Efficient electrocatalytic conversion of N2 to NH3 using oxygen-rich vacancy lithium niobate cubes
Shuhui Fan,Qi Wang,Yanan Hu,Qiang Zhao,Jinping Li,Guang Liu
Shanxi Key Laboratory of Gas Energy Efficient and Clean Utilization, College of Chemical Engineering and Technology, Taiyuan University of Technology, Taiyuan 030024, China
Keywords:Nitrogen reduction reaction Ammonia yield rate Oxygen vacancy LiNbO3 cubes Electronic structure
ABSTRACT Instead of the energy-intensive Haber-Bosch process,the researchers proposed a way to produce ammonia using water and nitrogen as feedstock,powered by electricity,without polluting the environment.Nevertheless,how to design efficient electrocatalyst for electrocatalytic nitrogen reduction reaction(NRR)is still urgent and challenging.Herein,a strategy is proposed to adjust the morphology and surface electronic structure of electrocatalyst by optimizing material synthesis method.LiNbO3(lithium niobate,LN)cubes with oxygen-rich vacancy and regular morphology were synthesized by hydrothermal synthesis and followed molten salt calcination process,which were used for electrocatalytic NRR under mild conditions.Compared with LN nanoparticles synthesized by solid phase reaction,LN cubes exhibit better NRR performance,with the highest ammonia yield rate(13.74 μg∙h-1∙mg-1)at the best potential of-0.45 V(vs.reversible hydrogen electrode,RHE)and the best Faradaic efficiency(85.43%)at-0.4 V.Moreover,LN cubes electrocatalyst also demonstrates high stability in 7 cycles and 18 h current–time tests.Further investigation of the reaction mechanism confirmed that the structure of oxygen vacancy could adjust the electronic structure of the electrocatalyst,which was conducive to the adsorption and activation of N2 molecule and also increased the ECSA of electrocatalyst,thus providing more active sites for the NRR process.
1.Introduction
Ammonia (NH3) is not only one of the most important basic chemical raw materials [1–4],but also an ideal hydrogen storage carrier and efficient liquid fuel[5–9].At present,industrial ammonia synthesis mainly relies on Haber-Bosch process[10–14],which generates huge energy consumption,and emits a large amount of greenhouse gases in the process of synthesis of raw hydrogen,causing serious environmental pollution [15–17].Therefore,researchers have proposed many green and sustainable ammonia synthesis technologies which are promising to replace the traditional Haber-Bosch process [18–20].Among them,electrocatalytic ammonia synthesis method can use environmental protection power generation device to supply energy,with mild reaction conditions and low energy consumption,is a promising new strategy[21–23].However,the electrocatalytic nitrogen reduction reaction(NRR) process is still facing many difficulties to reach the level of industrial production [24–26].This is mainly because,in thermodynamics,N2has a high bond energy (941 kJ∙mol-1),which requires a large amount of energy to destroy the inert non-polar molecular structure.While in kinetics,the low solubility of N2in water makes competitive hydrogen evolution reactions (HER)more likely to occur at negative potentials.Actually,to explore an efficient and stable electrocatalyst is one of the effective methods to improve the NRR performance.
At present,studies on NRR electrocatalysts mainly include Mo,Fe,Ti and other transition metal-based materials [5,27–36].The NRR activity of these materials is largely ascribed to the fact that the transition metal contains unfilled d orbital,which can accept lone pair electrons of N2and weaken the N≡N bond.But these electrocatalysts are more likely to bond with hydrogen atoms under negative potentials and also widely used in electrocatalytic HER processes.Niobium (Nb) has four single electrons and an empty d orbital and is located on the left side of the NRR volcanic diagram,which means that niobium has a better ability to bind nitrogen atoms than hydrogen atoms and has great potential for electrocatalytic NRR research.In this case,some reports about niobium series electrocatalysts have been carried out,mainly focusing on niobium oxides,such as Nb2O5[37,38],NbO2[39],Nb3O7(OH) [40] and Cu-Nb2O5@C [41].The performance of these catalysts,nevertheless,has not reached expectations,so it is necessary to modify Nb-based NRR electrocatalysts in order to improve the NRR activity.
