Inhibition mechanism of air nanobubbles on brass corrosion in circulating cooling water systems
Yuling Zhang,Shaolei Lu,Delie Li,Haiyang Duan,Congwen Duan,Jinghong Zhang,Songtao Liu
Department of Environmental Science and Engineering, North China Electric Power University, Baoding 071003, China
MOE Key Laboratory of Resources and Environmental Systems Optimization, College of Environmental Science and Engineering, North China Electric Power University, Beijing 102206, China
Keywords:Brass Air nanobubble Passivation film Bubble layer Corrosion inhibition
ABSTRACT Air nanobubbles(A-NBs)were used to inhibit the brass corrosion in circulating cooling water for the first time in the study.The results of mass loss method and electrochemical method showed that A-NBs had the obvious corrosion inhibition effect.The inhibition rate reached 52%at 35°C.The impedance and surface characterization results of corrosion samples indicated that the corrosion inhibition mechanisms of A-NBs mainly included adsorption of corrosion ions,promoting the formation of the passivation film on metal surface and the formation of the bubble layer and scale film on metal surface.A-NBs are potential excellent corrosion inhibitors.
1.Introduction
Corrosion[1],scaling[2],and microorganism growth[3]are the three main factors affecting the safe operation of industrial circulating cooling water systems.As a result of corrosion,metal pipes of a heat exchanger may explode and perforate,thus posing a serious threat to production safety [4,5].Current common corrosion inhibition methods include chemical corrosion inhibitor methods[6–8]and surface coating methods[9,10].Chemical corrosion inhibitors can be generally classified as inorganic inhibitors and organic inhibitors.Most inorganic inhibitors are toxic and organic inhibitors have the defects of difficult biodegradation and secondary pollution[11,12].It is therefore difficult for chemical corrosion inhibitors to achieve both high performance and environmental friendliness[13,14].The conventional surface coating method is a less economical method and a number of factors affect the coating quality and increase the difficulty in maintaining an extended period of time.Nanotechnology has been applied in corrosion control and has become a hot spot [15].
Nanomaterial coating is a commonly used nano-anti-corrosion technology.Common nanomaterials include graphene nano anticorrosion coatings,nano-modified acrylic resin anti-corrosion coatings,and nano-clay.Nanomaterial coatings can prevent corrosive particles from penetrating into the metal surface,so as to achieve the purpose of anti-corrosion[16].However,nanomaterial coatings are expensive and easily destroyed in complex environments.In recent years,bubbles with the diameter ranging from tens to hundreds of nanometers,namely,nanobubbles(NBs),have attracted wide attention.Compared with ordinary bubbles,NBs are characterized by long residence time in water[17,18],larger specific surface area [19],higher zeta potential [20],higher mass transfer efficiency [21],hydroxyl radicals-producing cavitation [22],and zero secondary pollution,and have been applied in many fields such as medical treatment [23],environmental pollution treatment[24–26],food processing[27],and agricultural production [28].
Air nanobubbles(A-NBs)have wide applications because of the cheap and easy air source.Their high oxygen mass transfer efficiency,cavitation formation of hydroxyl radicals,and other characteristics are conducive to the formation of a passivation film on metal surfaces,which can inhibit metal corrosion.A low-carbon steel suspension sheet suspended in the acid water circulation system of geothermal power plants realized the corrosion inhibition efficiency of 50% after the addition of air micro-nano bubbles (AMNBs) into acid geothermal fluids [29].However,in numerical simulation results,micron bubbles and Cl-worked together to accelerate the corrosion of metal pipes [30,31] and micro bubbles without the unique properties of nanobubbles gathered to generate large bubbles and then collapse,thus increasing shear force

