Synthesis of a novel magnetic biomass-MOF composite for the efficient removal of phosphates: Adsorption mechanism and characterization study
Aaron Albert Aryee,Chenping Gao,Runping Han,Lingbo Qu
College of Chemistry, Green Catalysis Center, Zhengzhou University, Zhengzhou 450001, China
Keywords:Magnetic biomass-MOF composite Adsorption Phosphate Mechanism
ABSTRACT The adverse effects of eutrophication have prompted the use of various remediation techniques for phosphate(PO43–)removal owing to it being the major causative agent.Herein,the influence of different solvents and ratios of 2-aminoterepthalic acid on the efficiency of magnetic biomass metal–organic framework composites based on the in situ growth of NH2-MIL-101 (Fe) onto magnetized peanut husks towards PO43–removal was assessed via the adsorption technique.The magnetic biocomposite labelled as MPN@NH2-MIL-101(Fe)exhibited the best efficiency owing to its mesoporous structures and presence of abundant oxygen and nitrogen possessing functional groups.Adsorption results confirmed MPN@NH2-MIL-101(Fe) to have a high adsorption capacity of (14.0 ± 0.3) mg∙L-1 at a PO43- concentration of 20 mg∙L-1 with an associated high stability within pH 2–10.The adsorption kinetics for the process was well described by both Elovich and pseudo-second-order kinetic models and was mediated by both internal diffusion and liquid film diffusion.The Temkin and Freundlich models fitted the equilibrium data well signifying occurrence of both physical and chemical adsorption on a heterogeneous surface.It is concluded that MPN@NH2-MIL-101(Fe)is a promising adsorbent for the effective removal of phosphate from a water body.
1.Introduction
Phosphorous is an essential nutrient which plays a significant role in the growth process as a lack of it in sufficient amount has been associated with stunted growth in living organisms [1,2].It is also a major component in the production of fertilizers therefore is likely to be found in effluents from agricultural processes.Although it is required for growth of plants,only about 10%–20%of phosphorous (usually in the form of phosphates) in fertilizers is absorbed by plants with the others being retained in the soil or washed off into surface waters.This may compromise the USEPA(U.S.Environmental Protection Agency) permissible limit of 0.1 mg∙L-1for phosphates in surface waters [3].Furthermore,its presence in aquatic environment can have adverse impact as it can lead to algae bloom which depletes dissolved oxygen and may result in conditions such as eutrophication [4,5].This occurrence further aggravates the issues associated with water scarcity and also impedes the attainment of the sustainable development goals (especially 6 and 13).
To ameliorate this situation,several remediation techniques which are categorized into three main groups vis-à-vis physical,chemical and biological have been developed and their efficiency towards the remediation of phosphate polluted water assessed[6,7].Although each of these methods have been associated with varying degrees of efficiency and other associated advantages,the physical method has received the most attention albeit its use in combination with the other processes have been reported.In this context,the adsorption process has garnered significant attention in the sequestration of pollutants in wastewater due to its associated benefits which include relatively cheaper cost and ease of operation and the ability to recycle the obtained pollutant[8,9].The effectiveness of the adsorption process is majorly hinged on the material applied as the adsorbent owing to the process being characterized as a surface science.In this regard,several adsorbents based on materials obtained from natural(such as biomass,activated carbon,chitosan,biochar) [7,10] and synthetic(graphene,metal–organic frameworks (MOFs) and polymers)[11–13]sources have been assessed for their usefulness in removing phosphates from wastewater.In most of these cases it was evident that the materials from synthetic sources usually show excellent features and relatively higher adsorption efficiency owing to the ability to alter their properties.In a bid to leverage on the benefits of these major sources for the development of adsorbents,we report for the first time a magnetic biocomposite based on peanut husk and MOFsviaa simple synthetic route using readily available precursors (i.e.peanut husk and iron) and its application for the efficient removal of phosphates in both synthetic and simulated wastewater using an actual water sample under benign environmental conditions.
MOFs are a group of highly porous and crystalline materials which are based on metal ions/clusters and multifunctional organic linkers [14].Their associated excellent features such as good stability,tuneable properties and a relatively large surface area have endeared their application for various purposes including sequestration of wastewater [15,16].These features and their efficiency have been reported to be significantly hinged on the metal precursors,type of organic linker and solvent used.In addition,linkers with functionalized group may also affect the crystal growth rate of the developed MOF thus having a direct influence of its physicochemical properties and subsequently on its efficiency [17].For instance,Xieet al.[18] reported that NH2-MIL-101(Fe) exhibited a higher adsorption capacity (i.e.124.4 mg∙g-1)in comparison with MIL-101 (Fe) (i.e.107.7 mg∙g-1) which the authors ascribed to the amine groups which contributes to the adsorption processviathe ion exchange,hydrogen bonding and electrostatic attraction.Similar reports confirmed the superiority of UiO-66-NH2over UiO-66 towards the removal of diclofenac[19] and mercury [20].Although,these representative studies may confirm the superior properties of MOFs with functionalized organic linkers in the removal of pollutants,the fine nature of the MOF material (which is a major contributor to its good porosity) makes it challenging for their recovery after the adsorption process.
