Effect of slag composition on corrosion resistance of high chromia refractory bricks for industrial entrained-flow gasifier
Jinghong Gao,Weiguang Su,,Xudong Song,Peng Lv,Jun Yang,Guangsuo Yu,3,
1 State Key Laboratory of High-efficiency Utilization of Coal and Green Chemical Engineering, College of Chemistry and Chemical Engineering, Ningxia University, Yinchuan 750021, China
2 Chinese Energy Ningxia Coal Industry Co., Ltd., Yinchuan 750021, China
3 Institute of Clean Coal Technology, East China University of Science and Technology, Shanghai 200237, China
Keywords:Entrained-flow gasifier Coal slag High chromia refractory Corrosion Permeation Viscosity
ABSTRACT The slag composition corresponding to different coals varies significantly,which directly affects the operation of industrial entrained-flow gasifier and the service life of refractory bricks.In this study,the corrosion resistance of several typical coal slags for gasification on high chromia refractory bricks was comparatively investigated by static laboratory crucible tests and thermodynamic simulations.The results demonstrated that the corrosion degree of high chromia refractory bricks by different coal slags was high-Ca/Na slag>high-Fe slag>high-Si/Al slag.The surface structure of the refractory was relatively flat after corrosion by high-Si/Al slag,and the primary corrosion reaction was the partial dissolution of the matrix by the slag.High-Fe slag was prone to the precipitation of iron phases as well as the formation of(Mg,Fe)(Al,Cr)2O4 composite spinel layer at the slag/refractory interface.The high-Ca/Na slag was susceptible to react with the refractory to yield a low melting point phase,which led to the destruction of the matrix structure of the refractory and an isolated distribution of particles.In addition,the monoclinic ZrO2 in the refractory reacted with CaO in the slag to formed calcium zirconate,which loosened its phase toughening effect,was the primary factor that aggravated the refractory corrosion.
1.Introduction
At present,although the energy structure is actively shifting towards efficient,clean,low-carbon or carbon-free renewable energy sources such as natural gas,hydropower,nuclear energy,solar energy,wind energy,etc.,coal still makes up more than 60%of China’s fuel and energy consumption [1,2].Therefore,it is expected that coal will continue to dominate the supply and consumption of energy in China for a long time to come.Entrainedflow coal gasification technology has the advantages of large processing capacity,high content of active components (H2+CO) in syngas and high carbon conversion rate.It is the core technology of clean and effective utilization of coal and the development trend of large-scale coal gasification technology[3–5].This technology is widely adaptable to different coal types,as long as the coal meets the requirements in terms of ash content,ash melting point,grindability and slurry formation,it can be used in combination with abundant and widely distributed bituminous coal or lignite [6,7].
The gasifier is the main equipment of the industrial entrainedflow gasification plant.The hot face refractory bricks of the combustion chamber in an industrial gasifier come into contact with high temperature,high pressure,low viscosity and high-velocity slag.The corrosive substances such as SiO2and CaO in the slag penetrate into the refractory through pores and cracks and lead to changes in its composition and structure,which is the internal cause of brick damage.In addition,fluctuations in temperature and pressure,as well as mechanical scouring by high-speed airflow are external factors that accelerate the degradation of refractory bricks[8–10].Due to the harsh operating environment in the gasifier,high chromia refractory is usually selected as hot face liners in commercial gasifiers,with the primary components being Cr2O3(≥86% (mass)) and ZrO2(2%-5% (mass)) [11,12].The corrosion of slag to refractory is still a severe issue in the actual operation of the gasifier even though high chromia refractory has superior corrosion resistance than other refractory.Moreover,high chromia refractory is inherently expensive and its wear can significantly increase the operating cost of a gasifier [13].Both the gasifier and the refractory brick are fixed for a certain period of time due to economic and product factors.As a result,the feedstock coal is

2.2.Corrosion test
The corrosion tests of different coal slags on high chromia refractory bricks were carried out under a buried carbon atmosphere according to the static crucible method,and the specific experimental process was shown in Fig.1.First,the high chromia crucible was placed in a corundum sagger and filled with graphite powder and activated carbon around it.Then about 15 g of equal amounts of MHJ,JJT and ZD coal slags were added to the crucible,respectively.The specimens were put into a high temperature slagresistant furnace and heated at 1350 °C for 5 h.The air outlet was closed during the experiment to simulate a weak reducing atmosphere.The furnace was cooled to room temperature after the corrosion test and the crucible was removed.The crucible was cut along the central axis to observe the corrosion and permeation of slag on the specimen,and the position with obvious slag permeation was selected for curing and polishing.Static crucible tests were performed at least three times for each sample for further analysis and discussion.Corresponding to MHJ high-Si/Al slag,JJT high-Fe slag and ZD high-Ca/Na slag,the post-corrosion specimens were marked asSSA,SFandSCN,respectively.