The reported modification methods mainly include surface/interface engineering,crystal surface control and amorphous,defect engineering,etc.Among them,oxygen vacancies with many applications can adjust and modify the local coordination environment of metal center,leading to solve the potential obstacles of N2adsorption and activation on the surface of the electrocatalyst.By capturing metastable electrons in the antibonding orbital of the adsorbed nitrogen molecule,a large number of N≡N bonds can be split,which is conducive to the subsequent catalytic reaction,such as R-WO3NSs [42],BCC PdCu/C [43],np-Ovs-NiO/MoO3[44]and MoO3-x/MXene [45].
Herein,by changing the synthesis method,the morphology of LiNbO3(LN) can be effectively adjusted from severe agglomeration to regular shape of LN cubes.The resultant LN cubes with abundant oxygen vacancies can expose more reaction sites,thus leading to adsorb and activate nitrogen efficiently and demonstrate excellent NRR activities.The highest ammonia yield rate of 13.74 μg∙h-1∙mg-1can be obtained at an optimal potential of-0.45 V and the best Faradaic efficiency is 85.43% at -0.4 V,which is better than the solid-phase synthesis of LN nanoparticles(9.88 μg∙h-1∙mg-1,59.59%).
2.Experimental
2.1.Materials
Niobium pentaoxide (Nb2O5,99.99%),lithium sulfate monohydrate (Li2SO4,AR),potassium hydroxide (KOH,AR),potassium chloride (KCl,GR),sodium sulfate anhydrous (Na2SO4,99.5%),sodium hydroxide (NaOH,AR),salicylic acid (C7H6O3,AR),sodium citrate dehydrate (C6H5Na3O7∙2H2O,AR),sodium nitroferricyanide dihydrate (C5FeN6Na2O∙2H2O,99.98%),sodium hypochlorite solution (NaClO,available chlorine ≥4.00%–4.99%),ammonium chloride (NH4Cl,99.8%),hydrazine monohydrate (N2H4∙H2O,98%+),pdimethylamino-benzaldehyde (C9H11NO,99%),nessler reagent(K2HgI4),potassium sodium tartrate tetrahydrate (C4H4O6-KNa∙4H2O,AR),sodium nitrite (NaNO2,99.99%),sodium nitrate(NaNO3,AR),sulfanilic acid (C6H7NO3S,99%),phosphoric acid(H3PO4,85%(mass)),1-(1-naphthyl)ethylenediamine dihydrochloride (C12H16Cl2N2,AR),hydrogen dioxide (H2O2),ethanol (C2H5OH,≥99.9%),nafion®N-117 membrane,nafion solution (5% (mass)),N2(99.999%),Ar(99.999%).The above reagents were used without further purification.The ultra-pure water used in all experiments was purified by the Millipore system.
2.2.Preparation of the samples
2.2.1.Synthesis of LN nanoparticles
LN nanoparticles was prepared with solid phase combination method.A certain amount of Li2CO3and Nb2O5(Li:Nb=1:1)were weighted and dispersed evenly in a mortar with ethanol and ground until the ethanol volatilized completely to get the wellmixed precursor powder.Then the precursors were heated to 800 °C for 3 h at a rate of 15 °C∙min-1to get white powder and lithium niobate (LN) nanoparticles was obtained.
2.2.2.Synthesis of LN cubes
Firstly,KNbO3precursor was prepared,Nb2O5(0.092 g) and KOH solution (8.897 g KOH and 14 ml H2O) were mixed as raw materials and placed in the polytetrafluoroethylene tank and stirred for 30 min.The perovskite mineral KNbO3can be obtained after the reaction kettle is placed in an oven at 150°C for 5 days.KOH is not only a source of potassium,but also a catalyst for chemical reactions under alkaline conditions.And then,KNbO3and Li2SO4powders were fully mixed in a molar ratio of Nb:Li=1:6,placed in a clean agate mortar and ground evenly.KCl,which was the total mass of the first two,was added and ground evenly again.The mixture was reacted in a muffle furnace at 500°C for 3 h.The obtained products were washed repeatedly with deionized water and dried completely.

Fig.1. (a) Diagram of LN cubes synthesis process.(b) XRD patterns and (c) FTIR spectra for LN cubes and LN nanoparticles.(d) SEM image and (e) EDS elemental mapping images of Nb and O for LN cubes.(f) TEM image and (g) HRTEM image of LN cubes.