To address such challenges,MOFs are usually conjugated with other materials to form composites [21,22].The incorporation of biomass into MOFs in this regard can be an innovative way in increasing the yield of adsorbent produced as it has been reported that the production of pristine MOFs is usually associated with a small yield.This can ameliorate the issue of cost,longer time to achieve an adequate amount of MOFs for the adsorption process as well as the minimization in the amount of these biomass materials which end up at the landfill sites [23].A recent review presented by Liuet al.[24] revealed that significant progress has been made in the development of bio-MOF and MOF/biomass multifunctional materials for various applications.From this report it was found that most of these biocomposites were based on other types of MOFs such as the University of Oslo based MOFs (UiO-66) and zeolitic imidazolate framework.The few reported magnetic biocomposites were also obtainedviapyrolysis at higher temperatures orviathe addition of pristine Fe3O4into the synthesis medium.Although these may achieve magnetic biocomposites,their associated challenges which may include the release of toxic gases from the pyrolysis process,high reactivity of pristine Fe3O4and their associated toxicity to aquatic organisms due to leaching and instability may limit their practical applications;therefore,the need to explore other synthetic routes.
Peanut husk (a natural polymeric material) is one of the most abundant waste materials generated owing to the global production and consumption of peanut husk.To dispose them off,peanut husks are usually burnt on the farmland to generate ash for agricultural purposes or damped at landfill sites.These practices have been associated with some environmental challenges therefore necessitating the need to explore other options for its recyclability and further disposal.In this regard,the unique properties of peanut husk which includes having good thermal and chemical stability in a broad pH range as well as possessing abundant oxygen containing functional groups make it an ideal candidate for the development of adsorbents [25].However,its efficiency and difficulty in retrieving it after the adsorption process have been limiting factors to its industrial applications.To address this,the peanut husk was endowed with magnetic propertiesviathe coprecipitation method to facilitate its easy retrieval.This was then used as a support for the development of a magnetic MOF biocomposite using 2-aminoterephthalic acid and Fe3+as organic linker and metal ionviathe hydrothermal method.The formed MOF which belongs to the Materials of Institute Lavoisier (MILs) have the advantages of abundant adsorption sites,high stability and permanent porosity[26].The carboxylic acid groups and nitrogen groups of this Fe based MOF can enhance its interaction with phosphatesviathe formation of a coordination bond leading to their enhanced and selective uptake.Moreover,from our checks,there is yet to be a report on the application of peanut husk as a substrate in the synthesis of a biomass-MOF composite.
The efficiency of MOFs has been reported to be dependent on the organic linker,metal ions and the solvent used.To assess the impact of the solvent and the precursors on the effectiveness of the developed biocomposite for the removal of phosphates in solution,different solvents and ratios of the precursors were used.In addition,an in-depth characterization of the developed biocomposite was carried out using scanning electron microscopy (SEM)with energy dispersive X-ray spectroscopy(EDX),X-ray diffraction(XRD),vibrating-sample magnetometry (VSM),Fourier transform infrared (FTIR) spectrometry and N2adsorption–desorption isotherms.The adsorption kinetics,diffusions,isotherms,and thermodynamics were investigated using well defined models to understand the interactions that may occur at the adsorption interface.The adsorption mechanism was further elucidated through a combination of various analytical techniques,including X-ray photoelectron spectroscope (XPS),FTIR and the pH of point of zero charge (pHpzc).The selectivity,recyclability and practicability of MPN@NH2-MIL-101 (Fe) for remediation of real water samples were also evaluated.This study can be a model for the development of other novel and efficient adsorbents based on biomass and MOF materials for other environmental remediation and management applications.
2.Materials and Methods
2.1.Materials
Chemicals of analytical grade were used in this study without further purification.These included 2-aminoterephthalic acid(NH2-BDC,which was purchased from Energy Chemicals,China),ferric chloride (FeCl3∙6H2O),sodium hydroxide (NaOH),ferrous sulphate (FeSO4∙7H2O) (which were purchased from Aladdin,China) and solvents such asN,N-dimethylformaldehyde (DMF),water and ethanol.Sodium chloride (NaCl),magnesium nitrate(Mg(NO3)2),sodium sulphate (Na2SO4),and sodium bicarbonate(NaHCO3) were purchased from Tianjin Chemicals,China.All reagents were utilized as received.Deionized(DI)water(resistance 18.2 MΩ,Milli Q plus,Merck Millipore Co.,Germany) was used in all experiments.The peanut husk was obtained from a local farm in Zhengzhou,Henan,China.The raw peanut husks were pre-treated by washing several times and soaked in water for about a week.This was then dried at 333 K to dry mass after which they were ground,passed through a 0.5 mm sieve and soaked in DI water for another 24 h.This mixture was then decanted and the obtained peanut husks oven-dried at 333 K for 48 h then kept in a sealed container for further use.
2.2.Preparation of adsorbents
The magnetic peanut husk which was used as the support for the development of the adsorbent was prepared by incorporating Fe2+and Fe3+into peanut husk pretreated with NaOHviathe coprecipitation method as reported in our previous study [27].About 0.2 g of this magnetic material(labelled as MPN-NaOH)was added to a solution prepared from dissolving 0.16 g of FeCl3∙6H2O and 0.07 g of NH2-BDC in 10 ml DMF.This mixture was stirred at room temperature for an adequate period of time after which it was transferred into an autoclave and heated at 423 K for 12 h.The brown precipitate which was obtained after the reaction was separated and washed several times with methanol after which it was dried in a vacuum oven at 353 K for 24 h.The final product was stored in an air-tight container and labelled as MPN@NH2-MIL-101 (Fe).Fig.1 is a schematic diagram illustrating the preparation process.A similar procedure was followed to prepare the pristine MOF material without the addition of MPN-NaOH.This obtained material was labelled as NH2-MIL-101 (Fe).
