Investigates of substrate mingling ratio and organic loading rate of KOH pretreated corn stover and pig manure in batch and semi-continuous system: Anaerobic digestion performance and microbial characteristics
Chenyang Zhu,Ruoran Qu,Xiujin Li,,Xiaoyu Zuo,Hairong Yuan,
1 State Key Laboratory of Chemical Resource Engineering, Department of Environmental Science and Engineering, Beijing University of Chemical Technology, Beijing 100029, China
2 Sinochem Commerce Co., Ltd, Beijing 100045, China
Keywords:Substrate mingling ratio Organic loading rate Co-digestion Corn stover Pig manure Microbial community
ABSTRACT The effects of substrate mingling ratio (SMR) (1:1,1:2,1:3,3:1,and 2:1) and organic loading rate (OLR)(50–90 g total solids per liter per day) on anaerobic co-digestion performance and microbial characteristics were investigated for pig manure (PM) and pretreated/untreated corn stover in batch and semicontinuous anaerobic digestion (AD) system.The results showed that SMR and pretreatment affected co-digestion performance.The maximum cumulative methane yield of 428.5 ml∙g-1 (based on volatile solids (VS)) was obtained for PCP13,which was 35.7% and 40.0% higher than that of CSU and PM.In the first 5 days,the maximum methane yield improvement rate was 378.1%for PCP13.The daily methane yield per gram VS of PCP13 was 11.4%–18.5% higher than that of PCU13. Clostridium_sensu_stricto_1,DMER64,and Bacteroides and Methanosaeta,Methanobacterium,and Methanospirillum had higher relative abundance at the genus level.Therefore,SMR and OLR are important factor affecting the AD process,and OLR can affect methane production through volatile fatty acids.
1.Introduction
Anaerobic digestion (AD) can generate clean and renewable energy from waste biomass and is expected to be an effective,sustainable,environmental-friendly,and achieve carbon–neutral by 2060 [1].China is one of the largest planting and breeding countries in the world,with approximately 915 million tons of crop straw and 5.9 billion tons of animal manure according to 2022 statistical data from China’s National Bureau of Statistics.Corn stover(CS) and pig manure (PM) are the two most important wastes,accounting for 40.7% and 58.8% of the total waste,respectively.This waste is a good substrate for AD system.
CS is a lignocellulosic biomass with a complex and cross-linked internal structure that leads to poor hydrolysis resistance [2].The C/N ratio range of CS is 45–100[3,4],which is higher than the recommended range(20–30)for AD[5].PM is a rich N substrate,and its C/N ratio is less than 10 [6].The co-digestion of two or more types of substrates can be performed to balance the C/N ratio and overcome the disadvantages of mono-substrate in practical operation [7].The substrate mixing ratio (SMR) of co-digestion is an important parameter for improving biogas or methane yields[8].Yeet al.[9]found that the highest biogas yield of 674.4 ml∙g-1(based on volatile solids(VS))was obtained at the ratio of 0.4:1.6:1 for kitchen waste,PM,and rice straw (RS) [9].RS and cattle dung(CD) were used as co-digestion substrates at ratios of 1:1,1:1.5,and 1:2 (RS:CD,total solids (TS) ratio),and optimized biogas production (360 mlN∙(gVS)–1) was obtained at 1:1 ratio of RS and CD[10].Liet al.[11]investigated the effect of substrate ratio at ratios of 1:2,1:1,and 2:1 (RS:PM,VS ratio) on the anaerobic mesophilic co-digestion of RS and PM and noted that the optimal ratio was 1:1 with 479.0 ml∙g-1biogas yield[11].The change trend of biogas yields increased first and then decreased with increasing RS proportion.However,an opposite trend appeared in co-AD with swine manure and RS,biogas and methane production decreased with increasing RS ratios (25%,33.3%,and 50%,TS ratio) [7].According to literature,SMR is very wide-ranged,however,there is still no consensus on the optimal SMR for different substrates.