2.3.Characterizations
Crystal structure of the obtained samples was tested by X-ray diffraction(XRD) pattern on Bruker D8 Advance with Cu Kα radiation.Scanning electron microscopy(SEM)were conducted on Hitachi SU8010 and energy dispersive X-ray spectrometry (EDS) were recorded by IXRF SDD 3360.Transmission electron microscopy(TEM) and high-resolution transmission electron microscopy(HRTEM) were performed on JEM-2100F.X-ray photoelectron spectroscopy (XPS) analysis were acquired by VGESCALAB250 and performed using the C 1s line at 284.6 eV.Electron paramagnetic resonance (EPR) spectra were conducted on BRUKER A300.Fourier transform infrared (FT-IR) spectra were obtained on BRUKER-QUINOX-55 IR spectrophotometer.UV–vis spectra were acquired by a spectrophotometer (PerkinElmer Lambda 650).
2.4.Electrochemical measurements
Electrochemical measurements for NRR were tested by BioLogic VMP3 workstation.The NRR reactor was performed with H-type electrolytic cell which include three electrodes(working electrode,Ag/AgCl reference electrode and platinum net counter electrode)and separated by Nafion 117 membrane with 60 ml of 0.1 mol∙L-1Na2SO4electrolyte in the anode and cathode chamber,respectively.The working electrode was carbon paper loaded with 0.3 mg catalyst (loading mass density was about 0.3 mg∙cm-2).All the potentials needed to convert to the reversible hydrogen electrode with the following formula:
3.Results and Discussion
LN nanoparticles was synthesized by solid phase synthesis method and LN cubes was synthesized by hydrothermal synthesis and followed molten salt calcination process [46,47],as shown in Fig.1(a).Then,a series of physical characterization of two lithium niobate materials were carried out.Firstly,X-ray diffraction patterns of LN nanoparticles and LN cubes(Fig.1(b))were performed,and the diffraction peaks of two kinds of lithium niobate materials correspond to the standard card (JCPDS:20-0631).The diffraction peaks at 23.7°,32.7°,34.8°,40.1°,42.5°,48.5°,53.2°and 56.1°correspond to the(0 1 2),(1 0 4),(1 1 0),(1 1 3),(2 0 2),(0 2 4),(1 1 6)and(1 2 2)crystal plane of LN,respectively.However,LN nanoparticles have some relatively weak signal peaks belonging to LiNb3O8at 14.9°,21.5°,24.5°,30.3°,which could be attributed to the failure of complete contact between Nb2O5and Li2CO3in the solid phase reaction.The above phenomenon also indicates that the reaction of synthesis of LN cubes by hydrothermal-molten salt method is more complete than that of solid phase synthesis of LN nanoparticles.The characteristic peaks of Li-O bond and Nb-O bond can be detected in the FTIR spectra of LN nanoparticles and LN cubes,and the peak positions are basically not offset,as seen in Fig.1(c).Then,a series of morphologies of LN nanoparticles and LN cubes were characterized.As displayed in Fig.1(d) and Fig.S5,scanning electron microscopy (SEM) images show that the LN cubes have a more regular shape and a more dispersed distribution,compared with the obvious agglomerated lump morphology of LN nanoparticles,which implies that LN cubes could expose more active sites.In addition,from the energy dispersive X-ray spectrometry (EDS) elemental mapping diagram (Fig.1(e)),Nb and O elements are uniformly distributed.As illustrated in Fig.1(f),transmission electron microscopy (TEM) images further prove the morphology regularity of LN cubes.high-resolution transmission electron microscopy (HRTEM) image (Fig.1(g)) shows clearly visible lattice fringes,and the lattice spacing of the LN cubes is 0.266 nm,corresponding to the (1 1 0) plane of the LiNbO3cubes.