Using the same procedure as described for MPN@NH2-MIL-101(Fe),different solvents vis-à-vis water and DMF/water solution(1:1)were used to develop the adsorbents labelled as MPN@MOFWand MPN@MOFW/Drespectively.To assess the influence of the amount of the NH2-BDC on the adsorption capacity,a similar procedure as reported for the development of MPN@NH2-MIL-101(Fe)was used with the amount of NH2-BDC reduced to 0.02 g.This formed adsorbent was labelled as MPN@MOF1.
2.3.Characterization studies
The nature of MPN@NH2-MIL-101(Fe) was assessed using analytical techniques under standard protocols as described in our previous study [27].
2.4.Batch adsorption and statistical studies
The batch method was used to assess the efficacy of the adsorbents towards phosphate removal in an aqueous medium.Unless stated otherwise,about 0.012 g of MPN@NH2-MIL-101 (Fe) (or other studied adsorbents) was mixed with 10 ml of phosphatesat a concentration of 20 mg∙L-1(as P) for 4 h at 298 K.The adsorbent was then recovered using a magnet and theconcentration in the solution analyzed using the molybdenum antimony anti-spectrophotometry method at a maximum wavelength of 700 nm.The adsorption capacity(q,mg∙g-1)of the adsorbent and its associated removal efficiency (p,%) can be assessed using the expressions:
wherem(g)andV(L)are the mass of adsorbent and volume ofsolution.C0andC(mg∙L-1) are the initial and finalconcentration.
Environmental factors such as salts (0.02–0.1 mol∙L-1of NaCl,Mg(NO3)2,Na2SO4,and NaHCO3),pH (2–10),temperature (298–318 K),contact time (5–360 min) and initial PO43–concentration(10–70 mg∙L-1) were assesed due to their ability to influence the adsorption process.The reproduceability of the results from this study was assessed by repeating the adsorption experiments and the obtained data analyzed using the average and standard deviation functions from the Microsoft Excel software after which the relative error was determined.
2.5.Adsorption studies using actual wastewater
The practicability of this developed adsorbent was assessed using a real water sample spiked withto make a final concentration of 15 mg∙L-1.Then after different masses of MPN@NH2-MIL-101 (Fe) were added and their removal efficiency assessed.To confirm their efficiency,the absorbance spectrum ofsolution before and after the adsorption process was recorded using the UV–Vis spectrophotometer.
2.6.Regeneration and stability studies
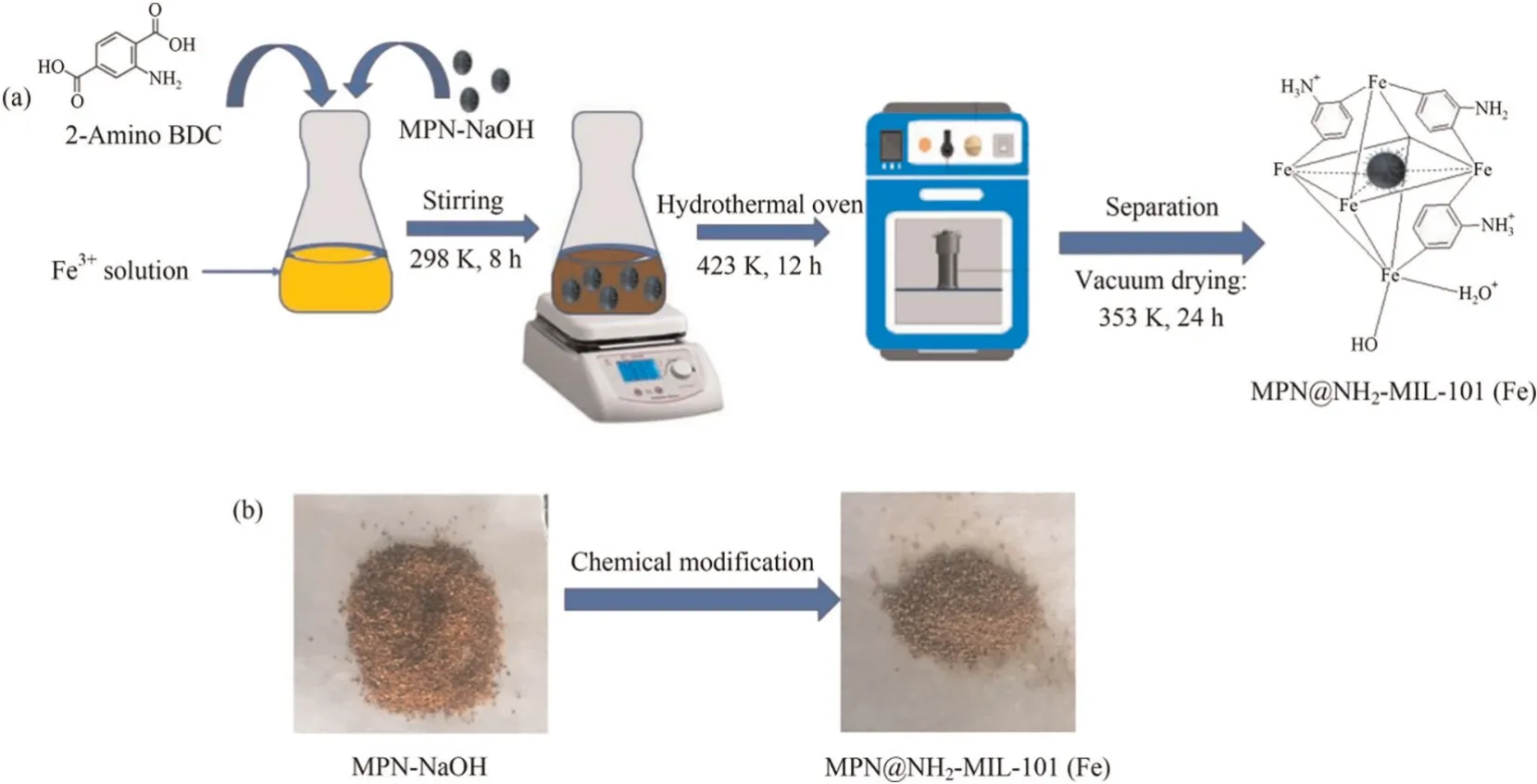
Fig.1. (a) Schematic diagram showing the preparation of MPN@NH2-MIL-101 (Fe).(b) Images of MPN-NaOH and MPN@NH2-MIL-101 (Fe).