To improve biogas or methane yield in co-digestion,lignocellulosic biomass was pretreated and the organic loading rate(OLR) increased in the AD system.Pretreatment of lignocellulosic biomassisnecessaryto destroyits structureandimprovebiodegradability [12].Among the various pretreatment methods,chemical pretreatment has attracted increasing attention.NaOH,KOH,Ca(OH)2,and ammonia solution are commonly used as alkaline chemical reagents [12].Owing to the nutritional supplementation of K+to microorganisms and the selective disruption of OH–to the lignocellulose structure,KOH pretreatment is used to enhance biogas production from lignocellulose material[13].Methane yield in co-digestion of KOH-pretreated wheat straw and PM increased by 128%compared to that of the untreated group[14].Moreover,OLR is related to AD capacity,and it is one of the major factors affecting AD performance.Too low or too high OLR could lead to low AD efficiency or cause acid accumulation,resulting in instability or even irreversible failure of AD[15].Liet al.[11]found that biogas production of 413 ml∙g-1was achieved at an OLR of 3–8 g∙L–1∙d–1in continuous co-digestion of RS and PM with SMR of 1:1 and OLRs of 3.0,3.6,4.2,4.8,6.0,8.0,and 12.0 g∙L–1∙d–1,which was severely inhibited at 12.0 g∙L–1∙d–1due to the accumulation of volatile fatty acids(VFAs).However,change in the trend of methane yield was unclear at 8.0–12.0 g∙L–1∙d–1of OLR.A gradual increasing trend in the daily biogas production was observed in completely stirred tank reactor(CSTR)for co-digestion of CS and chicken manure (CM) with increasing OLRs from 65 to 100 g∙L–1∙d–1[16].The optimal OLR differed for different substrates[17].Therefore,although there are few studies on the methane potential in co-digestion of different animal manure with different crop straw under different SMR and OLR condition,there is still no consensus on the optimal SMR and OLR for the codigestion of CS and PM in literature.
It is well known that anaerobic microorganisms play an important role in the AD process.Under different OLRs,the microbial community structures are significantly different in co-digestion system of CS and CM[16].At the phyla level,Bacteroidetes,Firmicutes,Proteobacteria,Spirochaetes,Chloroflexi and Cloacimonetes were the dominant phyla at OLR of 2–8 g∙L–1∙d–1for co-digestion of CS and PM[18].Wanget al.[19]found that five phyla,Bacteroidetes,Proteobacteria,Firmicutes,Fibrobacteres and Spirochaetes appeared in CSTR of co-digestion of cow manure and CS [19].The major archaea in the AD system were Euryarchaeota,Halobacterota,and Thermoplasmata.Methanomicrobia,Methanobacteria,andThermoplasmatawere the predominant genera belonging to the Euryarchaeota phylum[20].However,bacteria and archaea differed widely under different substrates,OLR,and other conditions.
Considering the above information,the main objectives of this study were to (1) investigate the co-digestion performances of CS and PM with different SMR and pretreatments in batch experiments,(2)analyze the effect of different OLRs on co-digestion performance in the CSTR system,and (3) compare the differences between the microbial community structure at different OLRs in the CSTR system.
2.Materials and Methods
2.1.Materials and inoculum
CS came from the Shunyi District,Beijing,China.First,it was naturally air-dried in an open field,then was shredded into 4 cm lengths,and ground into a size of 0.85 mm by a hammer mill(YSW-180,Yanshan Zhengde Co,Beijing,China).The inoculum and PM were collected from a mesophilic biogas plant using PM as the AD substrate in the Shunyi District,Beijing,China.Pig hair and other impurities were removed from PM,then was put in a–20 °C refrigerator until use.The characteristics of CS,PM,and inoculum are listed in Table 1.
