Vanadium(V)reduction by using a by-product of the yellow phosphorus industry
Baibin Yang,Shihong Chen,Haowen Ren,Yang Qiu,Chong Chen,Yong Guo,Chunhui Luo,Qiang Zhao,,Wei Yang
1 School of Chemical Engineering, Sichuan University, Chengdu 610065, China
2 State Key Laboratory of Hydraulics and Mountain River Engineering, College of Water Resource &Hydropower, Sichuan University, Chengdu 610065, China
Keywords:Vanadium Phosphorus-iron slag Thermodynamics Kinetics Reduction
ABSTRACT Release of vanadium(V)from industry has threatened the environment and human health.In this paper,a removal method of vanadium(V) is proposed using a by-product of the yellow phosphorus industry(phosphorus-iron) as a reducing agent.The thermodynamics analysis shows that the Gibbs free energy is always negative from 0 to 100 °C,indicating a spontaneous process.Effect of the phosphorus-iron slag/sulfuric acid dosage and temperature on the removal efficiency is comprehensively studied,and the kinetics parameters are calculated based on a quasi-first order reaction kinetics model.Results indicate that vanadium(V) can be entirely reduced by using phosphorus-iron slag,the frequency factor and apparent activation energy are 3.23 × 109 min-1 and 64.50 kJ∙mol-1 for vanadium(V) reduction.Based on above results,a lab-scale reactor is constructed and achieves a removal efficiency of~100%and a treatment capacity of 200 ml vanadium(V) solution (2 g∙L–1) within 3 h.This work demonstrates the feasibility of vanadium(V) reduction using phosphorus-iron slag as a reducing agent in applications.
1.Introduction
Vanadium is a transition metal element that commonly exists in the lithosphere with different oxidation states,concomitant with minerals,oil and coal [1,2].Naturally,chemical weathering of minerals can release vanadium into the aquifer,while human activities including the combustion of vanadium-containing fossil fuel,the discharge of mining wastewater,the utilization of vanadium-related hardening steel,pigments,catalysts,and pharmaceuticals are primarily anthropogenic sources [3–5].Vanadium(V) (V(V)) is the most common state among different oxidation states of vanadium.In mining and smelting areas,the content of V(V)is about 13%–35%and 81%–97%in contaminated soil and soil leachate,respectively [6].In fact,trace amounts of vanadium is crucial for the growth of biological cells,but excessive vanadium will lead to toxic effect [7].Generally,vanadium with a higher valence state can severely damage the living body [8].Previous study suggested that V(V) had a strong inhibition effect on the enzyme Na and K-ATPase,and the high toxicity of V(V) has been proved in mice in the past years [9].A high dosage of V(V) can damage human organs,including morphological and functional lesions [4,10,11].Differently,V(IV) exhibits a less toxicity and can be precipitated naturally under neutral condition,implying that the transformation from V(V) to V(IV) is a promising avenue to realize the alleviation of V(V)toxicity and consequently achieve the V(IV) removal through precipitation [12,13].
In the past decades,several strategies have been reported for V(V) removal,including physical and chemical adsorption [14,15],ion exchange [16,17],chemical precipitation [18,19],microbial reduction [20,21],electrochemical reduction [22,23],etc.Among them,adsorption,ion exchange and microbial reduction method will produce V containing solid waste (with a limited V content),from which the utilization of V source is not environmentally friendly and unfeasible.Chemical reduction and precipitation is one of the most promising avenues in water pollution control,due to the high removal/reaction rate and stable operation [24].The waste water with a high V(V) concentration (>1000 mg∙L–1)can also be treated using suitable chemical reducing agent and precipitate.Ferrous sulfate,iron filings,ammonium salt,sulfur gas and sulfite are the widely used reducing agents [25].For instance,Zhanget al.and Wanget al.[26,27] proposed a method for V(V)removal from mining ditch water (4.66–6.85 mg∙L–1) using ironbased materials(e.g.,ferric oxyhydroxide,ferric groundwater treatment residual)as a reducing agent.The V(V)in mining ditch water(initial vanadium concentration 6.85 mg∙L–1;pH 7.02–7.83) was reduced by reducing agent (17 g in packing columns).The proposed method realized a removal efficiency higher than 85%.Although with attractive features,the chemical precipitation still suffered from the high cost of treatment equipment and reagents.Within this context,it is of great significance to realize V(V)removal through a cost-effective and high-efficient method.