The economic viability of the adsorbent is enhanced if it is fairly stable in aqueous solution as well as can be reused for further adsorption cycles with good removal efficiencies.The study to assess the reusability efficacy of MPN@NH2-MIL-101 (Fe) for further adsorption studies was carried out by using the spent adsorbents obtained from the adsorption study under the following conditions:m=0.012 g,C0=20 mg∙L-1,T=298 K,pH~5.60.The spent adsorbents obtained after the adsorption process were retrieved from the solutions using a magnet,washed with water and then dried in an oven at 333 K for 12 h.Under the same experimental conditions,the efficiency of different eluent solutions visà-vis 0.1 mol∙L-1HCl and NaOH were used for desorption offrom MPN@NH2-MIL-101 (Fe) and the subsequent use of the regenerated adsorbent for further adsorption cycles was assessed.The regeneration efficiency (r,%) was expressed as a ratio of the adsorption capacity of MPN@NH2-MIL-101 (Fe) before and after the regeneration process as expressed below:
wherem0andmdare the mass ofon MPN@NH2-MIL-101 (Fe)before desorption and the reduced mass ofon MPN@NH2-MIL-101 (Fe) after desorption,respectively.qris the adsorption amount in the next adsorption after each desorption (d) andqeis the initial adsorption capacity.
3.Results
3.1.Adsorption performance
3.1.1.Preliminary studies to assess the efficiency of adsorbents obtained under different synthesis conditions
In a bid to select the best adsorbent for this study,the adsorption capacity of the formed adsorbent were assessed under the following experimental conditions:m=0.012 g,pH=5.60,T=298 K,C0=20 mg∙L-1,t=6 h.From the results of this study,the adsorption capacity for MPN@NH2-MIL-101 (Fe),MPN@MOFW/D,MPN@MOFW,MPN@MOF1,NH2-MIL-101 (Fe),MOFWwas found to be 11.40,3.13,3.81,7.08,17.5,2.01 mg∙g-1respectively signifying the order of NH2-MIL-101 (Fe) >MPN@NH2-MIL-101(Fe) >MPN@MOF1>MPN@MOFW>MPN@MOFW/D>MOFW.It is worthy to note that,a very low adsorption capacity was recorded when water or a mixture of water and DMF(1:1)is used as the solvent in the development of the adsorbent (MPN@MOFWand MPN@MOFW/D) or the pristine MOF (i.e.MOFW).This could mean that water may not promote the crystal growth and nucleation of these MOFs under the studied conditions and even if they do,may result in poor physicochemical features as well as other MOF phases which may significantly affect their removal efficiency.In addition,reducing the amount of NH2-BDC to 0.02 g resulted in a decreased removal efficiency of the formed adsorbent(MPN@MOF1)therefore signifying the important role of the organic linker on the efficiency of the adsorbent.The reduction in the adsorption capacity after the addition of the magnetized peanut husk could be due to its ability to reduce the surface area of NH2-MIL-101 (Fe) by blocking some of its pores.However,challenges associated with the removal of NH2-MIL-101 (Fe) after the adsorption process as well as to find alternative use for peanut husk formed the basis for the choice of MPN@NH2-MIL-101 (Fe)for further application in this study.The SEM images of the formed adsorbents are shown in Fig.2.Based on the images,it could be suggested that the the MOF is connected to the magnetic peanut husk byin situgrowth which may have occurredviathe complexation mechanism.
3.1.2.Effect of MPN@NH2-101(Fe)dose
The adsorbent dose is usually considered in the assessment of the efficiency of adsorbents as it relates directly to the number of active sites present for the adsorption process[28].Under the studied conditions,it was found that an increase in MPN@NH2-MIL-101(Fe) dose from 0.004–0.02 g resulted in an increase in itsqefrom(15.9 ± 0.3)– (10.1 ± 0.2) mg∙g-1which translated to a removal efficiency of (32.4 ± 1.0)%– (99.1 ± 0.1)% (Fig.3(a)).This is due to the increase in availabilty of active sites with an increase in its dose which enhances the removal ofwith a concomitant increase in the unsaturation of these active sites [29].
The presence of the MOF moeity on MPN@NH2-MIL-101 (Fe)was found to be beneficial as its adsorption capacity was higher in comparison with pristine peanut husk (PN),pristine Fe3O4paticles (MP),magnetized peanut husk (MPN) and MPN-NaOH (Fig.3(b)).This corroborates our research objective of finding an alternative for this waste material.