where υ0is the corrosion rate (mm∙a-1)of the test sample of blank group;υ1is the corrosion rate(mm∙a-1)of the test sample of A-NBs group.
The polarization curve and the impedance of the corrosion brass samples were determined with the CHI-604E electrochemical work station and the three-electrode system.The working electrode,reference electrode,and auxiliary electrode were respectively a corrosion brass sample,a saturated calomel electrode,and a platinum electrode.The three electrodes were immersed in simulated circulating cooling water to determine the open circuit potential(OCP).The OCP result was recorded after it was stable.Under the potential of OCP ± 1 V and the potential scanning rate of 0.01 V∙s-1the polarization curve was determined.The frequency of EIS test was set to 10-2–105Hz to measure the impedance.
The corrosion inhibition rate η2is calculated as follows:
whereIcorrandare the self-corrosion currents (A∙cm2) of test samples of blank group and A-NBs group corrosion.
2.4.3.Research methods of corrosion inhibition mechanism
The ion adsorption mechanism was investigated with the conductivity and zeta potential change.The generation mechanism of the passivation film on brass samples was investigated in terms of dissolved oxygen,pH change,and EIS.The chemical composition of the passivation membrane was investigated with EDS and XPS.The surface corrosion and the formation of calcium carbonate film of corrosion samples were investigated by SEM.The hydrophobicity of corrosion samples were investigated by using the contact angle test.
2.4.4.Characterization of A-NBs and corrosion samples
The particle size and solution potential of A-NBs were measured with a Zetasizer Nano ZSE (Malvern,USA).The scanning electron microscope (SEM) of SIGMA 500 (ZEISS,Germany) was used to observe the surface appearance of corrosion samples.The elemental composition of corrosion products on the sample surface was determined by energy dispersive X-ray spectroscope(EDS).Chemical composition of corrosion products was analyzed by ESCALAB 250xi X-ray photoelectron spectroscope (XPS).OCA-20 contact angle measuring instrument was used to measure the contact angle of the sample surface and determine its hydrophobicity.
3.Results and Discussion
3.1.Effect of circulating cooling water temperature on particle size and zeta potential of A-NBs
A circulating cooling water system is characterized by the large temperature difference between the inlet and outlet of the heat exchanger.Temperature change affects the particle size distribution and zeta potential of A-NBs in circulating cooling water and directly determines its role in the cooling water system.The influences of water temperature on the size and zeta potential of A-NBs were investigated within the operating temperature range of the circulating cooling water system.The results are shown in Fig.1.
With the increase in water temperature,the particle size of ANBs in both deionized water and 1000 mg∙L-1CaCl2solution decreased and the particle size distribution gradually became more concentrated (Fig.1(a) and (b)).At the same water temperature,the particle size range of A-NBs in 1000 mg∙L-1CaCl2solution was smaller than that in deionized water.With the increase in water temperature,the zeta potential of A-NBs decreased (Fig.1(c)).The zeta potential of A-NBs in 1000 mg∙L-1CaCl2solution decreased more significantly than that in deionized water and even became positive at 70 °C.
The effect of temperature on A-NBs size was further analyzed.With the increase in water temperature,the number of largesized A-NBs decreased and the proportion of small-sized A-NBs in the solution increased,suggesting that the average size of ANBs decreased.The decrease in the number of large-sized A-NBs was ascribed to the change of gas–liquid properties caused by temperature change.The increase in temperature decreased the viscosity,density,and surface tension of solution and the resistance to the rise of A-NBs was reduced,so the large-sized A-NBs were more likely to rise to the gas–liquid interface [37].In addition,with the increase in temperature,the gas–liquid interface became more unstable and the larger-sized A-NBs at the interface were more likely to collapse[38,39].With the increase in temperature,a large number of large-sized A-NBs collapsed,so the proportion of largesized A-NBs decreased,as confirmed by the decreased average size of A-NBs.The effect of temperature on zeta potential of A-NBs was analyzed below.When the water temperature rose,large-sized ANBs collapsed,thus increasing cation concentration in the solution and cations in the diffusion layer of small-sized A-NBs,as manifested by the weakened electronegativity of A-NBs in the solution[40,41].
The temperature difference in circulating cooling water was not conducive to the long-term stable existence of A-NBs,so it was feasible to maintain a certain concentration of small-sized A-NBs in the solution by continuously supplying A-NBs to the system.In this way,the role of A-NBs in the solution could be ensured.
3.2.Effects of temperature on corrosion inhibition of brass in A-NBs solution
After the water temperature was set according to the working conditions of the circulating cooling water system,the influence of A-NBs on the corrosion of brass samples at different temperatures was investigated with the mass loss method(Fig.2).At different temperatures,the corrosion rate of brass samples in the A-NBs group was slower than that in the blank group and the inhibition effect on brass samples of the A-NBs group was significant.With the increase in temperature,the corrosion rate of brass samples in A-NBs group and blank group showed an upward trend and the corrosion inhibition effect of A-NBs gradually decreased from 52% at 35 °C to 31% at 55 °C.The above results showed that the temperature rise was not conducive to the inhibition effect of ANBs.
The steady-state open circuit potential (OCP) of the corrosion samples of blank group and A-NBs group at different temperatures is shown in Table 3.The OCP value of corrosion samples in A-NBs group was significantly larger than that in blank group.The OCP values of corrosion samples in both A-NBs group and blank group decreased with the increase in temperature.The polarization curves are shown in Fig.3 and the corrosion parameters are provided in Table 4.At different temperatures,the self-corrosion current of the corrosion samples of A-NBs group was lower than that of the blank group,indicating that A-NBs had a certain corrosion inhibition effect on brass samples.The self-corrosion potential of the corrosion samples of A-NBs group moved towards the positive direction,indicating that A-NBs was an anodic corrosion inhibitor.With the increase in temperature,the self-corrosion current of the corrosion samples of blank group and A-NBs group gradually increased and the self-corrosion potential moved towards the negative direction,indicating that the corrosion rate of the brass samples of blank group and A-NBs group increased with the increase in temperature.With the increase in temperature,the inhibition effect η2of A-NBs decreased from 57% at 35 °C to 35% at 55 °C,as confirmed by the results of mass loss method.
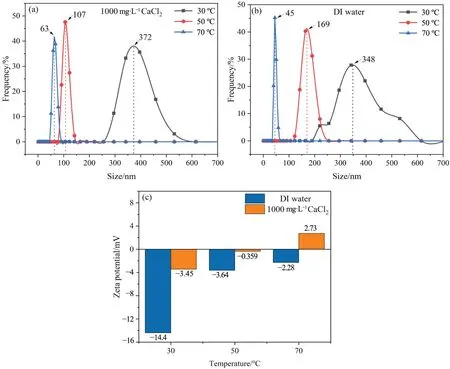
Fig.1. Effects of temperature on physical and chemical properties of A-NBs:(a)bubble size in 1000 mg∙L-1 CaCl2 solution,(b)bubble size in deionized water,and(c)bubble zeta potential of 1000 mg∙L-1 CaCl2 solution and deionized water.