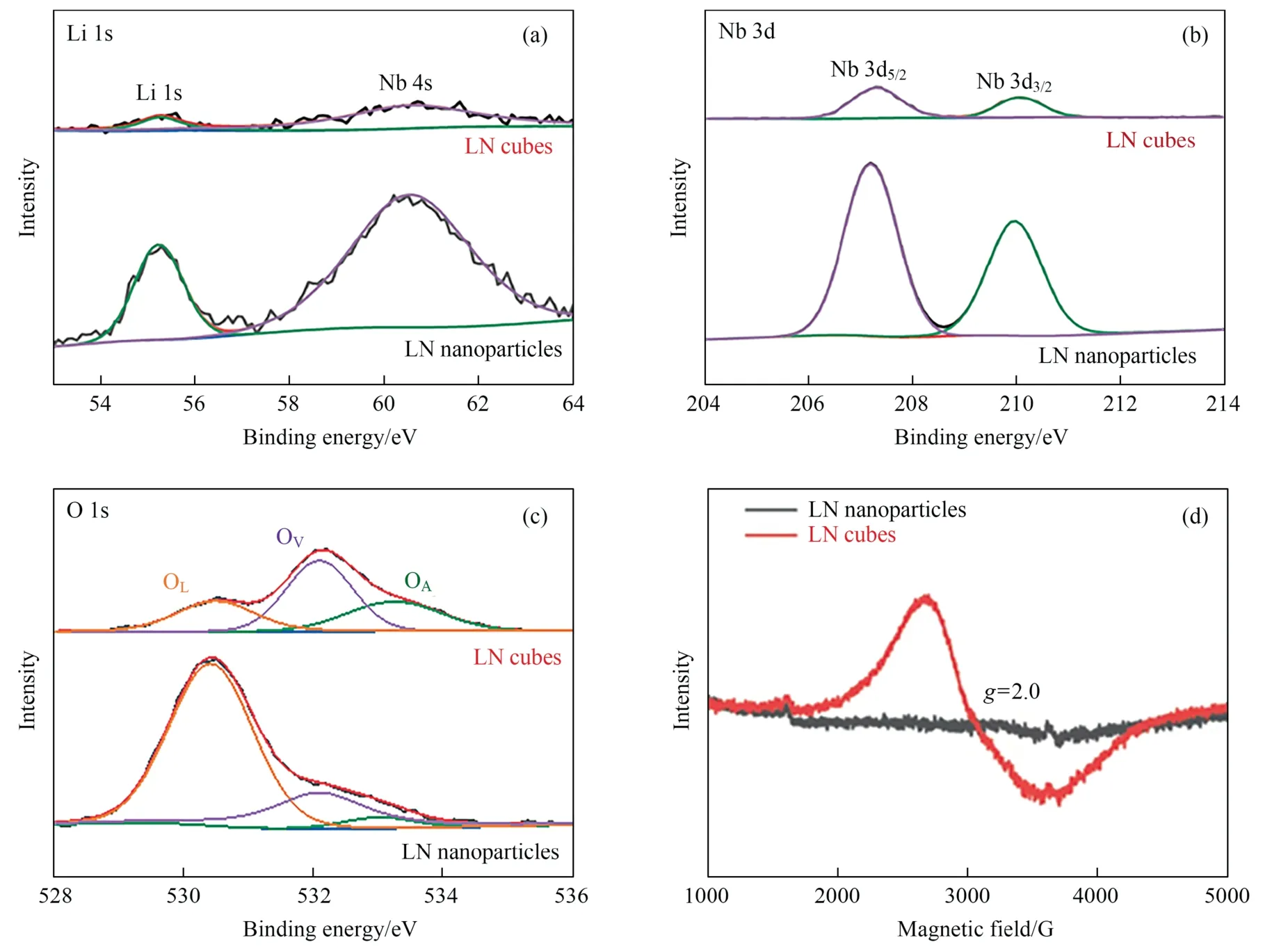
Fig.2. High-resolution XPS spectra of(a)Li 1s;(b)Nb 3d;(c)O 1s regions for LN nanoparticles and LN cubes.(d)Electron paramagnetic resonance spectra of LN nanoparticles and LN cubes.