2.2.Experimental methods
CS was pretreated with 4% KOH (dry base of CS) at an ambient temperature ((20 ± 2) °C) for 3 days with 6 times CS (dry base TS)moisture content according to the results of the previous research[21].AD was performed in a 1 L blue cap bottle with 0.8 L working volume in batch experiments.AD substrate of 50 g∙L–1(TS) and inoculum of 15.0 g∙L–1(mixed liquid suspended solid,MLSS) were added to the digester [22].AD SMR (dry basis TS) of PM and pretreated CS were 1:1,1:2,1:3,3:1,and 2:1 and were denoted as PCP11,PCP12,PCP13,PCP31,and PCP21,respectively.Meanwhile,experiments of PM and untreated CS with the same SMR were performed to compare the pretreatment effects,and were recorded as PCU11,PCU12,PCU13,PCU31,PCU21,respectively.Moreover,only PM,untreated CS,and pretreated CS were set as the control groups and denoted as PM,CSU,and CSP,respectively.The single inoculum was used to deduct the effect of the inoculum.All the digesters were placed in a water bath incubator at (35 ± 1) °C for 50 days,and were manually shaken 2–3 times a day.Each AD experiment group was repeated three times,and the mean value of the triplicates was used as the experimental result.

Table1 Characteristics of CS,PM,and inoculum
Based on the results of the batch AD experiment,PCP13 was used for lab-scale semi-continuous AD in a CSTR.In addition,PCU13 was set as the control.The working volume of the laboratory-scale CSTR was 8 L.The temperature of CSTR was maintained at (35 ± 2) °Cviaa circulating water pump.Each CSTR was equipped with an agitator to mix the substrates at 60 r∙min-1every 2 h for 10 min.The AD time was 140 days.To better analyze the changes in the AD system,the AD process was divided into four phases in CSTR system,including start-up phase,phase I (21–60 days),phase II (61–100 days),and phase III (101–140 days).At first,different substrates were put into the CSTR at the startup phase with substrate concentration of 50 g∙L–1∙d–1,and inoculum of 20 g∙L–1(MLSS)was added to CSTR.The daily biogas production (DBP) was recorded every day.After AD for 20 days,the raw material was fed into CSTR once a day from 21 days at the phase I.OLR was 50 g∙L–1∙d–1.And then,OLR was increased to 70 (phase II) and 90 g∙L–1∙d–1(phase III) from 50 g∙L–1∙d–1(phase I).The hydraulic residence time (HRT) was 30 days,and each OLR was run for 40 days.DBP was recorded daily,and ammonia nitrogen(AN),total alkalinity concentration(TAC),and VFAs were measured every five days in all experiments.The daily methane yield(DMY),DMY per gram of VS (DMY-VS),and DMY of per L reactor volume(DMY-V) were calculated according to DBP,methane content(MC),and reactor volume.
2.3.Analytical methods
The water replacement method was used to record DBP.Biogas components were measured with a gas chromatography (GC,SP2100,Beifen,China) equipped with a 2 m × 3 mm TDX-01 column and a thermal conductivity detector (TCD).Methane production was calculated according to MC and DBP at standard conditions (101.325 kPa,0 °C).TAC,TS,VS,and MLSS were determined by the APHA standard method [23].An elemental analyzer(Vario EL/micro cube elemental analyzer,Germany) was used to analyze the total carbon (TC) and total nitrogen (TN) of CS and PM.AN was measured using a HANNA environmental testing photometer(HI83206,China).Ethanol and VFAs were analyzedviagas chromatography(GC-2014,Shimadzu,Japan),which was equipped with a flame ionization detector (FID) and a DBWAX123-70302 column (30 m × 0.25 mm × 0.25 mm).Nitrogen was used as the carrier gas.A CHN868 pH meter (3-Star,American Thermal Orion Meter)was used to measure the pH of all samples.A Fiber Analyzer(ANKOM,A2000i,USA)was used to determine the cellulose,hemicellulose,and lignin contents of CS.