Fig.1. Schematic diagram of slag corrosion test.
2.3.Characterization
The phase composition of the corroded specimens was examined by X-ray diffractometer(XRD,D8 ADVANCE A25)with copper radiation(Cu-Kα,λ=0.15406 nm)at 40 kV/40 mA in the 2θ range of 10°–85°.The chemical composition of the coal slags were analyzed using X-ray fluorescence spectrometry(XRF,ARL PERFORM’X).The microstructure and morphology of each specimen after corrosion tests were observed using scanning electron microscopy (SEM,TESCAN MIRA LMS),and the material composition and elemental distribution were acquired by semi-quantitative EDS analysis.In addition,the corrosion processes of high chromia refractory by coal slag with different compositions were simulated using FactSage 7.3 thermodynamic software.
3.Results and Discussion
3.1.Simulation of corrosion behavior
The reaction between oxides in the slag and the refractory is an important cause of corrosion and permeation behavior [31–34].Accordingly,the Gibbs free energy (ΔG⊖) values for the possible chemical reactions between the coal slag and the high chromia refractory bricks under test conditions were first calculated by FactSage 7.3 software using the reaction module.The FactPS and FToxid databases were selected,and the temperature range was 1000–1600 °C with a 50 °C interval.
In view of the complex oxide composition of the refractory and coal slag,only chemical reactions between simple oxides were taken into consideration evaluate potential reactions.All conceivable chemical reactions between simple oxides are listed in Table 3,where R1-R11 indicate interactions between the major simple oxides and R12-R20 represent interactions between the other simple oxides.The ΔG⊖values in Fig.2(a) are all negative,which demonstrates that the chemical reactions between the major simple oxides can all proceed spontaneously.The positive value of ΔG⊖for R19 in Fig.2(b)suggests that the reaction is thermodynamically infeasible.As a result,all reactions can occur except for the MgO in the coal slag which does not react with the small amount of Fe2O3in the refractory brick.
3.2.Corrosion resistance
The cross-sectional macroscopic images of the specimens after corrosion tests are shown in Fig.3.In each specimen,a distinct slag-refractory interface can be seen.The high chromia crucible mouth is almost completely uncorroded by the coal slag,and the slag seems to diminish in bulk after melting and heating.Moreover,it is evident that the permeation depth of the specimen only slightly increases while the residual height falls as the slag alkalinity rises.There are more slag residues in specimenSSA,a small amount of slag residues inSF,and almost all of the slag inSCNpermeated into the high chromia refractory bricks.The permeation depth of the slag into the brick could not be directly identified due to the gray-black color of both the slag and the refractory.In order to ascertain the precise permeation depth of slag,the distribution of slag elements at various distances from crucible surface was examined using EDS in the following section.
