack of sufficient catalytic sites.The yield increased significantly to 78% by adding the molar ratio from 0.25:1 to 1:1.This tendency was due to the increasing solubility and the contact frequency between catalyst and substrate were strengthened by adding the catalyst concentration.No drastically promotion could be achieved by further risingn(catalyst)/n(substrate) to 3:1,certificating that ratio ofn(catalyst)/n(substrate) to 2:1 is the appropriate proportion for the reaction.As is shown in Fig.2(d),it was unexpected that CO2pressure has a drastically positive influence on the reaction.The yield of quinazoline-2,4(1H,3H)-diones was merely around 15% under atmospheric at 30 °C,prompting sharply to 77% at 0.5 MPa and realized almost 100%yield at 2 MPa.It is mainly because the higher CO2concentration could penetrate into the reactant system under pressure and this could effectively enhance the transformation of 2-aminobenzontrile to quinazoline-2,4(1H,3H)-diones.Herein,it drew a conclusion that the [TMG][2-OPy] exhibits promising catalytic performance at 90 °C under atmospheric conditions with the molar ratio ofn(catalyst)/n(substrate) to 2:1.
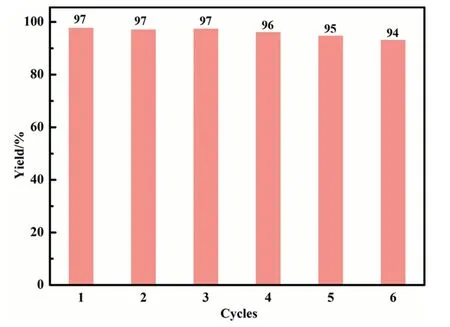
Fig.3. Reusability of[TMG][2-OPy]in CO2 carboxylation with 2-aminobenzontrile.
In order to further highlight the superiority,the comparison between [TMG][2-OPy] and the published ones was conducted in Table 2.It is straightforward that these references require either harsh reaction conditions or the introduction of solvents.Thus,this work realized the advantages of enabling to conduct CO2carboxylation under milder conditions without any solvent or co-catalyst.
3.4.General applicability
To evaluate CO2carboxylation catalyst versatility,a range of substrates containing halogen-group and methyl-group were selected to evaluate the universality of [TMG][2-OPy] in Table 3.It is concluded that the substrates with para-or metasubstituent could afford a high yield of over 95% because of their low steric hindrance effect and mass transfer resistance.It is noticeable that ortho substitute groups achieved relatively low yield round 70%-80%,which is owing to the strong electronwithdrawing effect of the substrates.All the substitute chemical structures were determined by1H and13C NMR spectrum(Fig.S15-Fig.S32).The results emphasized that [TMG][2-OPy]unfolded excellent activity towards multiform substitutes,exhibiting a comprehensive applicability.
3.5.Catalyst recyclability
Recycling performance test was estimated in a six-cycle evaluation (Fig.3).The result exhibited that the product could retain 94% yield after six runs.The recycled [TMG][2-OPy] was subsequently analyzed by FT-IR,NMR and TGA spectrum,and the chemical structure was barely changed according to the FT-IR (Fig.S35)and NMR spectrum (Fig.S33,S34).The TGA curve (Fig.S36)revealed a 15% loss in mass and started to decompose at around 130°C.The results corroborated that[TMG][2-OPy]catalyst is collectable with prominent chemical structure stability.
3.6.Reaction kinetics
The reaction kinetics model activated by [TMG][2-OPy] is further investigated.The ordinary format of the reaction equation was depicted in Fig.4(a),where [Amino],[CO2],and [Cata.] stand for the 2-aminobenzonitrile,CO2,and[TMG][2-OPy]concentration,respectively.The symbol of α,β,γ is representing the corresponding orders of the carboxylation.It could be considered as first order reaction on the basis of 2-aminobenzonitrile concentration.Eq.(1)is simplified as Eq.(2) with an assumption that CO2concentration remain constant because of continuing injection.It is further depicted as Eq.(3) and Eq.(4),wherekis the pesudo-first-order observed rate constant for 2-aminobenzonitrile,trepresents reac-tion time,andxrepresents conversion yield.Thekvalue was calculated according to the curves of ln (1-x)versustime from 50 °C to 90 °C,which data was recorded from Fig.S37 to S41.The calculation of the activation energyEawas based on the Arrhenius equation.The fitting curve of lnkversus 1/Twas exhibited in Fig.4(c),with anEanumber of 68 kJ∙mol-1.