As a by-product of the yellow phosphorus industry,phosphorus-iron (Fe-P) mainly composes of FeP and Fe2P,which exhibited a morphology of irregular nanoparticles in the grey powder products.Both of FeP and Fe2P are inter-metallic compounds consisting of Fe and P,which has been used as Fe and P sources for the preparation of LiFePO4in our previous work [28,29].In China,about 140 thousand tons of Fe-P slag are produced in the yellow phosphorus industry.Although Fe-P slag can serve as Fe and P resources,its application was still hindered by the high reaction kinetic resistance of the transformation or oxidation reaction at room temperature.Attributing to a low chemical valence of constituent element,Fe-P exhibits a good reducibility and has a tremendous potential serving as a reducing agent for V(V) reduction.Moreover,the cost of Fe-P powder in 2000 mesh size is as low as~300 USD∙t-1[28,30].Therefore,it is promising to realize the reduction of V(V) by using Fe-P as a reducing agent in large scale applications.
In this paper,we reported a method for V(V) removal in wastewater using by-product of yellow phosphorus industry as a reducing agent.The reaction mechanism of V(V) reduction was first studied,and the thermodynamics processes were further calculated based on simulation.In addition,the reduction of V(V)was experimentally evaluated at different H2SO4concentrations,Fe-P slag dosages and temperatures.Finally,a procedure was proposed to demonstrate the feasibility of V(V) removal using Fe-P slag as a reducing agent in lab-scale reactor.
2.Materials and Methods
2.1.Chemicals
Fe-P slag powder was collected from the yellow phosphorous factory and treated by fluid energy grinding (2000 mesh,equates to 6.5 μm) to obtain the powder sample with an average particle size of 8.0 μm.Sulfuric acid (98%,H2SO4),sodium metavanadate(99.99%),ammonium ferrous sulfate hexahydrate (99.5%,(NH4)2-Fe(SO4)2∙6H2O),sodium carbonate (99.8%,Na2CO3) andN-phenyl anthranilic acid (AR) were from Kelong Chemical Group,China.Water was purified by an HK-UP-LH-20L water system (resistivity 18.3 MΩ∙cm) before use.
2.2.Reduction and measurements V(V)
V(V) reduction was conducted in a round bottom flask(1000 ml).Fe-P slag(4.7 g)and concentrated H2SO4(36.3 ml)were added into vanadium solution (2 g∙L–1,500 ml,calculated by the mass of vanadium element),and the obtained mixture slurry was stirred at 1200 r∙min-1.In the subsequent sensitivity analysis,the dosage of Fe-P slag and H2SO4increased in multiple,for instance,9.4 g Fe-P slag can be abbreviated as 2x Fe-P slag,72.6 ml H2SO4can be abbreviated as 2x H2SO4.After 2 h’s reaction,the mixture was centrifuged at 5000 r∙min-1for 5 min,and the clear liquid and precipitate were collected.KOH solution(1 mol∙L–1) was added to the clear liquid,and the pH value was adjusted to 9.After 2 h’s evaporation,the precipitation containing V(VI)was obtained from the above mixture solution.The obtained clear liquid and precipitate were used for further analysis.
V(V)concentration in the solution was measured based on titration method [31–33].In detail,the mixed acid solution was first prepared with 25%(vol) H2SO4and 5%(vol) H3PO4,and 20 ml of the mixed acid solution was added into a volumetric flask(150 ml).After that,the indicator solution of V(V) was prepared by adding 0.2 g ofn-phenylanthranilic acid into Na2CO3solution(2 g∙L–1).Subsequently,10 ml of the sample and 50 μl of V(V)indicator solution were added into 20 ml of the above mixed acid solution.Finally,a standard(NH4)2Fe(SO4)2solution(0.01 mol∙L–1)was added into the above solution for titration,along with the color change from purplish red to bright green.The V(V) concentration of the solution was determined by following equation [31,32,34]:
whereV[(NH4)2Fe(SO4)2] is the volume of standard (NH4)2Fe(SO4)2solution used for titration (m3).