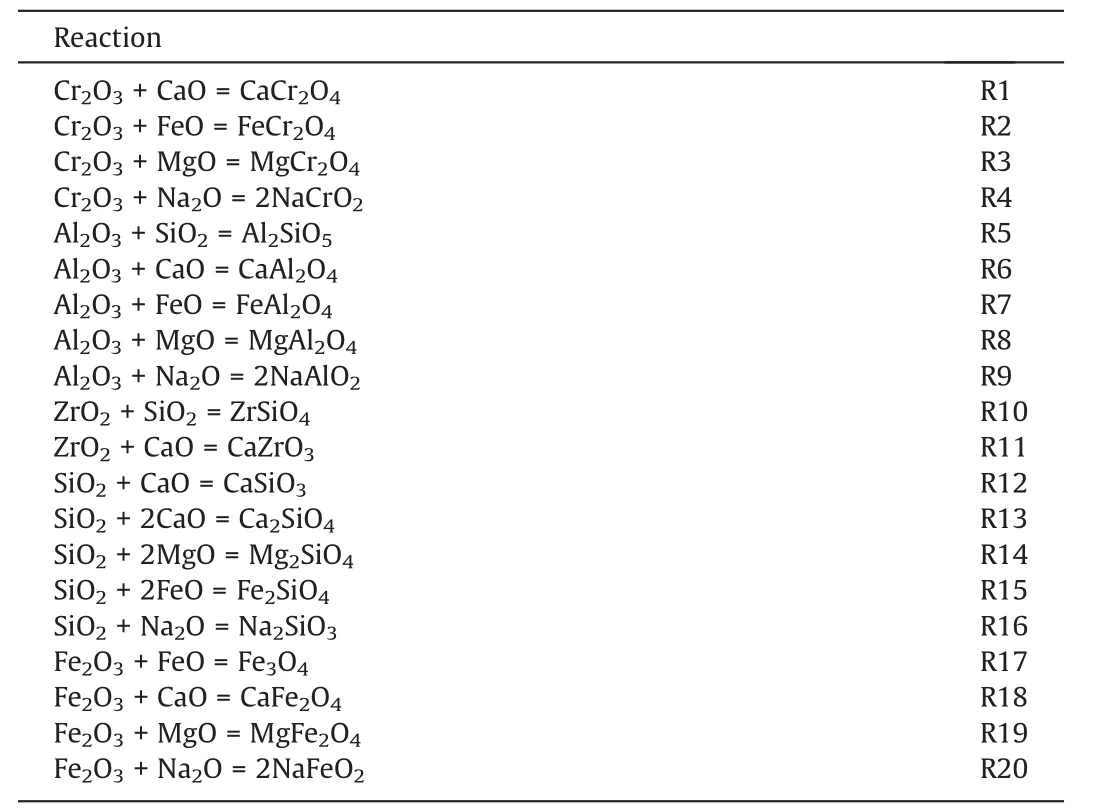
Table3 Possible reactions between the slag and the refractory
3.3.Chemical composition and phase evolution
The chemical composition and XRD patterns of the residual slag on the surface of different specimens after corrosion tests are shown in Table 4 and Fig.4,respectively.For all specimens,the main crystalline phase is spinel as well as a mixture of corrosion products of Al2O3,SiO2and CaO.There are also a considerable number of intense diffraction peaks caused by Cr2O3.On the one hand,the surface residual slag is removed from the specimen by mechanical means after the corrosion test,and a part of high chromia refractory may have been shed during this process.On the other hand,Cr2O3in the refractory also reacts with the slag and partially dissolves into the slag.The main phase in theSSAspecimen corroded by high-Si/Al slag is corundum (Al2O3),spinel and anorthite(CaAl2Si2O8).The relatively weak intensity of the diffraction peak of Cr2O3indicates the low solubility of Cr2O3in the high-Si/Al acidic slag.The differences of peak intensities of compounds with spinel structures in different specimens are also very obvious.The intensity of the spinel structured phase chromite (FeCr2O4) is the largest in the specimenSFcorroded by the high-Fe slag.It indicates that the amount of composite spinel solid solution formation increases with the FeO content in slag.In addition to the common spinel phase,spinel with embedded sodium ions has been found.The spinel phase with Na ions is present only in theSCNspecimen with the highest Na2O content.A significant amount of nepheline(NaAlSiO4) and anorthite (CaAl2Si2O8) is generated when the contents of Na2O and CaO in the slag are high.The presence of feldspar material will reduce the viscosity of slag,which will make it more likely to penetrate refractory and cause serious corrosion.