Fig.2. Corrosion of brass samples in blank group and bubble group at different temperatures and corrosion inhibition efficiency (CIE) of A-NBs.

Fig.3. Polarization curves of the corrosion samples of blank group and bubble group at different temperatures.
The results of mass loss method and polarization curve showed that A-NBs had the obvious inhibition effect on brass corrosion in circulating cooling water,but the inhibition effect decreased with the increase in circulating cooling water temperature.The inhibition effect might be interpreted as follows.Firstly,the zeta poten-tial of A-NBs in the simulated circulating cooling water was extremely high and corrosive ions could be adsorbed onto its surface,thus reducing the concentration of free corrosive ions and pitting corrosion of brass test pieces.Secondly,A-NBs as an anodic corrosion inhibitor could promote the formation of a passivation film on the surface of brass samples so as to mitigate corrosion.Thirdly,according to the results in Section 3.1,when the temperature rose,large-sized A-NBs easily rose and collapse and the density of A-NBs decreased,thus decreasing the corrosion inhibition effect of A-NBs.

Table3 Steady-state open circuit voltage of the corrosion samples of blank group and A-NBs group at different temperatures
3.3.Inhibition of A-NBs in circulating cooling water on corrosion of brass samples
3.3.1.Adsorption of corrosive ions
The types and concentrations of corrosive ions are the main factors of the corrosion property of a solution.In circulating cooling water,the types of corrosive ions are fixed,so the concentrationsof corrosive ions are the main factor to be considered.The influence of ion concentration can be explored in terms of the conductivity of the solution.When the conductivity is low,the corrosion is weak.Fig.4 shows the conductivity of simulated circulating cooling water in blank group and A-NBs group at different temperatures.After A-NBs were introduced into the A-NBs group,the conductivity firstly showed a significant downward trend,reached the lowest value after 5 min of aeration,then gradually increased,and finally tended to be stable.With the increase in temperature,the effect of A-NBs on the reduction of solution conductivity decreased.In other words,the inhibition effect of A-NBs on brass corrosion decreased.

