Preparation and evaluation of controllable drug delivery system: A light responsive nanosphere based on β-cyclodextrin/mesoporous silica
Yi Wu ,Hongzhou Shang,Shisheng Lai ,Yali Di ,Xiaoran Sun ,Ning Qiao ,Lihua Han,Zheng Zhao,Yujin Lu
1 College of Chemical Engineering, North China University of Science and Technology, Tangshan 063210, China
2 College of Materials Science and Engineering, North China University of Science and Technology, Tangshan 063210, China
3 College of Pharmacy, North China University of Science and Technology, Tangshan 063210, China
4 Department of Cardiology, Tangshan Central Hospital, Tangshan 063000, China
Keywords:Silica Drug delivery system Light-responsive β-CD Nanomaterials Support
ABSTRACT A novel light responsive nanosphere was constructed,and it was used as a drug carrier to investigate the loading and release properties of the Quercetin (QU).In this paper,mesoporous silica nanoparticles(MSN)were used as a substrate,and 3-aminopropyl triethyoxysilane was used as a surface modification agent to introduce —NH2,and the azobenzene-4,4′-dicarboxylic acid (AZO) was used as light responsive agent to introduce the group of —N=N—,and then β-cyclodextrin (β-CD) was combined with AZO through host–guest interaction to construct light responsive nanoparticles (MSN@β-CD).The structure and properties of the carrier were analyzed by FTIR,BET,XPS,TGA,XRD,SEM and TEM. In vitro drug release studies showed the release rate of QU@MSN@β-CD(dark)was 12.19%within 72 h,but the release rate of QU@MSN@β-CD(light 10 min)was 26.09%,exhibiting a light-responsive property.The CCK8 tests demonstrated that MSN@β-CD could significantly decrease the toxicity of QU.Therefore,the controllable light-responsive drug delivery system has great application prospects.
1.Introduction
Stimulus-responsive drug delivery system(SDS)is composed of stimulus-responsive material and drugs.It can realize regular and targeted delivery according to the changes of the stimulus source.Based on the source of the stimulus,SDS can be divided into two categories: endogenous stimuli (such as pH-responsive [1–4],temperature-responsive [5–8],reduction-responsive [9–11],enzyme-responsive [12–15]) and exogenous stimuli (such as light-responsive [16–18] and ultrasound-responsive [19,20]).
Among exogenous stimulation sources,light sources have the advantages of being clean,non-invasive and efficient,and can achieve precise control in time and space,which has become a hot spot for research on SDS.365 nm UV light has the advantages of fast response,high sensitivity and non-toxic in short time irradiation,which has been widely studied[21–23].Azobenzene(AZO)is a photo isomeric compound of with—N=N—,the trans structure of the molecule changes to a cis structure,accompanied by changes of physicochemical properties such as the dipole moment.For example,azobenzene is often present in a trans conformation and less hydrophilic;converted into a more hydrophilic cis conformation under 365 nm light [24,25].β-cyclodextrin (β-CD) is usually used as a pharmaceutical excipient,so it has good biocompatibility [26,27].In addition,β-CD has a special cavity structure,under the shielding of the C—H bond,the inner cavity appears hydrophobic,while the presence of the external hydroxyl group makes the inner cavity externally relatively hydrophilic,with hydrophobic inner cavity and hydrophilic outer cavity,which enables it to form inclusion compounds with AZO compounds through host–guest interaction forces.Thus,it has become one of the most important research objects in the field of supramolecular chemical light control in recent years [28–30].