3.1.3.Effect of pH and salts
The solution pH as confirmed from many reported studies may arguably be the parameter which significantly affect reaction processes in water chemistry due to its ability to regulate the nature of the adsorbate present and the surface properties of the adsorbent.From the results of the study as shown in Fig.3(c),the pH of the solution influenced the adsorption capacity of MPN@NH2-MIL-101(Fe)towardsalbeit this was not large as compared to that reported for our previous adsorbents [4,30].This showed that MPN@NH2-MIL-101 (Fe) could be efficient for the removal ofwithin a broad pH range (i.e.2–10).Theqeof MPN@NH2-MIL-101 (Fe) increased gradually from (10.4 ± 0.2)– (14.0 ± 0.3)mg∙g-1as the solution pH was increased from 2–8.After this point,it marginally reduced to (10.7 ± 0.1) mg∙g-1as the pH reached 10.These observed phenomena could be linked to the protonation and deprotonation process which occurs.As the pHpzcof MPN@NH2-MIL-101(Fe)was found to be 4.05,it was expected to be very effective for the removal ofat pH
The assertions made with regards to the underlying mechanisms can be confirmed from the influence of some common ions found in wastewater on the adsorption process.From the results as displayed in Fig.3(d),it can be found that the presence of ions generated by the salts had little impact on theqeof MPN@NH2-MIL-101 (Fe) at relatively high concentrations.The lowestqeof MPN@NH2-MIL-101 (Fe) in the presence of these salts was found to be (8.28 ± 0.07) mg∙g-1when the concentration of NaHCO3was 0.1 mol∙L-1.This can be due to the ability of theion to compete withfor active sites on MPN@NH2-MIL-101 (Fe) as well as its ability to change the pH of the solution to an alkaline region which can be inferred from Fig.3(a) to be not favourable for the adsorption process.This could also infer that the uptake ofproceededviainner spere mechanism [9].
3.1.4.Effect of adsorption time and fitting of kinetic models
The adsorption time may also give an indication of the efficiency of the adsorbent for practical purposes as it may relate to cost of carrying out the process.To understand the adsorption rate,the uptake ofonto MPN@NH2-MIL-101 (Fe) as a function of time was assessed at three different concentrations of(i.e.10,20,30 mg∙L-1) at 298 K.As displayed in Fig.4(a),the uptake ofsignificantly increased at the initial stages of the reaction and required different times to achieve equilibrium based on the initialconcentration present albeit all the processes achieved equilibrium within 240 min.
It required a relatively shorter equlibrium time(i.e.80 min)for lowerconcentration (i.e.10 mg∙L-1) whereas this time increased to about 120 and 180 min atconcentrations of 20 and 30 mg∙L-1.The high adsorption rate at the initial stages was due to the availabilty of unoccupied active sites which coupled with the initialconcentration acted as driving forces to suppress the barriers to the adsorption process [27,31].However,with an increase in time,these sites gets occupied thus decreasing the driving force resulting in a reduction in the intraparticle diffusion rates leading to the attainment of equilibrium [32].
To further comprehend the nature of the mechanisms underlying the adsorption process,the pseudo-first order kinetic model(PFO),pseudo-second order kinetic model (PSO) and the Elovich model was fitted to this experimental data using the OriginPro software.The non-linearized forms of these models as used in this study is shown below:

Fig.4. Effects of (a) contact time and subsequent fitting of kinetic models,(b) PFO,(c) PSO,and (d) Elovich,and (e) intraparticle diffusion for the uptake of onto MPN@NH2-MIL-101 (Fe).
whereqtandqe(mg∙g-1) are the adsorbedat timetand at equilibrium,respectively;k1andk2refer to the first-order and second-order rate coefficient respectively whereasAandBare Elovich constants.
To assess the suitability of these models and the best fit to the experimental data,the determined coefficient (R2) and the sum of squared errors (SSE) were evaluated using the expressions:
whereqeandqcare the experimental and calculated adsorption capacities of the model andnis the number of observations.
The fitted curves as shown in Fig.4(b)–(d) and their obtained parameter values shown in Table 1 suggest that PSO and Elovich model could both describe the adsorption process connoting the presence of chemisorption process playing a significant role in the uptake ofonto MPN@NH2-MIL-101 (Fe).This assertion was based on their high regression coefficient value(0.922 ≤R2≤0.979 for PSO and 0.938 ≤R2≤0.993 for Elovich)and smaller SSE values (0.153 ≤SSE ≤3.12 for PFO and 1.42 ≤SS E ≤7.80 for Elovich).Although this method may not be an utopian way of selecting the best method,it has been observed to be very useful as can be confirmed by most reported studies which used this method in determining the best fitted models [33–36].
The role of pore filling underlying the process was evaluatedviathe assessment of intraparticle diffusion and film diffusion associated with the movement of thefrom the solution phase to the surface of MPN@NH2-MIL-101 (Fe).This assessment was carried out using the intraparticle diffusion expressed below:
Fig.4(e)is the curves associated with the intraparticle diffusion model fitted to the experimental data whereas the values of the associated parameters are also shown in Table 1.The observed steep slopes at the initial stages of the adsorption process with higher recordedKt1values (as compared withKt2values) which increased with concentration confirm the assertion that the highconcentrations coupled with available adsorption sites act as driving forces to promote high adsorption rates [32,33].The involvement of boundary layer diffusion as the rate controlling step in the adsorption process together with intraparticle diffusion can be inferred from the fitted lines not passing through the origin(0,0).This observation signifies the role of pore filling as an active mechanism for the process.