The standard solution of(NH4)2Fe(SO4)2was prepared and used for calibration to mitigate the measurement errors.In detail,10 ml of (NH4)2Fe(SO4)2standard solution and 50 μl of V(V) indicator solution were added into 20 ml of the mixed acid solution(25%(vol) H2SO4and 5%(vol) H3PO4).Titration is carried out using the same method as described above.The (NH4)2Fe(SO4)2concentration is determined by following equation [31,32,34]:
whereVis the volume of (NH4)2Fe(SO4)2for titration (m3).The titration accuracy (1%) was ensured by obtaining a titration curve withR2=0.999 before the experimental test(Fig.S1 in Supplymentary Material).
2.3.Reactor construction for V(V) reduction
The reactor was constructed based on a double-jacketed glass reactor (20 mm inner diameter and 300 mm in length),in which Fe-P slag was added as the reducing agent for V(V) reduction.The reactor was designed as mentioned in our previous work[30].The experiment was performed at a constant temperature,which was maintained by pumping hot water into the reactor shell.During the experiment,V(V) solution slowly flowed through the Fe-P slag packing from top to bottom of the reactor.After the reaction,the filtrate solution was collected for further analysis.
The pH value was measured using a pH meter (PHS-3E),which was calibrated using a standard solution (pH 4 and pH 9.18).The elemental composition and valence states of precipitated solid samples were analyzed using a X-ray photoelectron spectroscopy(XPS) based on a Kratos Axis Ultra DLD (Kratos,England) with an Al Kα source.The vanadium and iron concentration in the liquid after the reaction was detected using ICP-OES.The V(V)concentration in the liquid was determined by titration,while V(IV)concentration in the liquid was calculated by the concentration difference between V(V)and V(IV).X-ray diffractometer equipped with Cu Kα radiation was used to acquire X-ray diffraction (XRD) patterns from 10° to 90° at 5 (°)∙min-1.
2.4.Thermodynamic analysis
The possible reactions in this system were analyzed,and the thermodynamic parameters were obtained based on the software of HSC chemistry 6.0.All input substances and products in the simulation are regarded as ideal chemicals,while assuming all species in this system are mixed and defined to be in the same phase.The Gibbs free energy change (ΔG0) and enthalpy change (ΔH0) of the reactions were calculated to provide a better understanding of thermodynamic properties [35,36].The main thermodynamic parameters of the species are summarized in Table S1.And the basic thermodynamic equations was expressed as Eqs.(S1–S3).
3.Results and Discussion
3.1.Chemistry of V(V) reduction using Fe-P
In this work,V(V) in solution reacted with Fe-P slag under an acid condition followed by alkalization and precipitation.The reduction of V(V)was confirmed by XPS analysis of the precipitate sample,including chemical components and valence states.XPS spectrum of samples was obtained before and after the reaction.As depicted in Fig.1(a),it can be found that V 2p of samples exhibited two peaks at around 524 eV and 517 eV,which can be ascribed to V 2p1/2and V 2p3/2,separately [37,38].For the initial sample without reaction,the V 2p spectrum showed a 2p3/2peak assigned to the valence state of V5+.However,the V 2p spectrum of filter cake yielded two subpeaks at 517.4 eV and 516.3 eV,which can be attributed to V5+and V4+,indicating the presence of V4+.The P 2p of the Fe-P was depicted in Fig.1(b),one can see that the spectra of P 2p exhibited three peaks identified at 133.5 eV,129.7 eV and 129.0 eV,which can be corresponded to P-O,P 2p1/2and P 2p3/2,respectively.After the reduction reaction,the P 2p1/2and P 2p3/2peaks diminished,implying the consumption of Fe-P [30,39].Furthermore,from the spectra of Fe 2p,it can be found that two pairs of peaks were identified at 728.5/715.0 eV and 714.9/711.5 eV,ascribing to Fe 2p1/2and Fe 2p3/2.Significantly,a peak of Fe-P was found at 707.0 eV,which disappeared after the reaction(Fig.1(c)).These results suggest that the V(V) was successfully transformed into V(IV) by oxidizing P and Fe in the Fe-P reducing agent.In addition,the obtained Fe-P slag was characterized by XRD.The XRD patterns of Fe-P slag were illustrated before and after the reduction of V(V) (Fig.1(d)).According to the XRD patterns,the Fe-P powder composed of orthorhombic FeP (JPCDS 78-1443) and hexagonal Fe2P (JPCDS 01-1200).It showed that the structure of Fe-P slag was maintained before and after the reaction,indicating that the oxidation products of Fe and P were not deposited or accumulated on the surface of Fe-P slag.