Mesoporous silica nanoparticles (MSN) have the advantages of large specific surface area,easy surface modification,good biocompatibility and stability.But its was application limited by its own defects such as poor dispersion and responsiveness.Therefore,the development and application of functional MSN has become a main research hots pot of stimulus response materials[1,31–34].Herein,a light-responsive drug delivery system(MSN@β-CD) was prepared.Among,AZO was covalently modified on the surface of MSN,and then β-CD was combined with AZO through host–guest interaction to construct light responsive nanoparticles (MSN@β-CD).QU was used to investigate thein vitrodrug release properties.The biosafety of the carrier was detected by hemolysis and CCK8 test.Preparation and evaluation of the controllable drug delivery system of MSN@β-CD provide an effective reference for exogenous stimulus drug release.The specific process is shown in Fig.1.
2.Materials and Methods
2.1.Materials
The following materials were obtained from commercial suppliers and used as received.Cetyltrimethylammonium bromide(CTAB),tetraethyl orthosilicate (TEOS),azobenzene-4,4′-dicar boxylic acid(AZO),(3-aminopropyl)triethoxysilane(APTES),Quercetin (QU),1-ethyl-3-3-dimethylaminopropyl carbodiimide hydrochloride (EDC-HCl),1-hydroxy-5-pyrrolidinedione (NHS)and 4-dimethylaminopyridine (DMAP) were purchased from Shanghai Macklin Biochemical Co,Ltd.(Shanghai,China).Sodium hydroxide(NaOH)and ethanol were obtained from Tianjin Yongda Chemical Reagent Co.,Ltd.(Tianjin,China).Cell counting kit-8(CCK8) was purchased from Beijing Zoman Biotechnology Co.,Ltd.(Beijing,China).
2.2.Characterization
Fourier transform infrared (FTIR) spectra of the samples were recorded between 4000–500 cm-1using Vertex80/70v FTIR spectrophotometer.Thermo-gravimetric analysis (TGA) was carried out with a STA449F3 thermogravimetric analyzer from 25 °C to 800 ℃with a heating rate of 10 °C∙min-1under N2atmosphere.The surface morphology was studied by using S4800 field emission scanning electron microscope (SEM).JEM-2100F transmission microscopy(TEM)was used to observe the internal morphology and structure of the samples.Small angle XRD pattern was obtained between 0–10 °C using Bruker D8 Advance XRD diffractometer.X-ray photoelectron spectroscopy (XPS) was employed with a 250xi XPS analyzer to determine the chemical elemental composition of nanoparticles and the combined state of element C and element N.Specific surface area,pore size and pore volume of the materials were analyzed by the Tristar II 3020 specific surface area analyzers.The absorbance values of the samples at 250–550 nm were determined by using a UV-1900i spectrophotometer.The photo-responsive test was demonstrated by ZF-1 Ultraviolet analyzer.Themo Fisher Scientific CO2incubator was used to grow cells.The cytotoxicity results were studied by Flash E96 enzyme immunoassay analyzer.
2.3.Synthesis of the nanoparticles
2.3.1.Synthesis of MSN
1.0 g CTAB,3.5 ml 2 mol∙L–1NaOH solution and 480 ml deionized water were added to a three-neck flask and stirred magnetically at 80 °C for 30 min.And then 5.0 ml TEOS were added slowly dropwise to the above mixture solution,continuing reaction for 3 h.After the solution was cooled to room temperature,the product was collected by centrifugation (7300 r∙min-1,10 min),and washed 3 times with deionized water and anhydrous ethanol alternately.Finally,the product of CTAB@MSN was obtained by vacuum drying for 12 h.
To maintain the orderliness of the mesoporous structure,the acid extraction method was used to remove the template agent CTAB.1.0 g CTAB@MSN was placed in a mixture of hydrochloric acid–methanol (VHCL:VM=1:100) and refluxed at 60 °C for 12 h.And then,CTAB@MSN was separated,washed and dried once again.
2.3.2.Synthesis of MSN-NH2
0.5 g MSN and 50 ml toluene were added into the flask and sonicated to make it well dispersed and then refluxed at 80°C for 12 h after adding 5 ml APTES.Finally,the product of MSNs-NH2was separated,washed and dried.