3.4.Microstructure analysis
Figs.5–7 shows the microstructure and EDS analysis of the cross-sections of the specimens after corrosion by different coal slags.It is clear that the microstructures of different specimens are very diverse.The cross-sectional images of all specimens can be divided into four major regions:residual slag layer,spinel layer,reacted layer and unreacted layer (i.e.,original brick layer).
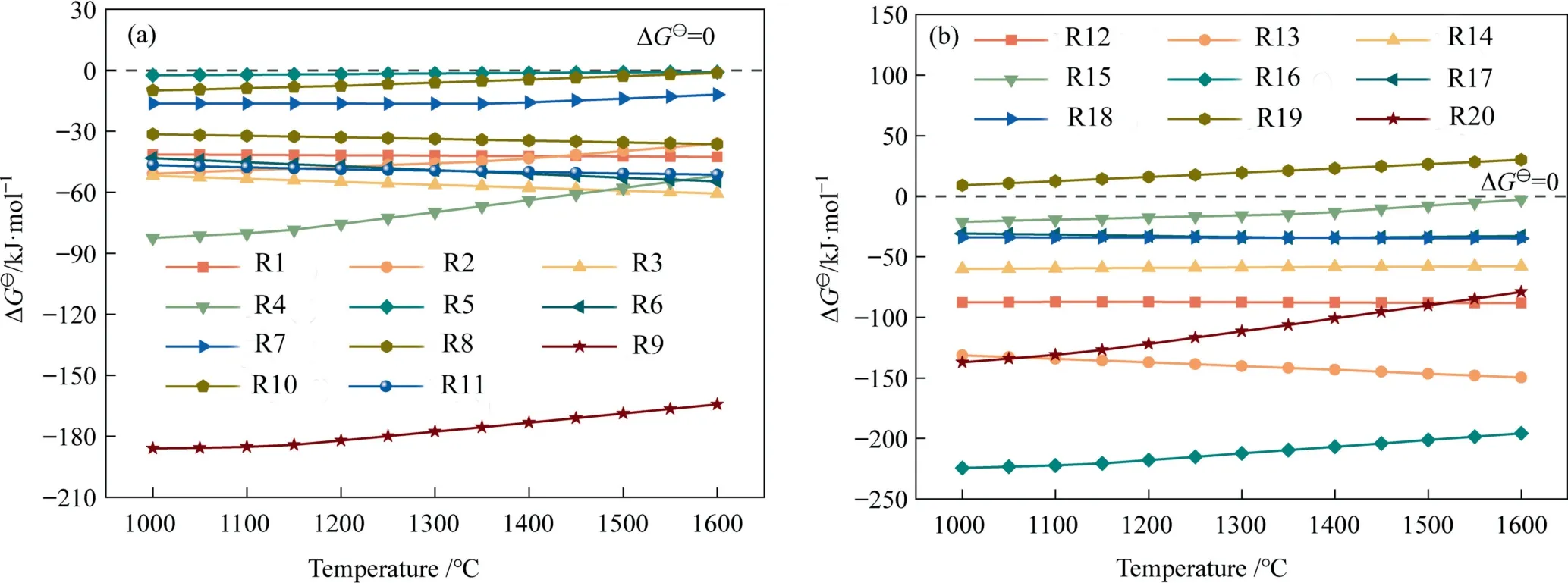
Fig.2. Gibbs free energies in the temperature range from 1000 °C to 1600 °C of (a) the main simple oxides and (b) other simple oxides.

Fig.3. Cross-sectional macroscopic images of the crucible specimens after corrosion tests with different slags.