3.2.Thermodynamic analysis
As discussed above,the V(V)was reduced to V(IV) by oxidizing P and Fe in the Fe-P reducing agent.Based on the analysis of products,the reduction reaction between V(V) and Fe-P can be expressed as below:
According to our previous work,Fe-P can be considered as a mixture of FeP and Fe2P,which was described as Fe1.5P.Therefore,the overall reaction can be expressed as below:
The thermodynamic parameters of the reactions (3) and (4)were evaluated based on thermodynamics simulation,and the reaction system composed of the following species: FeP,Fe2P,VO,H+,V4+,Fe3+,POand H2O.The ΔH0and ΔG0of above reactions were calculated and plotted in Fig.2.It is clear that the ΔG0exhibited a negative value for the reaction(3)and reaction(4)from 0–100 °C,indicating a spontaneous and exothermic reaction between V(V) and Fe-P slag.Notably,the ΔG0value gradually changed from–1261.9 to–1240.0 kJ∙mol-1and from–1669.5 to–1618.4 kJ∙mol-1for reaction FeP/V(V)and Fe2P/V(V),respectively.A nearly unchanged ΔG0indicated a constant spontaneity of above reactions.In addition,one can see that the ΔH0gradually decreased from–1608.3 to–1053.3 kJ∙mol-1and from–2212.3 to–1438.4 kJ∙mol-1for reaction FeP/V(V) and Fe2P/V(V) from 0 to 100 °C.These results indicated that the reaction between V(V) and Fe-P is a spontaneous exothermic chemical process.

Fig.1. (a) XPS spectra of V 2p,(b) P 2p and (c) Fe 2p of Fe-P slag and filter cake,and (d) XRD patterns of Fe-P before and after reaction.

Fig.2. ΔG and ΔH for the reaction of FeP/V(V) and Fe2P/V(V).
3.3.Influencing factor
According to the reaction equations,one can see that a higher concentration of H+benefits the reduction reactions of V(V).In addition,the reactions occurred on the interface of solid (Fe-P)and liquid phase (solution),a higher dosage of Fe-P can provide a larger interface for reactions and improve the total reaction rate.Based on the thermodynamic calculation,it concluded that the reduction of V(V) with Fe-P is an irreversible exothermic reaction,the increase of temperature can improve the rate constant and the reaction rate.Therefore,the concentration of H2SO4,the dosage of Fe-P and temperature were chosen as three factors to evaluate their effect on the removal of V(V).
3.3.1.Influence of Fe-P dosages on the removal efficiency of V(V)
The effect of Fe–P stoichiometric dosages on V(V) removal was first evaluated.In general,the reaction significantly relies on the interfaces between Fe-P and V(V)containing solution;an increased Fe-P dosage inevitably facilitates the reaction and improves the removal efficiency.As expected,it can be found that the dosage of reducing agent exhibited a remarkable influence on V(V)reduction (Fig.3(a)).In detail,a V(V) removal efficiency of 54%,84%,100% and 100% was realized with 5,10,15 and 20 times of Fe–P stoichiometric dosage.It should be noted that the removal time was shortened from 2 h to 1.25 h by increasing stoichiometric dosage from 15 to 20 times,with a removal efficiency of~100%.Considering a remarkable effect of Fe-P on the reduction reaction of V(V),flexible utilization of Fe-P is preferred according to the actual V(V) concentration in wastewater and the expected period.