2.3.3.Synthesis of MSN-AZO
Firstly,0.3 g 4,4′-azobenzenedicarboxylic acid (AZO) was dissolved in 30 ml dimethylformamide (DMF).Then 0.5 g 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride(EDC-HCl),0.3 gN-hydroxy succinimide (NHS) and catalytic amount of DMAP were added one by one with a magnetic stirrer at room temperature for 12 h under N2protection to activate carboxyl groups.Finally,0.1 g MSNs-NH2was added to the above solution and the reaction was continued for 12 h.The product was collected by centrifugation(12500 r∙min-1,10 min),washed several times with deionized water and methanol,and dried under vacuum to obtain the light yellow powder MSN-AZO.
2.3.4.Synthesis of MSN@β-CD
25 mg MSN-AZO and 23 mg β-CD were dispersed in 10 ml anhydrous ethanol solution and reacted at 25°C for 12 h.The product of MSNs@β-CD was collected by centrifugal separation,washing twice with deionized water and methanol,and vacuum drying at 50°C.
2.4.Test of drug absorption performance
2.4.1.Standard curve of QU
The stock solution of QU of 1.01 mg∙ml-1concentration was prepared.From this,different concentration solutions (1.01,2.02,3.03,4.04,5.05,6.06,7.07,8.08,9.09 and 10.1 μg∙ml-1) were prepared using anhydrous ethanol.The standard solution was measured at 374 nm.Finally,the standard equation was established by the least square method.
whereAis absorbance of the solution,Cis of concentration of QU in solution,and correlation coefficient is 0.9992.
2.4.2.Test of drug adsorption
One-pot method was used as for QU adsorption and the encapsulation of β-CD.The relationship between different concentrations of quercetin solution (QU) and the adsorption performance of MSN@β-CD was investigated.25 mg MSN-AZO were dispersed in 10 ml QU solution with different concentration separately,and stirred magnetically at 40°C for 12 h.Then 23 mg β-CD was added and the reaction was continued to carry on for 12 h.At the end of the reaction,the drug concentration in the supernatant was measured by UV–vis spectrophotometer,and the equilibrium adsorption capacity (qe) was calculated.
whereC0is the initial concentration of QU,mg∙L–1;Ceis the residual concentration of QU,mg∙L–1;Vis the volume of solution,L;mis the mass of MSN@β-CD,g.
2.5.In vitro drug release experiments
2.5.1.Sustained release performance of QU@MSN@β-CD
5 mg of QU@MSN,QU@MSN-AZO and QU@MSN@β-CD were dispersed in 2 ml phosphate buffer solution (PBS,pH 7.4) and encapsulated into dialysis bags (MWCO 8000–14000).Then the dialysis bags were placed in a centrifuge tube containing 35 ml of PBS(pH 7.4)solution.The above centrifuge tubes were magnetic stirrer at dark.Another copy of QU@MSN@β-CD was placed under UV light for 10 min and then placed in a dark place with continued magnetic stirring.3 ml the release medium was removed separately at the indicated times and the QU content was measured by UV–Vis spectrophotometer,while 3 ml fresh release medium was added.The cumulative release percentage (CRP) of each sample was calculated.
Among,Veis the volume of PBS removed;V0is the total volume of PBS;Ciis the QU concentration of thei-th solution taken out,mg∙L–1;MQUis the carrier contained the total mass of QU,mg;nis the number of times the liquid removed.
2.5.2.Light controlled drug release performance of QU@MSN@β-CD
To further verify the light response performance of the carrier,the drug release performance of QU@MSN@β-CD was investigated under different light exposure times.4 parts of 5 mg QU@MSNs@β-CD were dispersed in 2 ml PBS (pH=7.4) and encapsulated into dialysis bags (MWCO 8000–14000).Then the above dialysis bags were placed in beakers with 35 ml of PBS (pH 7.4).After a 15-watt,365 nm UV lamp exposure (15 min,30 min,60 min,and 120 min),the absorbance was measured using a UV spectrophotometer.