LN cubes and LN nanoparticles were further analyzed by X-ray photoelectron spectroscopy(XPS)in order to analyze the influence of different synthesis methods on the electronic structure of lithium niobate materials.As shown in Fig.S6,similar characteristic peaks are found in the XPS survey spectra of LN nanoparticles and LN cubes.In the spectra of Li 1s region (Fig.2(a)),the characteristic peaks of Li 1s and Nb 4s corresponding at 55.15 eV and 60.52 eV can be observed.Nb 3d peaks (Fig.2(b)) located around 210.0 eV and 207.2 eV can be deconvoluted into Nb 3d3/2and Nb 3d5/2respectively,which can verify the presence of Nb5+and the valence state of Nb is not changed by different synthesis methods.In addition,as shown in Fig.2(c),it can be found that the characteristic peaks at 530.51 eV and 533.28 eV correspond to lattice oxygen species (OL) and adsorbed oxygen species (OA),respectively,while the characteristic peak at 532.1 eV corresponds to vacancy oxygen species (OV).At the same time,significant differences in oxygen species distribution in the LN cubes and LN nanoparticles were also observed.By calculating the vacancy oxygen ratio of LN nanoparticles and LN cubes respectively(Table S1),the proportion of vacancy oxygen in LN cubes are as high as 47.70%,which is much higher than that of LN nanoparticles synthesized in solid phase method(24.01%).This result indicates that the LN cubes synthesized by hydrothermal fusion salt method has abundant oxygen vacancies.Unpaired electrons in LN cubes and LN nanoparticles were determined by electron paramagnetic resonance(EPR)to further confirm the presence of oxygen vacancy(Fig.2(d)).The result indicates that the LN cubes generate a strong signal peak atg=2.0,while the LN nanoparticles do not show a signal peak.Therefore,it can be proved that the LN cubes have abundant oxygen vacancies,which is consistent with the conclusion obtained by XPS spectroscopy.LN cubes with abundant oxygen vacancies can greatly improve the ability to activate nitrogen and promote the nitrogen reduction reaction.

Fig.3. (a)LSV curves of the LN cubes in Ar-saturated and N2-saturated electrolyte.(b)I-t curves at various potentials.(c)UV–vis absorption spectra and(d)NH3 yield rate and FE of LN cubes at various potentials.
The electrocatalytic NRR activities of LN cubes were investigated in 0.1 mol∙L-1Na2SO4solution.As shown in Fig.3(a),linear sweep voltammetry (LSV) curves obtained with Ar-saturated and N2-saturated electrolyte show the difference in current density,indicating the occurrence of nitrogen reduction reactions in the potential range of -0.8–0 V.And then,the potential gradient was set in the range of -0.35 to -0.6 V for electrochemical NRR tests.The current density-time (I-t) curves of the LN cubes at various potentials can be clearly seen in Fig.3(b),with almost no attenuation within 2 h,implying the stability of NRR for LN cubes.Fig.3(c)exhibits the ultraviolet–visible light absorption spectrum(UV–vis)obtained after the reaction of Indophenol blue chromogenic agent method,suggesting the different NH3yield amounts with various potentials.As shown in Fig.3(d),ammonia yield rate and Faradaic efficiency(FE) were calculated by substituting absorbance data at 655 nm into the standard curve.The results demonstrate that the best ammonia yield rate of LN cubes electrocatalyst is 13.74 μg∙h-1∙mg-1obtained at -0.45 V and a very high FEis 85.43% obtained at -0.4 V.Its performance exceeds that of most reported transition metal-based catalysts (see Table S2).At the same time,the electrochemical NRR performance of LN nanoparticles (Fig.S7) was also tested and found that the highest ammonia yield rate of LN nanoparticles is 9.88 μg∙h-1∙mg-1at -0.45 V,and the FE is 59.59%at the same potential,which is lower than that of LN cubes (Fig.S8).This phenomenon proves that the regular morphology and abundant oxygen vacancies obtained by changing the synthesis method could improve the electrocatalytic activity of lithium niobate.In addition,the raw material(Nb2O5)and the precursor(KNbO3,KN)were also used as NRR electrocatalysts for electrochemical NRR tests (Fig.S9).At the same potential of -0.45 V,the ammonia yield rate and FE of Nb2O5are 2.12 μg∙h-1∙mg-1and 4.75%,and those of KN are 4.64 μg∙h-1∙mg-1and 2.05%,respectively.Based on the above data,it can be inferred that LN is a kind of NRR electrocatalyst with excellent catalytic performance.