2.4.Kinetic analysis and synergistic index
The modified Gompertz model[24] (Eq.(1)) was used to simulate the batch experimental data.
whereB(t),Bm,μmrefer to the simulated cumulative methane production at timet,the simulated maximum methane production at the end of AD and the maximum methane production rate(ml∙g-1∙d-1,VS),respectively;t,λ,andeindicate AD time (day),the bacterial growth lag phase time (day),and an accuracy of 2.71828,respectively.
To evaluate the synergistic effect of batch co-digestion,the synergistic effect index (SEI) was calculated by Eq.(2) [25].
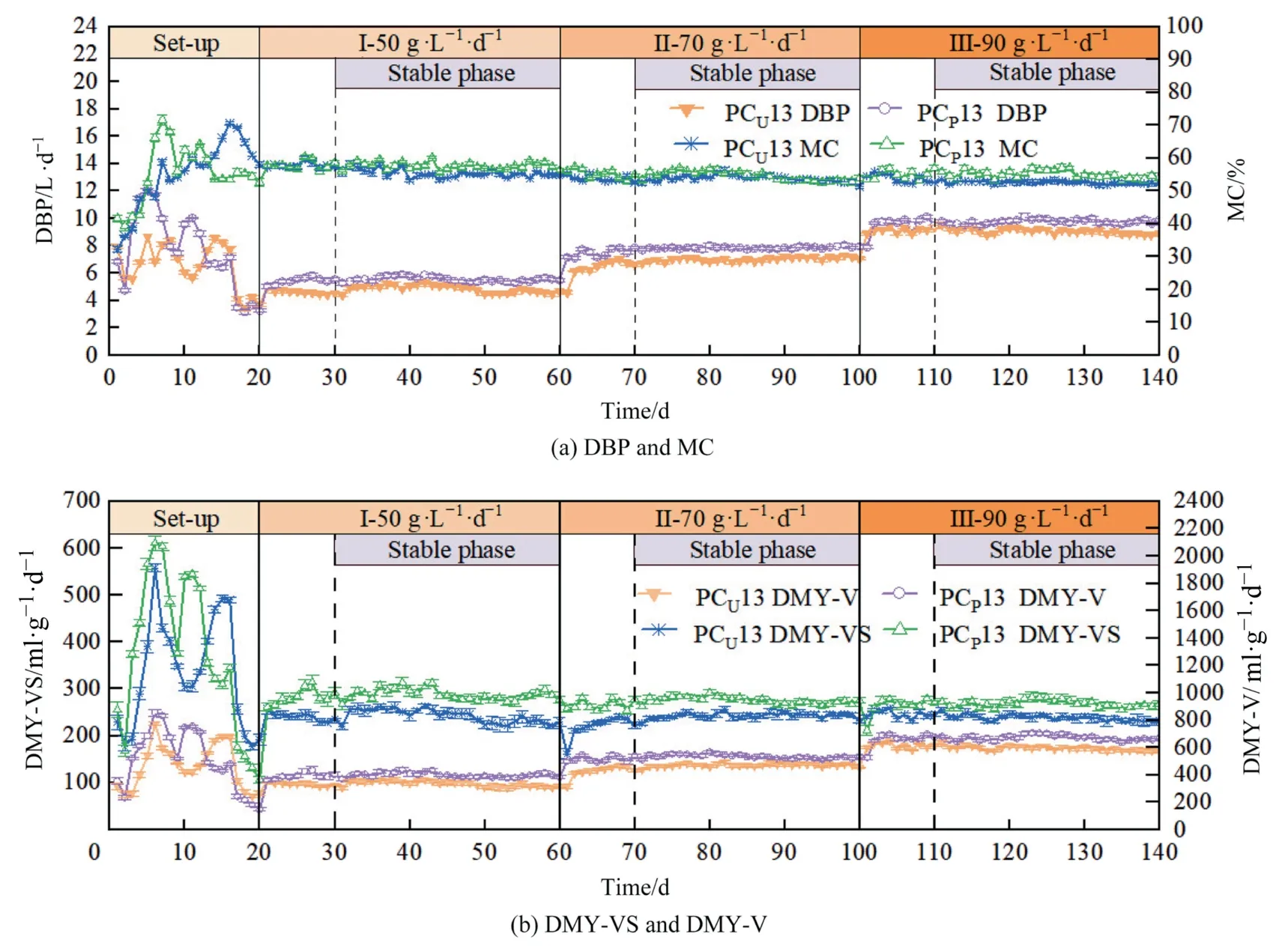
where,Gi,nandGo,iare the cumulative methane production from codigestion and the mono-digestion ofisubstrate,respectively,ml∙g-1(VS);is the weighted average and calculated based on the VS content (%VS) of the cumulative methane production of individual substrates,ml∙g-1(VS);nindicates the total quantity of substrates in the co-digestion.SEI>1 or SEI Microbial samples collected from CSTR at the end of each HRT of 50 g∙L–1∙d–1(TS,phase I) and 90 g∙L–1∙d–1(TS,phase III) for pretreated and untreated CS and PM groups on day 60 and day 140,which was named as PCP13_50,PCU13_50,PCP13_90,and PCU13_90,respectively.The samples were detected by Shanghai Meiji Biopharmaceutical Technology Co.,Ltd.Microbial DNA was extracted by FastDNASpin kit (MP Biologics,USA),and real-time PCR was performed by ABIGeneAmp®9700 (ABI,USA) and TransGenAP221-02 (China) and AxyPrepDNA gel extraction Kit(Axygen,USA).The primers of 338F (5′-ACTCCTACGGGAGGCAGC AG-3′),806R (5′-GGACTACHVGGGTWTCTAAT-3′) for bacteria and 524F_10_ext (5′-TGYCAGCCGCCGCGGTAA-3′) and Arch 958R(5′-YCCGGCGTTGAVTCCAATT-3′) for archaea were used to recognize bacteria and archaea in this study,respectively.The highthroughput 16S rRNA gene sequencing was fulfilled by Illumina MiSeq platform (Illumina Company).The microbial data were analyzedviathe Major Bio Cloud online Platform. The data processing and statistical differences were analyzed using Microsoft Excel 2019.The modified Gompertz model was nonlinear fitted and the graphs were drawn by Origin 2021.All data in this paper are plotted using Origin 2021 software.Statistically significant values are indicated by symbols:*,**,and***,indicatingP≤0.05,0.01,and 0.001,respectively. 3.1.1.Methane production DMP of the different experimental groups displayed 2–4 peaks value(Fig.1(a),(b))during the entire AD period.DMP peak value of co-digestion were 21.6–30.1 ml∙g-1(VS) and 17.5–22.6 ml∙g-1for pretreated groups and untreated groups,which were 3.2%–7.9%,2.9%–32.9%,and 9.4%–88.1% higher than those of CSP,CSU,and PM,respectively.For pretreated groups,the first DMP peak value reached 15.3,12.3,18.4 ml∙g-1for