Table4 Polarization curve parameters of the corrosion samples of blank group and A-NBs group at different temperatures

Fig.4. Conductivity changes of blank group and bubble group at different temperatures: (a) 1-d conductivity,(b) 2-d conductivity,and (c) 3-d conductivity.
The decrease in the conductivity of A-NBs group might be interpreted as follows.Firstly,A-NBs in simulated circulating cooling water had strongly negative zeta potential [37,42] and could adsorb cations [43].Secondly,the A-NBs cation adsorption layer can adsorb anions such as Cl-and SO42-[44,45],which cause pitting corrosion in brass [46].In other words,cations and anions in the solution were bound by the adsorption effect of A-NBs,thus decreasing the concentrations of corrosive ions,the conductivity,and the probability of chemical reaction between corrosive ions and test samples.In this way,the corrosion of samples was inhibited.When the temperature rose,large-sized A-NBs were easy to collapse,thus decreasing the density of A-NBs and increasing the concentrations of corrosive ions in the solution and the conductivity.Finally,the corrosion inhibition effect of A-NBs was weakened.Therefore,the adsorption of ions by A-NBs was significant for the corrosion inhibition of A-NBs in simulated circulating cooling water.
3.3.2.Promoting the formation of the passivation film
In the circulating cooling water system,the formation of a passivation film on the tubes of the heat exchanger was closely related to the types and concentrations of oxidants and water temperature.Both the blank group and A-NBs group had the same oxidant source,but their dissolved oxygen concentrations were different.The DO concentrations in simulated circulating cooling water of the two groups at different temperatures are shown in Fig.5.At different temperatures,DO concentration of the A-NBs group reached the maximum value after about 1 min and then slowly decreased to a stable value.With the increase in temperature,the DO concentrations in the blank group and A-NBs group gradually decreased,but the DO concentration in the A-NBs group was always higher than that in the blank group during the experimental period,indicating that A-NBs with a certain particle size could exist stably for a long time and contribute to the formation of the passivation film.
The A-NBs group met the conditions required for the formation of a passivation film.Firstly,when DO concentration in the simulated circulating cooling water rose to a critical point,it promoted the formation of the passivation film on the surface of brass samples [47].Secondly,with the rise in temperature,A-NBs were ruptured and produced highly oxidizing ∙OH,which also promoted the formation of a passivation film on brass surface [48,49].

Fig.5. Changes of dissolved oxygen concentration in blank group and bubble group at different temperatures:(a)DO concentration at 1 d of operation,(b)DO concentration at 2 d of operation,and (c) DO concentration at 3 d of operation.

Fig.6. EIS analysis on the surface of corroded brass samples in blank group and bubble group at different temperatures:(a)Nyquist plots,(b)Bode impedance plots,and(c)Bode phase plots.
In order to investigate whether A-NBs promoted the formation of passivation film on the surface of brass samples,EIS tests were conducted with the corrosion samples of blank and A-NBs groups at different temperatures (Fig.6).The equivalent circuit diagram for fitting Nyquist impedance spectrum is shown in Fig.7 and the parameters are shown in Table 5.In the Nyquist impedance spectrum,the semicircle represents the capacitance loops.A sample with the better corrosion resistance usually shows a larger capacitive loop in the Nyquist plots.At the same temperature,the capacitance loop of the corrosion samples of A-NBs group was much larger than that of the corrosion samples of blank group(Fig.6(a)).As the temperature rose,the capacitance loops of the corrosion samples of A-NBs group and blank group decreased continuously.Bode impedance plots (Fig.6(b)) directly showed that the absolute impedance value |Z| of the corrosion samples of ANBs group was significantly larger than that of the corrosion samples of blank group.With the increase in temperature,the absolute impedance value |Z| of the corrosion samples of A-NBs group and blank group decreased.The corrosion samples of A-NBs group had more stable and larger maximum phase angle in a wider frequency range than the corrosion samples of blank group (Fig.6(c)).The above EIS results confirmed that a passivation film was formed on the brass sample surface in A-NBs group.With the increase in temperature,the density of A-NBs decreased and the ability of A-NBs to promote the formation of passivation film on the sample surface declined,thus decreasing the inhibition effect of A-NBs in simulated circulating cooling water.
In order to prove the physical existence of the passivation film,with EDS,the element analysis was conducted on the surface of original sample and corrosion sample before and after cleaning(Fig.8).Surface elements of the original brass sample are mainly Cu and less C and O(Fig.8(a)).C,O,and Cu elements are more concentrated on the surface of the corrosion sample in blank group(Fig.8(b))mainly due to the formation of passivation film and calcium carbonate scale.Surface elements of the corrosion sample in A-NBs group were mainly C,O and Ca,and also included less Cu(Fig.8(c)),indicating that the surface of corrosion samples was mainly covered by calcium carbonate scale and few corrosion products.The elemental composition of the corrosion samples of blank group and A-NBs group after cleaning surface corrosion products is shown Fig.8(d) and (e).The surface elements of the corrosion samples of two groups were mainly Cu and also included less C and O,but the content of C and O on the surface of corrosion samples was higher than that of original sample due to the residual passivation film.Au element detected on the surface of the test sample was mainly caused by the step gold spraying in the test stage.