3.1.5.Effect of equilibriumconcentration and fitting of isotherm models
To further understand the adsorption capacity of MPN@NH2-MIL-101 (Fe) towards the removal of,differentconcentrations were used and the obtained equilibrium concentrations plotted against theqeof MPN@NH2-MIL-101 (Fe) (Fig.5(a)).As shown in this data,theqeof MPN@NH2-MIL-101 (Fe) increased with increasingconcentration which was due to their ability to act as driving force to overcome the resistances to the adsorption process.
Another observation is the insignificant effect of temperature on theqeof MPN@NH2-MIL-101 (Fe) towards uptake of.This may indicate that although chemical forces may play major roles in the process the impact of physical forces cannot be neglected thereby corroborating the significant effect of pore filling [6].To further understand the underlying mechanisms and also to aid in the design of the adsorption system for practical applications,the experimental data was fitted with the non-linear forms of the Langmuir,Freundlich,Temkin and Koble–Corrigan adsorption models as expressed below:

Table1 Parameters of fitted kinetic models

Fig.5. Effects of(a)equilibrium concentration and subsequent fitting of adsorption models,(b)Langmuir,(c)Freundlich,(d)Temkin,and(e)Koble–Corrigan for the uptake of onto MPN@NH2-MIL-101 (Fe).
whereqmandqeare the maximum adsorption capacity and the equilibrium adsorption capacity MPN@NH2-MIL-101 (Fe);KL(L∙mg-1) is a constant related to the affinity of the binding sites and energy of adsorption whereasCe(mg∙L-1) is equilibrium concentration andKFrelates to the adsorption capacity and 1/nto the adsorption intensity of MPN@NH2-MIL-101(Fe).ATandBTare Temkin constants,AandBare Koble–Corrigan constants.
Based on the closeness of the fitted curve to the experimental data(as shown in Fig.5(b)–(e)and the associatedR2and SSE values(as shown in Table 2),the Temkin and Koble–Corrigan model was found to be better fits to the experimental data.The Koble–Corrigan model is based on the Langmuir and Freundlich models therefore is usually used to explain processes which involve both monolayer and multilayer processes.Also,it has been reported that its ‘n’ value in the model may give an indication as to which of these individual models may be more pronounce in the studiedprocess.The obtainednvalue which was found to be 0.387 Table2 Parameters of fitted adsorption isotherm models for the uptake of PO43– onto MPN@NH2-MIL-101 (Fe) and thermodynamic analysis The feasibility of the adsorption process occurring and its reliability on temperature are usually obtainedviathe values of the associated parameters from the thermodynamic study.The corresponding thermodynamic parameters ΔG0,ΔS0and ΔH0were obtainedviathe following expressions: whereCad,e(mg∙L–1)is the concentration ofon MPN-NH2-MIL-101 (Fe) at equilibrium,the value ofKccan be obtained with the lowest experimental concentration of,R(8.314 J∙mol-1∙K-1)is the universal gas constant,T(K) is the absolute temperature.K0(g∙mg-1∙min-1) is the temperature independent factor. From this analysis as shown in Table 2,the adsorption was found to be spontaneous owing to the negative ΔG0values(i.e.–3.89 ≤ΔG0≤–4.94 kJ∙mol-1∙K-1) which decreased with an increase in temperature suggest the favourability of higher temperature in the adsorption process.This was confirmed by the positive ΔH0value (i.e.11.7 kJ∙mol-1) which suggests the process to be endothermic in nature.The magnitude of the ΔH0value further confirms the presence of physical and chemical forces in the adsorption process.The disorderliness at the interface of the adsorption process was found to decrease as can be inferred from the negative value of ΔS0(i.e.–0.0527 kJ∙mol-1∙K-1). In addition to the features described above,the reusability efficiency of adsorbents is usually considered for their economic benefits.In this study,both 0.1 mol∙L-1HCl and NaOH solutions could achieve a relatively good desorption efficiency of >50% with a regeneration efficiency of about >30% after three cycles of reusability study (Fig.6(a)). This observation may be due to factors which may include: (i)the ability of the eluents to corrode the surface of the adsorbent leading to the unavailability of some pores,(ii)changes in the surface functional groups and (iii) unavailability of some active sites for further adsorption process which may corroborate the presence of monolayer adsorption. In addition,the Fe leached into the solution was insignificant as compared to the amount of Fe in MPN@NH2-MIL-101 (Fe) (≪1%)suggesting that it still possessed a significant amount of its magnetic properties and may not release high amounts of Fe which could pose a threat to other organisms.Also,the nature of MPN@NH2-MIL-101 (Fe) before and after the adsorption process may make it possible for it to be decomposed into the elements Fe,C,N,O and P when disposed of into the soil which may help in plant growth. Table3 Comparison of maximum adsorption capacity for the uptake of onto different adsorbents Table3 Comparison of maximum adsorption capacity for the uptake of onto different adsorbents Fig.6(b)shows the influence of the mass of MPN@NH2-MIL-101(Fe)on the removal ofin a simulated wastewater using a real water sample drawn from a lake within Zhengzhou City.The results from this study confirmed that 1.6 mg∙L-1of MPN@NH2-MIL-101 (Fe) could achieve the WHO permissible limit forin surface waters even when present at high concentrations (i.e.15 mg∙L-1) [38].This confirms the high prospects for this biocomposite for practical application.The associated absorption spectra showing the influence of mass of MPN@NH2-MIL-101 (Fe) in this study are shown in Fig.6(c). Table 3 is a comparison of the monolayer adsorption capacity of MPN@NH2-MIL-101 (Fe) under the studied conditions towardswith other reported adsorbents.From this study,it can be seen that the developed MOF-biomass composite in this study exhibits good adsorption capacity with lowerconcentrations.This coupled with its unique features (such as the presence of amino groups and Fe2+/Fe3+) may also promote its application for other processes such as in photocatalysis. Fig.7. Electron cloud maps of(a)Fe and(b)N in MPN@NH2-MIL-101(Fe).