Table2 Comparison of the catalytic performance with the published catalysts
4.Understanding the Plausible Catalytic Mechanism
In order to better comprehend the catalytic mechanism,the interaction between[TMG][2-OPy]and CO2was initially confirmed by FT-IR analysis.It has been reported that [TMG][2-OPy] could chemically capture CO2through the cooperative interaction from two active sites: N-heterocyclic O-of and pyridine N,generating carbonate and carbamate intermediates,respectively [47].The FT-IR spectrum in Fig.5(a) revealed a new signal at 1788 cm-1after CO2absorption,and it was associated with the formation of asymmetric C=O vibration peak corresponding to the carbamate salt(NH—COO-)[48].Another new band at 1385 cm-1was belong to C=O stretching vibration bond of generating carbonate between aryl O-and CO2.In addition,the C—O group stretch signal at 1590 cm-1is transferred to 1600 cm-1as a result of formatting carbonate ions (O—COO-) [49].Furthermore,to monitor the carboxylation of CO2and 2-aminobenzonitrile,the reaction process was detected with FT-IR spectrum as a function of time.It is exhibited that the quinazoline-2,4(1H,3H)-diones characteristic peak at 1030 cm-1and 765 cm-1gradually strengthened when the reaction time extended from 0 h to 8 h.

Fig.4. (a) The reaction kinetic model equation;(b) the yield of product versus various reaction temperature and time;(c) Arrhenius plots for CO2 carboxylation with 2-aminobenzontrile;(d) the calculated Ea data b(a) based on Arrhenius equation.

Fig.5. (a)FT-IR spectra of[TMG][2-OPy]before and after interacting with CO2;(b)FT-IR spectra as a function of time from 0 h to 8 h;(c)13C NMR spectra of[TMG][2-OPy]before and after interacting with CO2;(d)1H NMR spectra of [TMG][2-OPy] before and after contacting with 2-aminobenzotrile.
As is shown in NMR13C spectrum,two new peaks appeared at δ=159.35 and 156.70,which are corresponding to the carbonyl carbon atom from pyridine and the carbonyl carbon atom from the combination between N-heterocyclic O-and CO2[50].It is obvious that the peak strength at 159.35 is much stronger than that at 156.70,which implied that N-heterocyclic O-is easier to combine with CO2than pyridine N,revealing the CO2capture and conversion by N-heterocyclic O-group could be activated by priority.Meanwhile,the original peak at δ=165.96,δ=164.11 transferred upfield to δ=162.71 and 163.26 after CO2absorption,also revealing the interaction between[TMG][2-OPy]and CO2.The two main changes were observed in Fig.5(d) by comparison of 2-aminobenzontrile1H NMR spectrum before and after contacting with [TMG][2-OPy].The proton peak of —NH2group belong to 2-aminbenzonitrile transferred downfield from 6.005 to 6.037.The hydrogen signal of 2-hydroxypyridine shifted from 7.410 to 7.498.This phenomenon was possibly attributed to the generation of hydrogen bond between 2-aminobenzonitrile and [TMG][2-OPy],which similar conclusions had been drawn in the previously reported work[51].To sum up,we confirm that the[TMG][2-OPy]has dual function groups for activating both CO2and 2-aminobenzonitrile simultaneously.

Fig.6. Plausible mechanism of CO2 carboxylation catalyzed by [TMG][2-OPy].
Referring to the experimental data and previous study,a feasible reaction mechanism of converting CO2into quinazoline-2,4(1H,3H)-diones is put forward (Fig.6).The 2-aminobenzonitrile was firstly interacted with [TMG][2-OPy] by generating hydrogen bonds (TS1),obtaining an intermediate TS2.Meanwhile,CO2was catalyzed by 2-hydroxypyridine anion and formed the compound with carbonate group.TS2 electron-rich nitrogen atom subsequently carried out a nucleophilic attack on the carbonate carbon atom,generating an unstable oxyanion intermediate TS3.It was transformed into TS4 through an intramolecular nucleophilic cyclization step immediately.TS4 was converted to TS5 spontaneously with corresponding intramolecular rearrangement steps.Finally,quinazoline-2,4(1H,3H)-diones was obtained and [TMG][2-OPy] was regenerated simultaneously by a proton transfer step of TS6.
5.Conclusions
In summary,a pattern of 2-hydroxypyridine based protic ILs[TMG][2-OPy] was successfully prepared through one pot neutralization method,applying in the area of CO2carboxylation to synthesis quinazoline-2,4(1H,3H)-diones as well as its derivates.The CO2absorption behavior and the reaction parameters were investigated.This work certificated that the [TMG][2-OPy] could activate 2-aminobenzonitrile and CO2gaseous simultaneously,obtaining high yield of 97% under ordinary pressure without any metal or solvent,making the catalyst essence environmentally friendly.Broad substrate applications and simply reusability strategy for six cycles were also proved.Further,the fundamental kinetics suited for CO2carboxylation was put forward and the activation energy data was calculated.Finally,the feasible synergistic catalytic mechanism was presented.This work highlights a facile pathway to synthesize[TMG][2-OPy]with multi-functional groups and high catalytic activity in the domain of transforming CO2into the valuable industrial products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22278202) and the Natural Science Foundation of Jiangsu Province (BM2018007.BK20210185).
Supplementary Material
The data that support the findings of this study are available in the Supplementary Information and from the corresponding author upon reasonable request.Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.003.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