2.6.Hemolysis test
3 ml margin venous blood of rabbit ear was added into the anticoagulation tube and the supernatant was removed by centrifuge(3000 r∙min-1,10 min).To obtain the deposited red blood cells,normal saline(NS)was used to wash solution until the supernatant was clarified.And then 2% erythrocyte suspension in NS was prepared.
1.25 ml erythrocyte suspension was mixed with different concentrations of carrier solution(0.2–1.0 mg∙ml-1),saline and deionized water in equal amounts,where the saline group was used as a negative control and deionized water was used as a positive control.All samples were incubated in 37 °C constant temperature water bath for 1 h,and then centrifuged (3000 r∙min-1,10 min).to calculate the hemolysis rate (HR),the maximum absorbance of the positive control group hemoglobin was determined by UV–Vis spectrophotometer,and the absorbances of the negative control group and the experimental group were measured at the above same detection wavelength the hemolysis rate.
2.7.Cytotoxicity test
In this experiment,the cell viability of breast cancer cells(MDAMB-231) was examined using the CCK8 method to evaluate the biosafety of MSN@β-CD.
Firstly,breast cancer cells in the exponential growth period were inoculated into 96-well plates at a density of 5000 per well and incubated at 37°C and 5%CO2for 24 h.Then the same concentration gradient of free QU,MSN@β-CD and QU@MSN@β-CD were added as the experimental group,while the pure culture medium was used as the blank group and the culture medium containing cells was used as the control group,with 5 replicate wells for each sample,continue incubation for 24 h.Finally,10 μl CCK8 solution was added to each well,and the incubation was continued for 4 h.The absorbance at 450 nm was measured using an enzyme marker.
3.Results and Discussion
3.1.Sample characterization
The FTIR spectra of MSN,MSN-NH2,MSN-AZO and MSN@β-CD were shown in Fig.2(a).In the curve of MSN,the peaks at 1076 cm-1,806 cm-1were attributed to the Si—O—Si asymmetric stretching vibration and the stretching vibration,respectively.In addition,the peak at 973 cm-1was the bending vibration peak of Si—O.In the curve of MSN-NH2,the peaks of 2933 cm-1,1560 cm-1and 1334 cm-1were considered as the stretching vibration peak of—CH2,the bending vibration of—NH2and the stretching vibration peak of—C—N,respectively.In the curve of MSN-AZO,the peak at 1471 cm-1may be the stretching vibration peak of the—N=N— and the benzene ring backbone.Besides,the absorption peak at 1635 cm-1was enhanced,probably due to the superposition of—C=The horizontal coordinate is changed to O in the amide group.According to the ‘‘inclusion attenuated infrared absorption effect”,the change in absorption intensity was the basis for the analysis of inclusion compounds by infrared spectroscopy [35].In the curve of MSN@β-CD,the peak at 1301 cm-1was the characteristic absorption peak of β-CD.In addition,compared to MSN-AZO,all absorption peaks in MSN@β-CD decreased and even some absorption peaks disappeared,suggesting the successful preparation of MSN@β-CD.
To further confirm the successful modification of the APTES,the surface elemental composition of MSN and MSN-NH2was analyzed by XPS,and the results were shown in Fig.2(b).In the curve of MSN,three elements C,O and Si were detected,where the presence of element C could be the residual template CTAB.However,four elements C,N,O and Si were found in the spectrum of MSN-NH2,where the detection of the N element and the decrease of the peak intensity of the Si element implied the successful grafting of APTES on the surface of MSN.
In order to verify the successful grafting of AZO on the surface of MSN,the UV spectra of AZO,the physical mixture of MSN and AZO and the covalent binding of MSN and AZO in the range of 250–550 nm were measured by UV spectrophotometer.It can be found from Fig.2(c),comparing with AZO and MSN+AZO,the peak position of N=N in MSN-AZO occurred a slight red shift.This may be the reason that O=C—Ar—N=N— was a large conjugate system,and the introduction of—NH2increases the electron cloud density of the conjugate system and reduces the energy required for the Kband jump of —N=N—,which indicates the successful modification of AZO.