(c)Elemental composition of MPN@NH2-MIL-101(Fe).(d)Hysteresis loop showing N2 adsorption–desorption isotherm on MPN@NH2-MIL-101(Fe).(e)XRD patterns of NH2-MIL-101(Fe),MPN@NH2 MIL-101(Fe)before and after the adsorption process.(f)FTIR spectra of MPN@NH2-MIL-101(Fe)before and after the adsorption of .(g)Hysteresis loop of MPN@NH2-MIL-101(Fe)showing its magnetization before and after the adsorption of (1 emu∙g-1=1 A∙m2∙kg-1,1 Oe=80 A∙m-1).(h) Hysteresis loop showing the pHpzc of MPN@ NH2-MIL-101 (Fe). The elemental mapping studies confirmed the uniform distribution of Fe (Fig.7(a)) and N (Fig.7(b)) in MPN@NH2-MIL-101 (Fe)suggesting the successful loading of the MOFs onto the magnetic substrate.Based on this study the composition of MPN@NH2-MIL-101 (Fe) was C=55.5%,O=24.7%,Fe=15.2%,N=4.7% as shown in Fig.7(c).The porosity of adsorbents play a crucial role in the adsorption process.An assessment of this feature of the adsorbentviathe BET method showed theSBETof the adsorbent to be 70.5 m2∙g-1with an average size width of 6.17 nm confirming the presence of mesoporous structures.This can be confirmed from the nature of the hysteresis loop of N2adsorption–desorption isotherm(Fig.7(d))which depicts a type IV isotherm that has usually been associated with mesoporous adsorbents.The XRD was further used to confirm the crystallinity of the formed adsorbents.As shown in Fig.7(e),NH2-MIL-101 (Fe) showed peaks which were consistent with reported diffraction patterns of NH2-MIL-101 (Fe)with good crystal structure [42].MPN@NH2-MIL-101 (Fe) was found to have similar diffraction peaks with NH2-MIL-101 implying that the presence of the magnetic support does not destroy the MOF structure.In addition some peaks (albeit significantly reduced) which matched well to the Fe3O4with a facedcentered-cubic structure (JCPDS No.75-0449) confirmed the successful synthesis of the magnetic adsorbent.The structure of MPN@NH2-MIL-101(Fe)remained fairly constant after the adsorption process which may infer its good stability. The FTIR analysis is useful in determining the functional groups on the adsorbent and their role in the adsorption process.From this analysis as shown in Fig.7(f),the overlap of the symmetric vibrations of the —OH and —NH2functional groups in MPN@NH2-MIL-101 was found at 3420 cm-1whereas the peak at 1652 and 1588 cm-1can be ascribed to the C=O group and the overlap of C=O and the benzene ring in NH2-MIL-101 (Fe).The C—N bond and the Fe—O bond (which results from Fe3O4) were found at 1257 and 581 cm-1respectively [43].These observations may further corroborate the successful loading of NH2-MIL-101 onto MPN-NaOH to form the magnetic adsorbent (i.e.MPN@NH2-MIL-101 (Fe)). Fig.8. (a)SEM image of MPN@NH2-MIL-101(Fe)after the adsorption of .(b)EDS spectrum showing the uniform distribution of P-element on MPN@NH2-MIL-101(Fe)after the adsorption process.(c)Wide scan of MPN@NH2-MIL-101(Fe) before and after the adsorption process.(d)High resolution of O 1s peak on MPN@NH2-MIL-101 (Fe)before and after the uptake of .(e)High resolution of Fe 2p on MPN@NH2-MIL-101(Fe)before and after the adsorption process.(f)High resolution of P 2p on MPN@NH2-MIL-101 (Fe). Fig.9. Schematic diagram showing the probable mechanisms underlying the uptake of onto MPN@NH2-MIL-101 (Fe). The magnetization properties were further confirmedviathe VSM analysis and the results shown in Fig.7(g).In this study,the vibrating-sample magnetometry (VSM,Quantum Design PPMS DynaCool,USA) was used to determine the magnetic properties at room temperature.The hysteresis curve of MPN@NH2-MIL-101(Fe)showed an S-shape with a coercetivity close to zero signifying the ease with which it can be retrieved although it has a significantly lower magnetization saturation value (Ms) of 4.52 emu∙g-1(1 emu∙g-1=1 A∙m2∙kg-1)as compared to 77.64 emu∙g-1recorded for pristine Fe3O4[34].The magnetic properties of MPN@NH2-MIL-101 (Fe) was observed to be intact albeit a reduction in itsMs(i.e.3.24 emu∙g-1) after the adsorption of.This confirms the ease associated with its removal using a magnet whereas the reduction in theMsvalue may be due to the shielding effect offered by the adsorbedmolecules as well as the leaching of Fe.However,the amount of Fe leached into the solution after the adsorption process was found to be~0.19 mg∙L-1which is below the permissible limit for lead in surface waters set by the USEPA[44].The isoelectric point can give an indication of the removal efficiency of the adsorbent towards different classes of pollutants.From this analysis,the pHpzc of MPN@NH2-MIL-101 (Fe) was recorded to be 4.05 (Fig.7(h)). Fig.8(a) shows the morphology of MPN@NH2-MIL-101 (Fe)after the