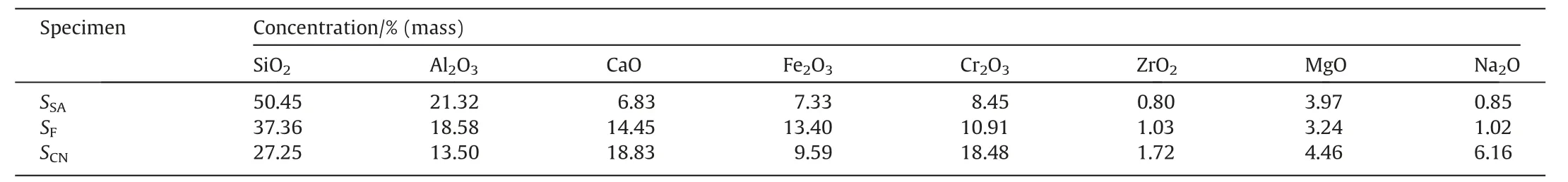
Table4 Chemical compositions of the residual slag on the surface of specimens after corrosion tests

Fig.4. XRD patterns of the residual slag on the surface of specimens after corrosion tests.
The microstructure and EDS analysis of the specimenSSAafter the corrosion test are displayed in Fig.5.The surface structure of the specimenSSAafter corrosion by high-Si/Al slag is relatively complete,with a thick slag layer on the refractory surface.The slag layer structure is relatively dense,and the brick is tightly bonded to it.According to the distribution of each element in the mapping image,Al,Si and Ca are primarily present in the slag layer in high concentration and in same distribution.The concentration of Fe in the spinel layer is significantly higher than that in other layers,which is in agreement with the results of the line scan.As a result,it can be judged that a thin discontinuous spinel layer is formed on the refractory surface.This discontinuous spinel layer may be generated directly by the reaction with the slag after the dissolution of(Al,Cr)2O3solid solution at the interface,or it may be spinel precipitated from the surface slag layer.Fig.5 depicts a highmagnification BEI image of the region I near the corrosion interface.The pores in this region are large and interpenetrating,which is mainly due to the production and dissolution of the low melting point phase [34].The fine ZrO2particles are sporadically distributed in the matrix shows that high-Si/Al slag corrosion did not result in the formation of a de-zirconium layer in the corroded region.The EDS analysis of point 1 shows that the chemical composition of the dark gray crystalline phase in Fig.5 contains mainly 63.43% Si,17.71% Al,8.03% Ca and 6.51% Cr (mass).Except for a small amount of dissolved Cr2O3,the chemical composition is roughly similar to that of the slag.This implies that the slag fills the pores between the (Al,Cr)2O3solid solution after permeation and that a slight dissolution reaction occurs.The concentrations of Al,Ca,Si and Fe are also much greater in the reacted layer than in the unreacted layer.However,the high-Si/Al acidic slag has a shallow permeation depth in the refractory owing to its high viscosity [35].

Fig.5. SEM images and EDS analysis of the corrosion area in specimen SSA.
Fig.6 shows the microstructure and EDS analysis results of specimenSFafter corrosion by high-Fe slag.The slag layer on the surface of specimenSFis thinner than that of specimenSSA,and an obvious de-zirconium layer and dense spinel layer are formed.The high magnification BEI image of region I shows that several layered crystals and pore structures are formed in the corrosion interface.Based on XRD and EDS analysis,the chemical composition of the layered crystals (point 1) in Fig.6 contains 70.58% Cr and 14.29% Fe (mass).The chemical composition of the dark gray long crystalline phase (point 4) contains 80.36% Cr and 14.62% Al(mass).According to the chemical composition and regular shape,it can be inferred that the layered crystals and the dark gray long crystalline phase are FeCr2O4spinel and (Al,Cr)2O3solid solution,respectively.The chemical composition of point 2 contains mainly 31.24%Cr,47.59%Ca and 12.65%Fe((mass)),which may be formed by the reaction of a large amount of CaO in the coal slag with Cr2O3in the refractory brick to form CaCr2O4.The white particle point 3 is the ZrO2grain embedded in the hole.The ZrO2in the matrix of refractory reacts with SiO2in the slag at high temperatures to form ZrSiO4,which in turn decomposes into ZrO2under the action of CaO in the slag.The ZrO2in the matrix gradually separates to generate a de-zirconium layer in this process [12].