PCP21,PCP31,and PCP13 at day 2,respectively.The maximum DMP value were 27.9,30.1,26.3,21.6,23.1,and 28.8 ml∙g-1for CSP,PCP11,PCP21,PCP31,PCP13,and PCP12,which were 4.1%–32.0% higher than those of untreated groups.The maximum DMP of PCP11(30.1 ml∙g-1) was 4.5%–39.4% higher than that of other pretreated group.The time of highest DMP peak appeared on the days 5–8,which was 1–3 days earlier than that of the untreated group.The DMP peak value of pretreated groups were higher than those of the untreated groups.This was attributed to the fact that the complex lignocellulosic structure of CS was disrupted after pretreatment,the easily degradable soluble small molecules were released from cellulose and hemicellulose [26].In the co-digestion of CS and PM groups,the change trends of DMP in the first 10 days was similar,possibly because the C/N ratio of CS(46.2) and PM (8.6) (Table 1) were not within suitable range (20–30) for AD [5].The co-digestion of CS and PM can balance the C/N ration.The second DMP peak appeared on day 11–20 for PCPgroups.In the PCUgroups,the appeared time of the second DMP peak was delayed. Cumulative methane yields (CMY) are listed in Fig.1(c) and Fig.1(d).During the first 35 days,CMY increased rapidly in the pretreated group,and the trend of CMY flattened from day 36 to day 50.Similar trends were observed in the untreated groups,however,the change time of CMY was 4–5 days later than that of the pretreated groups.For pretreated groups,CMY of co-digestion with different SMR of PM and CS were 394.7–428.5 ml∙g-1,which were 13.9%–23.7% and 31.7%–43.0% higher than those of CSPand PM,respectively,and were 10.8%–14.3% and 25.0%–35.7% higher than those of co-digestion of untreated groups and CSUgroup (315.7 ml∙g-1),respectively.The maximum CMY of 428.5 ml∙g-1was obtained for PCP13,which was 67.0% higher than of co-digestion with PM and CS (VS ratio 7:3) [27].A similar CMY improvement rate of 11.4%–59% compared to the control was obtained for codigestion of cattle manure and rape straw with pretreatment of H2SO4and steam explosion at 180 °C for 5 min [28].This was attributed to the that pretreated CS was more easily digested by microorganisms.Moreover,the C/N ratio(25.8) was more suitable for AD than that of PM and CS according to data of Table 1 for codigestion of PM and CS(1:3).Therefore,CMY was higher in the codigestion group after pretreatment. T90as an indicator of AD biodegradability efficiency,is defined as the time required to reach 90% of the total methane production during AD.T90of the pretreated groups were significantly shorter than those of the untreated groups(Fig.1(c,d)).T90was 30–32 days for pretreated group of co-digestion,which was reduced by 8.6%–14.3% compared to PM (35 days) and CSP(30 days),which was reduced by 11.1%–16.7% compared to CSU(36 days).This result was similar to the findings of Buragohainet al.