uptake of.In comparison to the SEM of MPN@NH2-MIL-101 (Fe) as shown in Fig.2(b),the surface of the adsorbent after the adsorption process slightly changed with lots of flakes appearing.These can be attributed to the adsorbedions which were uniformly distributed as was confirmed by the EDS spectrum Fig.8(b).The EDS analysis further revealed that the amount of P element on the adsorbent was about 1.8% of its composition;an indication of its successful adsorption.The XPS analysis can also be a valuable tool to study the underlying mechanisms for an adsorption process.From the wide spectrum of MPN@NH2-MIL-101 (Fe) (Fig.8(c)),the P 2p peak at 133.1 eV which only appeared after the adsorption process corroborates the successful uptake ofonto MPN@NH2-MIL-101 (Fe) whereas the peak at 711.11 eV which corresponds to the Fe 2p confirms the magnetic properties of the adsorbent.The molar O/C ratio on MPN@NH2-MIL-101(Fe)was also observed to increase from 42.3%–67.1%after the adsorption process signifying the increase in the polar groups on its surface [45].The high-resolution spectra of O 1s and Fe 2p peaks were further carried out using the XPSPeak41 software with a Shirley background to understand their role in the adsorption process. For the O 1s peak on MPN@NH2-MIL-101 (Fe) (Fig.8(d)),the peaks at 529.7,531.3 and 532.8 eV could be assigned to Fe—O,Fe—OH and H2O [34].After the adsorption process,these peaks were shifted to higher binding energies with reduced intensities as well as the emergence of a new peak at 533.3 eV which is attributed to bridging oxygen (P—O—P) peak.These results show that the surface hydroxyl groups played a significant role in the uptake ofviathe ligand exchange mechanism.This can be confirmed by the about 19% reduction in the intensity of the Fe—OH peak after the adsorption process which authors such as Lanet al.[35]and Haoet al.[46] have ascribed to the presence of complexation mechanisms.For the Fe 2p spectra of MPN@NH2-MIL-101 (Fe)(Fig.8(e)),the three binding peaks at 711.2,710.5 and 714.9 eV can be assigned to Fe 2p3/2whereas the peak at 724.6 eV is assigned to the Fe 2p1/2. The active roles of these functional groups in the adsorption process were also confirmed from the FTIR spectrum of MPN@NH2-MIL-101 (Fe) after the adsorption process (Fig.7(f)).For instance,the peaks at 1652 and 580 cm-1which are ascribed to the C=O and Fe—O bond recorded changes in their intensities as well as a shift in their position to 1626 and 583 cm-1after the adsorption.In addition,a broad and intense peak observed at 1054 cm-1which can be attributed to the P—O bond emerged after the adsorption process [47].These observations coupled with that made from the influence of salt and pH on the adsorption process could mean that probable mechanisms underlying this process are the electrostatic and ion-exchange with the latter likely to be the dominant.A schematic diagram showing the mechanisms underlying the uptake ofonto MPN@NH2-MIL-101 (Fe) is shown in Fig.9. A novel magnetic biomass-MOF composite based on NH2-MIL-101 (Fe) loaded onto magnetic peanut husks was synthesized and applied for the removal of phosphates in water samples.The efficiency of the prepared composite was found to be significantly dependent on the solvent and ratio of precursors used in the synthesis.Of the six synthetic routes employed,the biocomposite(MPN@NH2-MIL-101 (Fe)) prepared using 0.2 g MPN-NaOH,0.07 g NH2-BDC and DMF as the solvent was found to be the optimum.MPN@NH2-MIL-101 (Fe) was found to be very efficient within a wide pH range with high selectivity towards phosphates in the presence of some common ions found in wastewater.The adsorption process was well described by the Elovich and PSO kinetic models and was largely mediated by internal diffusion and liquid film diffusion.The adsorption isotherms ofonto MPN@NH2-MIL-101 (Fe) were consistent with the Freundlich and Temkin models which suggest the presence of physical and chemical adsorption on an heterogenous surface.The adsorption process was an entropy increasing spontaneous and endothermic process with MPN@NH2-MIL-101(Fe)recording a high adsorption capacity of (14.0 ± 0.3) mg∙g-1at lowconcentration which was controlled by the ion-exchange mechanism.Although this study may be a model for the development of other biomass-MOF composites for environmental applications,further studies which seek to optimize the efficiency of the adsorbent using benign solvents in the synthetic process are recommended to address the toxicity concerns associated with the use of DMF. CRediT Authorship Contribution Statement Aaron Albert Aryee:Conceptualization,Methodology,Formal analysis,Investigation,Writing– original draft.Chenping Gao:Formal analysis.Runping Han:Conceptualization,Visualization,Supervision,Writing– review &editing,Funding acquisition.Lingbo Qu:Funding acquisition. Data Availability Data will be made available on request. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements The research was supported by the National Key Research and Development Program of China (2018YFD0401402–04) and Zhongyuan Scholars Foundation (202101510005).
3.2.Thermodynamic study
3.3.Reusability and stability studies
3.4.Efficiency of MPN@NH2-MIL-101 (Fe) using a real water sample

3.5.Comparison with other reported adsorbents
3.6.Characteristics of MPN@NH2-MIL-101 (Fe)



3.7.Adsorption mechanism
4.Conclusions
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