According to the mapping results in Fig.6,the distribution patterns of Si,Ca and Na are the same mainly concentrated in the slag layer,with only a small amount permeates into the refractory to form a permeation layer.Ca and Al have the same elemental distribution in some regions indicate that they may be CaAl12O19(CA6)phases.The distribution pattern of Fe and Mg is also the same,mainly concentrated at the slag/refractory interface.The EDS line scan also shows that Fe is little in the slag layer and increases gradually from the slag towards the refractory,reaching a maximum peak in the spinel layer.This is due to the fact that Fe2O3is firstly reduced to FeO(R21)by reaction with CO when the slag resistance test is carried out in a weak reducing atmosphere.The FeO and MgO in the slag then penetrate into the refractory and react with the Cr2O3aggregate and Al2O3in the matrix to form FeCr2O4,MgCr2O4,FeAl2O4and MgAl2O4(R2,R3,R7,R8 in Table 3).Further dissolution subsequently occurs and eventually an (Mg,Fe)(Al,Cr)2O4spinel dense layer is formed(R2).The ratio of FeO to MgO in the slag and the content of FeO in the spinel are the key determinants in determining the thickness of the composite spinel layer.The formation of the dense layer is caused by slag permeation into the pores of the refractory,which theoretically prevents slag permeation into the interior of the specimen [23,26,36].However,the higher test temperature makes the slag viscosity much lower,so the permeation into the specimenSFis still more severe than that into the specimenSSA.

Fig.7. SEM images and EDS analysis of the corrosion area in specimen SCN.
The microstructure and EDS analysis of the specimensSCNafter corrosion by alkaline high-Ca/Na slag are depicted in Fig.7.It can be seen that the permeation depth of the alkaline slag into the refractory is significantly deeper and the overall continuous architecture of the specimen is disrupted as well as dissolution occurs at the surface.This indicates that the reaction between the slag and the matrix is significantly greater than that with the Cr2O3aggregate.Furthermore,the spinel structure on the surface of specimenSCNis not obvious compared with specimenSF.The permeation depth of the slag is viscosity dependent,and its permeation ability increases with decreasing slag viscosity.The reaction layer is permeated by the de-zirconium layer inward,where the pores are mostly filled with slag and have obvious annular fractures.The mapping results show that the permeation of Si and Ca is significantly enhanced with the increase of slag alkalinity.According to the line scan analysis,Na2O,CaO,SiO2and other substances will penetrate into the refractory,among which the Si and Ca contents decreases more obviously with the increase the permeation depth.However,the content of Na tends to decrease less with the permeation depth.This is due to the decrease in viscosity and surface tension of the slag as the Na2O content rises,which leads to a higher permeability [28].The morphological observation of region I revealed that the bonding strength of small grains is reduced after being dissolved by the liquid phase.It can be speculated by EDS analysis that Na2O forms Na2O-3CaO-6SiO2at the bright white spot with the slag.The CaO content in the alkaline slag is relatively high.The slag penetrates into the matrix and reacts with monoclinic ZrO2to form calcium zirconate.This deprives the refractory of its phase toughening effect and exacerbates the damage to some extent [37,38].

Fig.8. Corrosion depth diagram of the specimens after corrosion tests.