Reaction kinetics is a crucial parameter to identify the effect of factors(such as reactant concentration,temperature,pressure,etc.)on reaction rate.The apparent rate constant (kobs),as a crucial parameter to evaluate reaction kinetics between V(V) and Fe-P,can be calculated based on experimental results (detailed calculation method were in Supplementary Materials).The reduction reaction of V(V)can be expressed using pseudo first-order reaction kinetics model.According to the fitting results and kinetic model,thekobsvalues were calculated under different Fe-P stoichiometric dosages,as depicted in Fig.3(b).The results suggested that thekobswere 6.94×10–3,1.50×10–2,2.54×10–2and 3.22×10–2min-1at stoichiometric dosages of 5,10,15 and 20 times,respectively.According to the above results,the relationship between Fe-P dosage andkobswas plotted (Fig.3(c)) and the linear fitting was expressed as follows:

Fig.3. (a) Removal efficiency,(b) plot of ln(C0/C) versus time,and (c) apparent rate constant at different Fe–P dosages.
3.3.2.Influence of H2SO4 on the removal efficiency of V(V)
Apart from Fe-P slag,the effect of H2SO4on removal efficiency was also studied.As shown in Fig.4(a),one can see that 2,5,8 and 11 times increase in H2SO4stoichiometric concentration realized a V(V) removal efficiency of 54%,82%,99%,and 100%,separately.Similar to Fe-P slag,an increase of H2SO4stoichiometric concentration facilitated the V(V) reduction and improved the removal efficiency.The ln(C0/C) at different H2SO4stoichiometric concentrations was obtained based on pseudo first-order reaction kinetics model(Fig.4(b)).As shown in Fig.4(c),thekobswas calculated to be 6.94 × 10–3,1.40 × 10–2,2.45 × 10–2and 3.45 × 10–2min-1at H2SO4stoichiometric concentration of 2,5,8 and 11 times.According to the linear fitting,the relationship between H2SO4stoichiometric concentration and kobswas expressed as follows:
3.3.3.Influence of temperature on the removal efficiency of V(V)
As mentioned above,the ΔH0and ΔG0are temperaturedependent parameters,the variation of temperature will directly affect reaction rate and removal efficiency.As depicted in Fig.5(a),the V(V) removal was evaluated at different temperatures.It is clear that the increase of temperature from 25 to 30 °C led to an improved removal efficiency from 82% to 100%.It should be noted that,when further increasing the temperature to 35 °C and 40 °C,the removal time was shortened from 1.5 h to 1 h with a removal efficiency of~100%.The reaction kinetics of V(V) reduction was also evaluated by plotting ln(C0/C) over time at various temperatures on the basis of the pseudo first-order reaction kinetics model.Thekobswas calculated to be 1.40 × 10–2,3.01 × 10–2,4.00 × 10–2and 5.07 × 10–2min-1at the temperature of 25,30,35 and 40 °C (Fig.5(c)).This implies that a higher temperature remarkably improved the kinetics of V(V) reduction and the removal efficiency.Also,the relationship betweenkobsand temperature was potted and expressed as:
As previously described,the relationship between temperature andkobscan be expressed by using the Arrhenius equation[40,41]:
whereA,Ea,RandTare apparent frequency factors(s-1),activation energy (J∙mol-1),molar gas constant (8.314 J∙mol-1∙K-1) and temperature (K).Eq.(9) was evolved into the following equation:

Fig.4. (a) Removal efficiency,(b) plot of ln(C0/C) versus time,and (c) apparent rate constant at different H2SO4 stoichiometric concentrations.

Fig.5. (a) Removal efficiency and (b) ln(C0/C) over time,(c) apparent rate constant at different temperatures,and (d) relationship between–ln(kobs) and 1/T.