[10].These results indicate that SMR of co-digestion and pretreatment can improve biodegradability of the substrate,reduce the overall digestion time,and improve the efficiency of the AD process. Fig.1. Methane productions of different experiment groups. 3.1.2.Kinetic analysis The kinetic parameters of the different experimental groups fitted using the modified Gompertz model are listed in Table 2.Compared with the experimentally measured methane yield(Bexp),the differences between the predictedBmvalues (Table 2) were 1.2%–7.1%,revealing that theBmandBexpvalues were very close,and the modified Gompertz model was suitable to fit CMY of codigestion and single substrate.The μmvalues of pretreated and untreated groups were 10.0–16.4 ml∙g-1∙d-1(VS) and 10.0–14.4 ml∙g-1∙d-1,which were very close to the actual DMP peak values of 16.0–30.1 ml∙g-1∙d-1and 16.0–22.6 ml∙g-1∙d-1,respectively.The lag phase time of AD was λ,implying that the microbial community needs time to adapt in the digester[29].At the same PM:CS mixing ratio condition,λ value of the pretreated group was lower than that of untreated group,indicating that the pretreatment can increase the production methane speed.Moreover,compared with the pretreated control group (5.4 d and 2.0 d),λ of the codigestion groups decreased by 66.7%–92.6% and 10.0%–80.0%,respectively,indicating co-digestion of PM and CS obviously accelerate the digestion speed.More specifically,co-digestion groups with PM:CS ratio of 1:3 achieved the shortest λ value of 0.4 d,which is consistent with the experimental results.The correlation coefficient factors (R2) were in the range of 0.9958–0.9989,which indicated that the modified Gompertz model could accurately simulate the AD process. Table2 Kinetic parameters of different experimental groups 3.1.3.Synergistic effects Fig.2. The SEI and IRMY. To evaluate the synergistic effect of co-digestion of PM and CS,SEI and improved rate of methane yield(IRMY)are shown in Fig.2.SEI and IRMY of the pretreated groups (Fig.2(a)) were obviously higher than those of the untreated groups(Fig.2(b)).SEI of the pretreated groups increased from 1.2 (day 1) to 4.9 (day 5) and then rapidly reduced to 1.3 from day 6 to day 10,which was 35.3%–75.0% higher than that of the untreated group in the first 10 days,and SEI was 1.2–1.3 from day 11 to day 50.This indicates that codigestion of PM and CS had positive synergistic effects.Similar trends were observed in IRMY.The maximum IRMY values were 282.3%,186.2%,134.2%,387.1%,and 336.2% for PCP11,PCP21,PCP31,PCP13 and PCP12,respectively,which were 88.2%–165.5%higher