Fig.8 shows the depth of each layer of the specimens after corrosion by different slags.For specimensSSA,SFandSCN,the measured residual slag depth decreases with increasing slag alkalinity,being 6.1 mm,2.7 mm and 1.2 mm respectively.This indicates that the amount of slag involved in interfacial reaction and permeation increases with the content of alkaline oxides in the slag.In addition,a dense composite spinel interlayer are clearly visible at the slag/refractory interface of specimensSSA,SFandSCNwith depths of 0.013 mm,0.055 mm and 0.019 mm from EDS line scan results from Figs.5–7,respectively.As can be observed,the thickness of the spinel layer is mainly influenced by the FeO content.The increase in slag alkalinity will also lead to an elevated amount of liquid phase produced at high temperatures,so that more Cr2O3aggregates will dissolve in the slag.At the same time,the structure of the refractory is also damaged and the depth of the corrosion reaction layer gradually increases.Combined the above analysis shows that high-Si/Al slag has the weakest corrosion on high chromia refractory,followed by high-Fe slag,while high-Ca/Na slag has the strongest corrosion.
3.5.Corrosion mechanism and thermodynamic simulation
The above analysis shows that the corrosion resistance effects of high-Si/Al slag,high-Fe slag and high-Ca/Na slag on high chromia refractory bricks are significantly different.To further understand the mechanism of interfacial reactions during corrosion tests,the equilib module in the thermodynamic analysis software Factsage 7.3 was used to predict the evolution of the phases.The calculation temperature was set at 1350 °C and the oxygen partial pressure wasP(O2)=10–9MPa.It is worth noting that a series of reactions occur with high chromia refractory bricks when the coal slag permeates,and its component changes accordingly [38].Therefore,the thermochemical calculations in this paper were performed taking into account the variations of all these components.Fig.9 shows the flow chart of the simulation of the slag/refractory interaction process.First,the 0.9 initial coal slag and 0.1 high chromia refractory were used to perform the first step of the equilibrium calculations,and the generated liquid slag was recorded as slag #1.Then,0.8 reactions slag #1 and 0.2 refractory were used to conduct the second step of calculations and the produced slag was labeled as slag#2.This procedure was repeated multiple times to simulate the entire slag/refractory reaction.
The interaction between different coal slags and high chromia refractory is simulated by the calculation model in Fig.9,and the results are presented in Fig.10.According to Fig.10(a),Al2O3and a small amount of Cr2O3and CrO are dissolved into the slag during the interaction of the high-Si/Al slag with the refractory.Cr2O3is reduced to CrO,and the spinel phase formed by the reaction between the slag and refractory is dominated by MgCr2O4.It can be seen from Fig.10(b) that the simulated results of the phase composition of specimenSFare similar to those ofSSA,with the only difference being that the amount of spinel generated in specimenSFis greater than that ofSSA.The production of (Mg,Fe) (Al,Cr)2O4composite spinel increases with the rise of FeO content in the slag,which is similar to the experimental results.As demonstrated in Fig.10(c),the principal components of refractory,such as Cr2O3,Al2O3and ZrO2,obviously decrease with an increase of slag phase in the reaction system of high alkali slag and refractory.The contents of CaO,Na2O and NaAlO2in the liquid phase are all almost unchanged.Besides,the dissolution of ZrO2further increases the interfacial porosity and provides a large number of permeation channels for the high-temperature liquid slag.As a result,the high chromia refractory exhibits the worst corrosion resistance to high-Ca/Na slag compared to other selected slags.
The corrosion performance of various slags on high chromia refractory can be summarized based on corrosion tests and thermodynamic simulation results,as shown in Fig.11.The corrosion mechanism between high-Si/Al slag and the refractory is depicted in Fig.11(a).The specimenSSAafter being corroded by high Si/Al slag exhibits high resistance to slag corrosion with a relatively intact surface structure.However,the pores in the area near the corrosion interface become slightly larger,which is mainly due to a slight dissolution reaction of the aggregates after the slag permeates the refractory.The slag viscosity increases with the contents of the acidic oxides SiO2and Al2O3,thus most of the slag collects on the refractory surface with the shallowest permeation depth.Slag viscosity is usually required to be lower than 25 Pa∙s to ensure the smooth discharge of liquid slag in an entrained-flow gasifier[16,17].The higher viscosity of high-Si/Al slag will pose a certain threat to the liquid slag discharge process of the gasifier.Therefore,MHJ coal usually needs to be applied to the gasifier by means of coal blending.