The morphology and particle sizes of the MSN and MSN@β-CD were characterized with SEM and TEM.As can be seen from Fig.3(a) and 3(e),the MSN was spherical in appearance with an average particle size of (77 ± 9) nm.In Fig.3(c),the mesopore was clearly visible and well aligned,which proved the successful preparation of MSN.It can be seen from Fig.3(b) and 3(f),the appearance of MSN@β-CD was spherical,and the average particle size increased to (89 ± 11.5) nm.In addition,a clear shell layer could be seen in Fig.3(d),and the mesoporous became blurred,which suggesting that β-CD was successfully modified on the surface of MSN.

Fig.2. (a) FTIR spectra of MSN,MSN-NH2,MSN-AZO and MSN@β-CD.(b)XPS survey spectra of MSN and MSN-NH2.(c) UV spectra of MSN,AZO,MSN-AZO and MSN-AZO.
The specific surface area,pore size,and pore volume of MSN and MSN@β-CD were analyzed by BET analyzer,and the results were shown in Fig.4(a)and 4(b)and Table 1.It could be seen from Fig.4(a),the N2adsorption/desorption curves of MSN showed a hysteresis loop belonging to Langmuir IV isotherm in the relative high-pressure region [36].Meanwhile,combined with Fig.4(b),it can be seen that the MSN pore size was concentrated around 2.8 nm,indicating that the prepared MSN material was mesoporous.According to Table 1,the specific surface area,pore size and pore volume of MSN were 1002.35 m2∙g-1,2.86 nm and 1.34 cm3∙g-1,respectively.Compared with MSN,the specific surface area,pore size and pore volume of MSN@β-CD were decreased to 35.69 m2∙g-1,1.26 nm and 0.33 cm3∙g-1,respectively,which indicated that the β-CD was successfully modified on the surface of MSN.
TGA was used to examine the grafting amount of the organics,and the results were shown in Fig.4(c).It can be seen from Fig.4(c),the mass loss of MSN of at 25–150 °C was attributed to the volatilization of physically adsorbed water and residual solvent in the sample.The mass loss under 150–800 °C might be the degradation of stencil agent CTAB and dehydration condensation reaction between silicon hydroxyl groups.In the curve of MSNNH2,the total mass loss was about 17.6%.Compared with MSN,the mass loss increased by 7.8%,which was due to the decomposition of APTES modified on the surface of the MSN.The total mass loss of MSN-AZO at 25–800 °C was 24.7%,and the total mass loss rate at 300–500°C was due to the decomposition of AZO modified on the surface of the MSN,indicating that the grafting amount of AZO was 7.1%.The total mass loss of MSN@β-CD was further reduced,and the final residual mass was about 69.8%,indicating that β-CD grafting amount was 5.5%.
XRD was used to analyze the crystallographic structure of the samples and the results were shown in Fig.4(d).As can be seen in Fig.4(d),The MSN curve shows a clear diffraction peak appears near 2.14 °,implying the pores of the particles were regular mesoporous structures.Compared with MSN,the diffraction peak intensity and peak position of MSN-NH2,MSN-AZO and MSN@β-CD decreased and shifted to a certain extent,which may be due to the entry of the compounds into the pore channel,reducing the order of the pore structure and corresponding pore size.

Fig.4. N2 adsorption/desorption curves (a) and pore size distribution (b) of MSN and MSN@β-CD.The TGA curves of MSN,MSN-NH2,MSN-AZO and MSN@β-CD (c).XRD patterns of MSN,MSN-NH2,MSN-AZO and MSN@β-CD (d).Adsorption thermodynamic curve of MSN@β-CD (e).