Fig.7. Equivalent circuit diagram for fitting Nyquist impedance spectrum (Rs:solution resistance; Rp: polarization resistance;CPE: constant phase element).

Table5 EIS parameters of blank group and A-NBs group at different temperatures

Fig.8. EDS analysis of the surface of different corrosion samples: (a) original brass sample,(b) corrosion sample of blank group,(c) corrosion sample of bubble group,(d)corrosion sample of blank group after cleaning,and (e) corrosion sample of bubble group after cleaning.
After the calcium carbonate scale film was removed,XPS test was performed on the corrosion sample in order to determine the chemical composition of the passivation film (Fig.9).The two peaks near 932.4 eV and 952.3 eV in XPS spectrum of the blank group brass sample (Fig.9(b)) respectively corresponded to Cu2O[50] and Cu [51],indicating that a layer of Cu2O film was formed on the sample surface of blank group.In XPS spectrum of the brass sample of A-NBs group (Fig.9(d)),the two peaks near 932.4 and 952.3 eV respectively corresponded to Cu2O [50] and Cu [51] and the two peaks near 934.6 eV and 954.4 eV corresponded to Cu2(-OH)2CO3[51],indicating that two layers of passivation films were formed on the sample surface of A-NBs group: the inner layer of Cu2O and the outer layer of Cu2(OH)2CO3.The two layers might be caused by the high oxygen mass transfer efficiency of A-NBs and ∙OH generated by cavitation.The reactions for the formation of the passivation film are provided as follows:
The pH of the solution is also an important influencing factor of metal corrosion.After the introduction of A-NBs,the pH value of simulated circulating cooling water rose significantly (Fig.10).The cause for the pH rise is discussed below.A part ofin simulated circulating cooling water is hydrolyzed to generate H2CO3and OH-and H2CO3is decomposed into CO2and H2O when heated.As A-NBs rise to the gas–liquid interface,CO2generated in the solution is carried by A-NBs and rises,thus promoting hydrolysis ofand increasing pH.The rise in pH generates an environment for the stable formation of the passivation film.The environment may be interpreted in two aspects.First,the metal corrosion at low pH is mainly hydrogen depolarization corrosion and the lower pH leads to the more severe corrosion.When the pH value is high,the corrosion is mainly oxygen depolarization corrosion.When the pH value increases,the corrosion rate gradually slows down [52].Second,the passivation film on the surface of brass sample is mainly Cu2(OH)2CO3and Cu2O,which can only exist stably under alkaline conditions [53].Therefore,A-NBs not only promoted the formation of the passivation film,but also contributed to the stability of the passivation film.The reactions are provided as follows:

Fig.9. XPS spectra of the surfaces of different corrosion samples: (a) XPS spectrum of blank group,(b) high resolution Cu 2p spectrum of blank group,(c) XPS spectrum of bubble group,and (d) high resolution Cu 2p spectrum of bubble group.