Fig.9. Calculation flow diagram for simulation of slag-refractory interaction using FactSageTM 7.3.
Fig.11(b)displays the interaction mechanism between high-Fe slag and the refractory.The continuous structure of the specimenSFafter corrosion by the high-Fe slag has been disrupted,and a visible de-zirconium layer has formed.The reaction of FeO in the slag with Cr2O3and Al2O3in the refractory also produces a dense composite spinel layer at the slag/refractory interface.The edge of the spinel particle shows irregular protrusions,and the grown particles are connected together again to form a certain continuous structure.In theory,a continuous composite spinel layer prevents further permeation of the slag into the refractory brick,but the higher temperature considerably reduces the slag viscosity.Hence,the corrosion performance of refractory by high-Fe slag is more serious than that by high-Si/Al slag.
Fig.11(c)shows the corrosion mechanism between high-Ca/Na slag and the refractory.It can be observed that the matrix structure of the specimenSCNhas been destroyed and the particles are distributed in isolation.There are obvious ring cracks in the reaction layer.The viscosity and surface tension of the slag decreases significantly with the increase of Na2O content,which makes the slag penetrate more easily.Moreover,CaO in the slag reacts with monoclinic ZrO2to form calcium zirconate (CaZrO3),which loses its phase toughening effect[37,39].This is the main reason for aggravating the corrosion of refractory brick by alkaline slag.The corrosion degree of the refractory by high-Ca/Na slag is greater than that by high-Si/Al slag and high-Fe slag.The corrosion of refractory by high-Ca/Na slag has a great impact on the safety and efficiency of gasifier operation,which is a key factor constraining the largescale utilization of highly alkaline ZD coal.Although the above investigations have made relatively important discoveries on the corrosion mechanism by high alkaline slag,there are still some limitations.It is well known that alkali metals in coal are highly volatile during the gasification process,especially Na2O.However,we only explored the corrosion and permeation behavior of liquid phase molten slag on high chromia refractory in this paper due to limited time and conditions.The effect of gaseous volatile alkali metal gases on corrosion was not considered.Therefore,an important direction for the next work is to focus on the higher alkali metals in ZD coal and analyze in depth the specific corrosion mechanisms of liquid-phase and gas-phase alkali metals on refractory.It is expected to provide further recommendations for the selection of feedstock coal and the performance optimization of high chromia refractory.
4.Conclusions
In this paper,a comprehensive evaluation of the corrosion performance of different coal slags on high chromia refractory brick was summarized by a combination of static crucible corrosion tests and thermodynamic simulations.The following main conclusions were drawn:
(1) The differences in the chemical and physical phase composition of the individual coal slags lead to large variations in the corrosion degree of high chromia refractory.The deepest corrosion was achieved by high-Ca/Na slag,followed by high-Fe slag,and the shallowest by high-Si/Al slag.
(2) The surface structure of the refractory brick was relatively flat after corrosion by high-Si/Al slag,and the primary corrosion reaction was the slight partial dissolution of the aggregate by the slag.The (Mg,Fe) (Al,Cr)2O4dense composite spinel layer was formed at the slag/refractory interface after corrosion by high-Fe slag,which prevented further permeation of the slag to some extent.The high-Ca/Na slag was prone to react with the refractory to generate a low melting point phase,resulting in the destruction of the matrix structure and isolated distribution of particles.
(3) The primary factor for aggravated the corrosion of high chromia refractory by high alkaline slag was the reaction of monoclinic ZrO2in the refractory with CaO in the slag to form calcium zirconate,which loosen its phase toughening effect.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge financial support from the Joint Funds of the National Natural Science Foundation of China(U21A20318).
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