Fig.6. Variation of V(V),V(IV),Fe andconcentration during the reaction.
Since thekobshas been obtained at different temperatures,the plot of–ln(kobs) and 1/Twas obtained and depicted in Fig.5(d).The relational expression was obtained based on linear fitting(Eq.(11)).According to the above equation,theAandEavalues were estimated to be 3.23 × 109min-1and 64.50 kJ∙mol-1.

Fig.7. Variation of temperature during the reduction reaction of V(V).
The balance of V element in the reaction system was further evaluated during the reaction.During the reaction,both of Fe and P were served as reducing agent,the Fe and P was oxidized to Fe3+and.As shown in Fig.6,one can see that the variations of V(V) and V(IV) concentrations exhibited opposite trend with a basically unchanged total V content,suggesting a V balance before and after V(V) reduction.Furthermore,it is clear that the concentration of Fe andgradually reached up to 0.399 and 0.158 g∙L–1attributing to the consumption of Fe-P slag (consumption of 0.511 g).These results showed that the contents of V,Fe and P in the solution remain basically unchanged before and after the reaction.
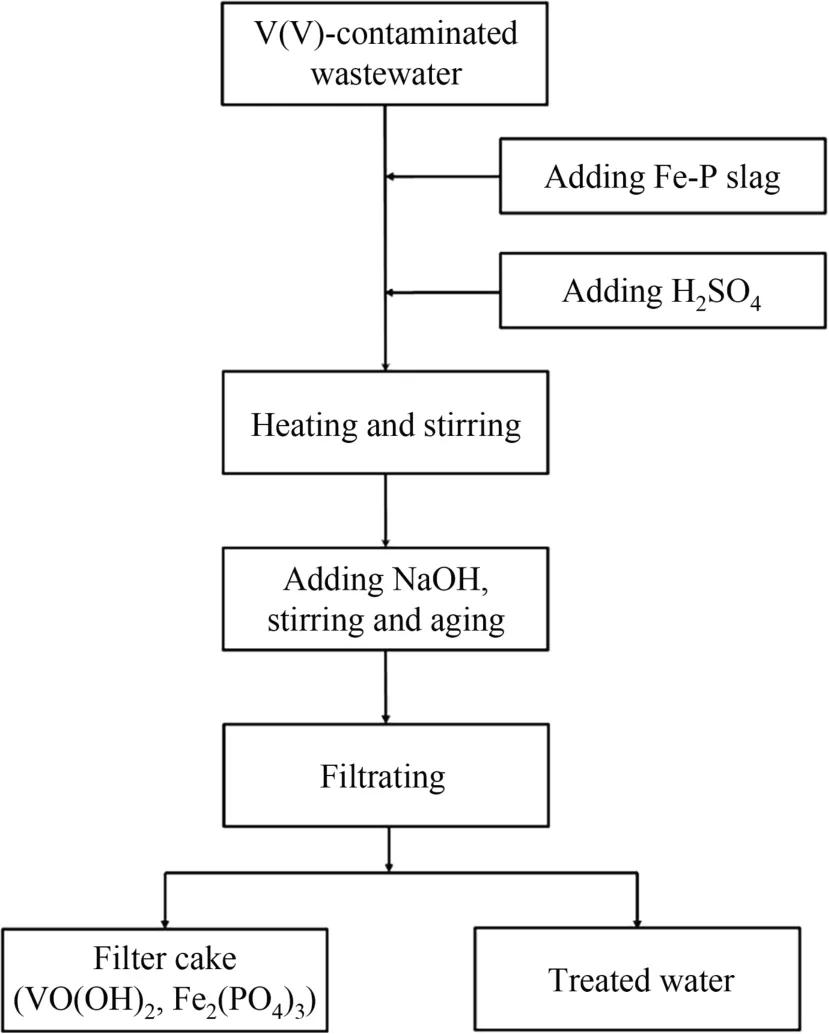
Fig.8. Procedure of V(V) removal for V(V)-contaminated wastewater.