Fig.10. pH change of blank group and bubble group at different temperatures.
According to the above analysis of film formation in conventional simulated circulating cooling water,its pH was generally greater than 8.When the pH was lower than 8,after bubble injection,the pH affected the particle size and zeta potential of A-NBs.In deionized water and 1000 mg∙L-1CaCl2,the particle size of ANBs became larger with the decrease in pH and the particle size distribution was more dispersed (Fig.11).The electronegativity of the zeta potential of A-NBs in deionized water and 1000 mg∙L-1CaCl2decreased as the pH decreased and the decreasing trend in deionized water was more obvious (Fig.11(c)).The larger particle size of A-NBs and the lower electronegativity of zeta potential indicated that the less stable A-NBs.Therefore,as the pH decreased,the stability of A-NBs decreased and the corrosion inhibition performance was decreased.
3.3.3.Formation of the calcium carbonate scale film

Fig.11. Effects of pH on physical and chemical properties of A-NBs: (a) bubble size in 1000 mg∙L-1 CaCl2 solution,(b) bubble size in deionized water,and (c) bubble zeta potential of 1000 mg∙L-1 CaCl2 solution and deionized water.

Fig.12. SEM images of the surfaces of different corrosion samples: (a) original brass sample,(b) corrosion sample of blank group,(c) corrosion sample of blank group after cleaning,(d) corrosion sample of bubble group,and (e) corrosion sample of bubble group after cleaning.
SEM images of the surface morphology of the original sample and the corrosion sample before and after cleaning are shown in Fig.12.The surface of the original sample was smooth without corrosion product and calcium carbonate scale adhesion (Fig.12(a)).Obvious corrosion pits could be observed on the surface of the corrosion sample of blank group before cleaning and a small quantity of calcium carbonate and corrosion products were adhered to the surface (Fig.12(b)).Dense and small sponge-shaped pits could be observed on the surface of the corrosion sample of blank group after cleaning(Fig.12(c)),indicating that the serious pitting corrosion occurred on brass sample in simulated circulating cooling water because the sample was exposed to circulating cooling water with high concentrations of Cl-and SO42-without any protection.The surface of the corrosion sample of A-NBs group was covered with a dense calcium carbonate scale film(Fig.12(d)),as confirmed by the EDS results in Section 3.3.2.The part of the brass sample beneath the scale film had no obvious corrosion,and no corrosion product was observed on the surface.After the scale film was cleaned,the surface of the brass sample was smooth without obvious pitting(Fig.12(e)),indicating that the calcium carbonate scale film might play a positive role in corrosion inhibition.
Calcium carbonate scale film plays multiple roles in inhibiting corrosion [54,55].The scale film blocks corrosive ions and improves surface hydrophobicity.In the anti-corrosion coatings of aluminum alloy[56],magnesium alloy[57,58],and carbon steel[59],the dense calcium carbonate scale film could effectively prevent the metal surface from contacting with the corrosion medium and calcium carbonate showed the calcite structure.In this study,the scale film(Fig.12(d))generated on the surface of the corrosion sample of A-NBs group could also effectively prevent the contact between Cu and corrosive ions such as Cl-and SO42-,thus weakening pitting corrosion on the surface of the sample.Based on the EDS element analysis(Fig.8(c)and(e))and SEM images of sample surface,it could be determined that the scale film was the calcium carbonate scale film with the aragonite structure.In general,the saturation index of calcite is higher than that of aragonite and its nucleation speed is faster.Calcite particles were firstly formed on the surface,but the introduction of A-NBs led to lattice distortion of calcium carbonate,which was conducive to the formation of aragonite.Therefore,a dense aragonite scale film was formed on the surface of brass sample.
The surface contact angles of the corrosion samples of blank group and A-NBs group were respectively 96.32° and 134.07°(Fig.13),indicating that the calcium carbonate scale film formed on the surface of the A-NBs group corrosion sample could enhance its surface hydrophobicity,reduce the contact area between Cu and corrosive ions such as Cl-and SO42-,and thus slow down the surface corrosion of brass sample.
3.3.4.Formation of the bubble layer