Since ammonia from the environment can easily contaminate the liquid in the experiment,a series of comparative experiments need to be set up to verify the reliability of experimental results.First,the high purity N2used in the reaction was tested to see if it contained other substances (such as NOx) that might affect the experimental results,as shown in Fig.S10.In order to verify that the experiment did not contain ammonia from air,the electrolytic reaction was carried out in Ar-saturated and N2-saturated electrolyte respectively under the same conditions.The experimental results show that the ammonia product comes from NRR process.Electrolytic reaction was further carried out under open circuit voltage to prove electric energy is the driving force of the NRR process.The positive effect of electrocatalyst on NRR process can be also evidenced by using blank carbon paper without electrocatalyst as working electrode.As shown in Fig.S11,there is only weak signal in the UV–vis absorption spectra measured in the above experiment and ammonia production is close to zero.In addition,three N2/Ar alternating cycle experiments were designed to prove that the ammonia products in each experiment was generated through NRR process rather than ammonia pollution in the environment as well as residual ammonia in the last experiment.The experimental results were shown in Fig.4(a) and Fig.S12,it is obvious that the ammonia yield rate and Faradaic efficiency obtained in N2atmosphere have little change and ammonia is hardly produced in Ar atmosphere.During the experimental process,in addition to avoid possible contamination in the process of electrolysis,we should also pay more attention to the possible ammonia pollution in the chromogenic reaction process.For example,the Indophenol blue reagent used for chromogenic reaction is polluted,which leads to unreliable data.Therefore,the Nessler´s reagent was further used as another chromogenic agent for the quantitative detection of ammonia yield to play a role of mutual verification.The results demonstrate that the ammonia yield rate and Faradaic efficiency measured by the two chromogens are basically the same with each other(Fig.S13).The above results fully prove that NH3was generated in the course of experiment by the NRR process.
The stability of the electrocatalyst was characterized by repeated experiments and current–time test within 18 h.Primarily,repeated experiments were conducted with LN cubes electrocatalyst at the optimal potential of -0.45 V and the results were shown in Fig.S14 and Fig.4(b).The current density of each experiment has little change and the ammonia yield rate as well as Faradaic efficiency obtained after 7 cycles are almost unchanged,indicating the LN cubes has stable electrocatalytic NRR activities.The long-term NRR stability of LN cubes were further characterized by current–time test within 18 hours at -0.45 V.As expected,the current density is unchanged within 18 h testing period(Fig.4(c)).The content of possible by-product N2H4in the synthesis process was also detected by the Watt-Chrisp method.As shown in Fig.4(d),there is no difference in N2H4content in the electrolyte before and after electrolysis,suggesting no by-product N2H4is generated,which prove that the synthesized LN cubes has high selectivity towards NRR process.

Fig.4. (a)NH3 yield rate and Faradic efficiency of LN cubes with alternating at the interval of 2 h cycles between Ar-saturated and N2-saturated electrolytes.(b)NH3 yield rate and Faradic efficiency of repeated experiment for LN cubes.(c) 18 h stability test diagram of LN cubes.(d) UV–vis spectra of N2H4 content test of LN cubes.
Collectively,LN cubes exhibit better NRR performance towards ammonia synthesis than that of LN nanoparticles prepared by solid phase method.This can be attributed to the regular morphology and abundant oxygen vacancies of LN cubes,which make it have a larger specific surface area for electrochemical NRR activity.The electrochemical surface area (ECSA) of LN nanoparticles and LN cubes were determined by electrochemical double-layer capacitance(Cdl)measurements.As shown in Fig.S15,theCdlvalue of LN cubes (0.39 mF∙cm-2) is significantly higher than that of LN nanoparticles(0.29 mF∙cm-2),which means that LN cubes can provide more active sites than LN nanoparticles during NRR process.
4.Conclusions
In this work,LN cubes with regular morphology and rich oxygen vacancies were synthesized by hydrothermal synthesis and molten salt calcination process,which was used as an efficient NRR electrocatalyst for electrocatalytic ammonia synthesis under mild conditions.The regular morphology and uniform dispersion state could expose more active sites.The construction of oxygen vacancies could adjust the electronic structure of the LN cubes,both of which are conducive to the adsorption and activation of N2molecule and improve the NRR activity of the electrocatalyst.Electrochemical tests demonstrate that LN cubes realize the best ammonia yield (13.74 μg∙h-1∙mg-1) at -0.45 V and the highest Faradaic efficiency (85.43%) at -0.4 V,and LN cubes also exhibit good stability and selectivity for NRR.Such LN cubes electrocatalyst with regular morphology and rich oxygen vacancies for high NRR activities and selectivity may provide important reference for the development of efficient electrocatalysts towards NRR.
Data Availability
Data will be made available on request.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (22075196,21878204),Key Research and Development Program of Shanxi Province (International Cooperation,201903D421073),Research Project Supported by Shanxi Scholarship Council of China (2022-050).
Supplementary Material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cjche.2023.03.009.
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