Table1 The pore structure parameters of MSN and MSN@β-CD
3.2.Absorption of MSN@β-CD
As can be seen from Fig.4(e),the adsorption of MSN@β-CD on QU gradually increased with the increase of solution concentration in the concentration range of 200–1200 mg∙L–1.When the concentration was 1000 mg∙L–1,the adsorption capacity remained basically the same and reached 188 mg∙g-1.

Fig.5. (a) Release curves of QU@MSN,QU@MSN-NH2,QU@MSN-AZO,QU@MSN@β-CD.(b) Release curves of QU @MSN@β-CD at different light time.
3.3.In vitro drug release experiments
3.3.1.Release performance between different drug delivery systems
In order to investigate the slow-release performance of QU@MSN@β-CD,the release performance of QU@MSN,QU@MSNAZO and QU@MSN@β-CD were investigated.As could be seen from Fig.5(a),the cumulative release of QU@MSN,QU@MSN-AZO and QU@MSN@β-CD(UV10 min)were 45.63%,36.62%and 26.09%after 72 h,respectively.It is suggested that with the increase of the modified groups,the slow-release performance of the carrier can be improved.In addition,by examining the cumulative release rate of QU@MSN@β-CD illuminated and unilluminated groups,we found that the release rate of QU@MSN@β-CD (unilluminated)was 12.19%,which is much lower than the 26.09% of QU@MSN@β-CD (UV356 nm,10 min),indicating that the carrier has a certain light-controlled drug release capacity.However,the total release of QU@MSN@β-CD (UV10 min) was lower,which might be the photo dislodged β-CD was recombined with the trans-azo bond in the PBS solution,and the QU was blocked by the β-CD in the pore,which resulted in a lower release.Meanwhile this phenomenon implied that QU@MSN@β-CD has the potential to be released on demand.
3.3.2.Drug release performance at different light time
To further demonstrate the light-controlled drug release performance of QU@MSN@β-CD,the absorbance values of the QU were recorded at different light time.From Fig.5(b),the absorbance of QU increased with the gradual increase of light exposure time,implying that the prepared QU@MSN@β-CD has a good light response performance.
3.4.Biosafety tests
3.4.1.Hemolysis test
The biosafety of MSN@β-CD was verified by anin vitrohemolysis assay,and the results were shown in Fig.6.It can be seen from Fig.6(a),the absorption peak at 576 nm was stronger,so the wavelength was chosen as the detection wavelength of hemoglobin.It can be seen in Fig.6(b),the hemolysis rate of MSN@β-CD was less than 5% at 0.2–1.0 mg∙ml-1,indicating that the carrier has favorable biocompatibility.
3.4.2.Cytotoxicity test
It can be seen from Fig.7(a),the cell viability was higher than 85% in the carrier concentration range of 2.5–20 μg∙ml-1,indicating favorable biocompatibility of MSN@β-CD.As can be seen from Fig.7(b),free QU has some killing power against breast cancer cells.However,the cell viability of QU@MSN@β-CD was higher than free QU at the same concentration,which demonstrates that QU was firmly encapsulated in MSN@β-CD even in the tumor complex environment,proving that the carrier has satisfactory stability.Meanwhile,the phenomenon of the cell viability of QU@MSN@β-CD was higher than free QU illustrates the ability of MSN@β-CD to reduce the toxic effects of drugs,which has a greater potential in the treatment of other diseases.