3.4.Lab-scale V(V) removal with a simplified industrial procedure
Based on the above analysis and discussion,it concluded that Fe-P could be a reducing agent for V(V)reduction.A scalable application for V(V)removal in industrial is possible due to the low cost of Fe-P slag.Notably,the thermodynamic study revealed that the reaction of V(V)reduction is spontaneous and exothermic.Considering an apparent influence of temperature on V(V) removal,the exothermic process cannot be ignored in the applications.To evaluate the exothermic process,the temperature variation was collected during the reaction under insulation conditions.The reactor was coated with a layer of insulating foam to prevent the heat losses,and the temperature was collected during the reaction.In the experiment,a mixture solution of V(V) and Fe-P slag with a room-temperature was added to the reactor.As reflected in Fig.7,the temperature of the mixture solution sharply increased up to~47 °C at the beginning of reduction reaction between V(V) and Fe-P slag.After that,the temperature of the solution gradually declined to~43 °C,attributing to a slight heat loss from incomplete insulation.It is confirmed that the reaction between V(V)and Fe-P slag is a spontaneous exothermic chemical process.Also,the increased temperature from V(V) reduction can further improve the kinetics and removal efficiency.

Fig.9. (a) Lab-scale reactor design for V(V) removal,(b) photo of a solution containing V(V),V(IV) and treated solution,and (c) V(V) removal over time in the reactor.
A simplified procedure was proposed to evaluate the feasibility of industrial application,as shown in Fig.8.First,the V(V)concentration can be roughly analyzed and evaluated,followed by adding proper mass of Fe-P slag and H2SO4successively according initial V(V) concentration.A special reactor equipped with stirring parts and temperature-controller is required to maintain the reaction efficiency.When the reaction is complete,the resulting solution will be alkalized by adding KOH and followed by precipitating V(VI).Finally,the mixture was filtrated to separate the V(VI)precipitates and clean water.
Importantly,we further proposed a screen-bowl reactor strategy for V(V) reduction using Fe-P as a filter cake.In this design,the V(V) removal was achieved using a double-jacked reactor(Fig.9(a)).The architecture of reactor was described in our previous work as an effective method for wastewater treatment [30].In the experiment,the bottom of the inner tube was packed with excessive Fe-P slag and temperature was maintained at~40 °C by pumping hot water through the reactor shell,building a similar condition to the exothermic process.During the reduction reaction between V(V)and Fe-P slag,the yellow V(V)solution slowly flowed through the inner tube,where the V(V)was transformed into V(IV)(blue color) (Fig.9(b)).To further remove the V(IV),the alkaline solution was added to convert V(IV) into VO(OH)2precipitation.The treated wastewater was finally obtained by separating VO(OH)2precipitation.As depicted in Fig.9(c),the proposed procedure and system achieved a V(V) removal efficiency of 100%,with a removal capacity of 200 ml of 2 g∙L–1V(V) solution within 3 h.
4.Conclusions
In this paper,a V(V) removal method was proposed using byproduct of Fe-P as a reducing agent.The thermodynamic simulation revealed a spontaneous reduction reaction of V(V) with an exothermic process,implying that the system can achieve a selfheating and hence the improvement of the reaction rate constant and the removal efficiency.The results demonstrated the feasibility of V(V) removal through the reduction reaction and precipitation.The influence of Fe-P slag/H2SO4dosages and temperature on the V(V)removal was evaluated,the apparent activation energy and frequency factor were calculated to be 64.50 kJ∙mol-1and 3.23 × 109min-1respectively.Significantly,the proposed procedure achieved a 100% removal efficiency and a capacity of 200 ml of V(V)solution(2 g∙L–1)within 3 h.These results suggested the feasibility of V(V) removal using Fe-P slag as a reducing agent and provide an alternative pathway for the resourceful utilization of industrial waste Fe-P.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation for Young Scientists of China (22108185,51906168,52276208).
Supplementary Material
Supplementary material to this article can be found online at https://doi.org/10.1016/j.cjche.2023.04.010.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