Fig.13. Surface contact angle of brass samples in blank group and bubble group: (a) blank group and (b) bubble group.
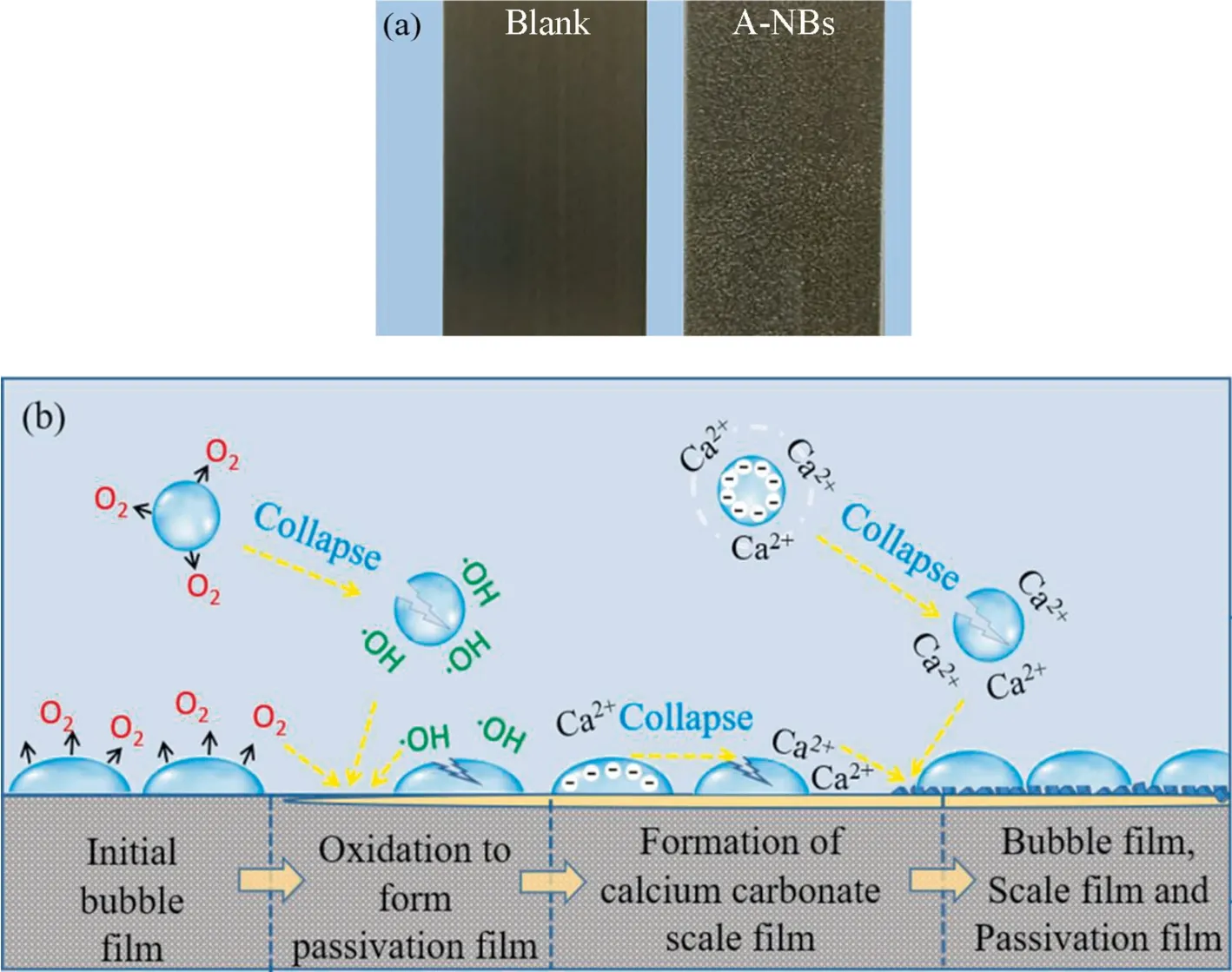
Fig.14. Bubble layer formed on the surface of brass sample by A-NBs: (a) diagram of the bubble layer and (b) action diagram of the bubble layer.
A-NBs could form a dense bubble layer on the surface of brass sample(Fig.14).In the study,the bubble layer was formed quickly at the initial stage of the contact between A-NBs and metal surface(Fig.14(a)) and the formed bubble layer had many roles (Fig.14(b)).Firstly,it could effectively reduce the direct contact area between the sample surface and corrosive ions (such as Cl-and) and alleviate pitting corrosion on brass surface.Secondly,it could increase the DO concentration near the sample surface and the ∙OH generated by ruptured bubbles could directly act on the metal surface so as to promote the formation of the passivation film on the brass surface.Third,the bubbles collapsed and led to the increased Ca2+concentration near the sample,which promoted the rapid formation of the calcium carbonate scale film.Fourthly,the outer layer of the calcium scale film adsorbed bubbles and the bubble layer reduced the friction between the solid phase surface and the fluid on solid phase boundary layer,thus leading to boundary slippage [60,61].In this way,A-NBs effectively slowed down the erosion of simulated circulating cooling water on brass surface and inhibited the thickening of the calcium carbonate scale film.In addition,A-NBs in water adsorbed scale cations and reduced scale precipitation.The rupture of A-NBs produced turbulence and reduced the adhesion of the scale to the inner surface of the system,thus inhibiting the thickening of the calcium carbonate scale film in the circulating cooling water system.
In the formation process of the bubble layer,A-NBs did not affect the working conditions such as temperature and flow rate of the circulating cooling water system,but they increased the pH of the solution and reduced the concentration of calcium ions.The increase of pH was conducive to the stable existence of the passivation film and the decrease of calcium ion was conducive to the inhibition effect on the thickening of the calcium carbonate scale film.In a word,A-NBs had the good corrosion inhibition effect,excellent scale inhibition effect,and certain sterilization effect.The addition of A-NBs could significantly reduce the required quantities of scale inhibitors,corrosion inhibitors,and fungicides,and even replace these agents and significantly cut off the operating cost.Therefore,the A-NB technology is green and cost-effective.
4.Conclusions