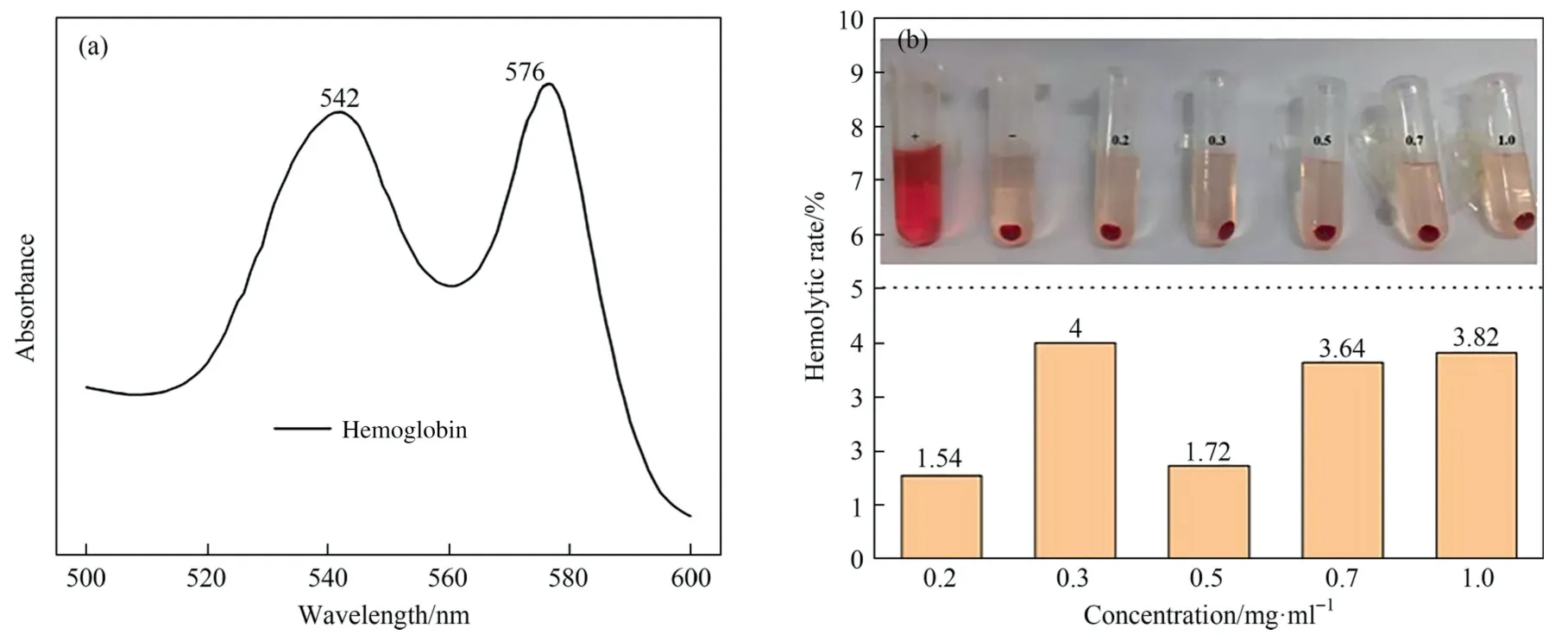
Fig.6. Absorption wavelength of hemoglobin (a) and in vitro hemolysis of MSN@β-CD (b).
4.Conclusions
In this paper,a novel light responsive drug carrier(MSN@β-CD)was successfully prepared,which was characterized by SEM,TEM,FTIR,TGA,UV,BET,XRD and XPS.QU was used as a mimetic drug to study the loading and release capacity of MSN@β-CD.In vitrodrug release showed that the QU@MSN@β-CD has a satisfactory slow release and light controlled drug release performance.The results of hemolysis experiments showed that the hemolysis rate of MSN@β-CD was less than 5% at 0.2–1.0 mg∙ml-1,indicating that the carrier has good biocompatibility.The cell viability was higher than 85%in the carrier concentration range of 2.5–20 μg∙ml-1,further confirming the good biosafety of MSN@β-CD.In addition,the cell viability of QU@MSN@β-CD was higher than the free QU,implying that MSN@β-CD has good stability and the ability of reducing the toxic effects of drugs.These data showed that MSN@β-CD had great potential as a drug delivery vehicle.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