As a typical emerging pollution-free technology,A-NBs have great corrosion inhibition potential.In this study,with simulated circulating cooling water as the experimental medium and the brass sample as the corrosion object,the inhibition effect of ANBs on brass corrosion at different temperatures was investigated and the corrosion inhibition mechanism was clarified.A-NBs showed the significant inhibition effect on the corrosion of brass sample in simulated circulating cooling water and the maximum inhibition efficiency reached 52% at 35 °C.However,with the rise in temperature,the stability of A-NBs decreased and the inhibition rate of brass corrosion decreased.Furthermore,the corrosion inhibition mechanism could be analyzed from the perspective of the existence space of A-NBs.At the beginning,A-NBs entering simulated circulating cooling water were evenly distributed and a bubble layer was immediately formed on the surface of brass sample.The bubble layer not only effectively reduced the contact area between corrosive ions and metal surface,but also increased the concentration of dissolved oxygen in the solid-phase boundary layer.Oxygen and ∙OH produced by bubble collapse directly reacted with Cu,thus prompting the formation of a passivation film on brass surface.The bubble collapse of the bubble layer increased Ca2+concentration in the boundary layer,so the calcium carbonate scale film was formed immediately to cover the passivation film.The calcium carbonate scale film had good hydrophobicity.Therefore,the passivation film and the scale film generated the double-layer protection on metal surface.The bubble layer attached to the outer layer of the scale film was helpful to inhibit corrosion and scale film thickening.In the main solution,A-NBs could adsorb cations and anions,effectively reduce the concentration of corrosive ions in the solution,and promote the solution to maintain a high pH value,thus creating the conditions for the stable existence of passivation film and scale film.Therefore,A-NBs has great potential in corrosion inhibition and the process optimization will be explored in the future.The study lays the basis for the application of A-NBs in the circulating cooling water system.
CRediT Authorship Contribution Statement
Yuling Zhang:Project administration,Experiment design,Writing– review &editing.Shaolei Lu:Corrosion inhibition experiment,Writing– original draft,Writing– review &editing.Delie Li:A-NBs characterization.Haiyang Duan:Samples characterization.Congwen Duan:Samples analysing.Jinghong Zhang:Application experiment.Songtao Liu:Resources,Validation.
Data Availability
The data that has been used is confidential.The raw/processed data required to produce these findings cannot be shared at this time as the data also forms part of an ongoing study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was supported by National Natural Science Foundation of China(52170074).The authors acknowledge Zeng Leyong’s Lab of Hebei University for their help.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