than those of the untreated groups.Similarly,the synergistic effect (SEI=4.42) was reported in the first 10 days of codigestion of CM and pretreated CS with the liquid fraction of digestate in a previous study[30].Therefore,co-digestion of PM and CS can adjust the C/N ratio to enhance methane yieldviaC/N balance and synergistic interactions [31].This was due to the C/N ratio of different SMR groups was 11.9–25.8 according to C/N ratio of single substrate (Table 1),and the C/N ratio after blending is more suitable for AD.In conclusion,in the batch AD experiment,the optimal SMR of PM and CS was 1:3 (PCP13) for pretreated CS. 3.2.1.Effects of OLR on biogas and methane yield Fig.3. DBP,MC,DMY and system stability under different OLRs. DBP and MC are two important parameters to reflect methane production capacity in CSTR.DBP and MC of different OLRs are shown in Fig.3(a).An increased trend of DBP was observed with increasing OLRs.The change range of DBP were 5.0–5.9,7.1–8.0,and 7.9–10.1 L∙d–1for 50,70,and 90 g∙L–1∙d–1(TS)of PCP13,respectively.The average DBP of PCP13 were 5.5,7.8,and 9.7 L/d for 50,70,and 90 g∙L–1∙d–1(TS),which were 14.8%,13.1%,and 6.9%higher than those of PCU13 at the same OLRs conditions,respectively.This indicates that pretreatment can effectively improve biogas production of co-digestion system.The average MC of PCP13 were 57.7%,54.7%,and 55.1%for 50,70,and 90 g∙L–1∙d–1(TS),which were 5.9%,3.4%,and 8.3% higher than those of the PCU13 at the same OLRs,respectively.Therefore,MC can be improved by pretreatment during the co-digestion process.Under different OLRs,DMY-V showed similar trends with DBP.The DMY-V of PCP13 appeared a continuously ascending trend (Fig.3(b)).DMY-V of PCP13 were 397.9,530.6,and 668.8 ml∙g-1∙L-1∙d-1(TS)for 50,70,and 90 g∙L–1∙d–1(TS),respectively.Conversely,DMY-VS of PCP13 gradually decreased with increasing OLRs.At OLRs of 50,70 and 90 gTS∙L–1∙d–1,DMYVS of PCP13 were 286.8,273.2,and 267.8 ml∙g-1∙d-1(VS),which were 18.5%,15.2%,and 11.4%higher than those of the PCU13,respectively.This is because under high OLRs conditions,the microbial growth speed cannot sustain the increase of organic matter,resulting in more organic matter that cannot be completely degraded.A similar phenomenon appeared in the co-digestion of CM and KOH pretreated CS at OLRs of 65,80,and 100 ml∙g-1∙d-1(TS)[16]. 3.2.2.Effects of OLRs on system stability The pH,AN,TAC,and VFAs of system stability parameters are important indicators affecting AD performances [1].The system stability parameters are presented in Fig.3(c).The appropriate range of pH in AD system is 6.5–7.8 [32].The pH of PCP13 were 7.14–7.29,7.01–7.20,and 7.24–7.34 for 50,70,and 90 g∙L–1∙d–1(TS)of OLRs,which were 1.4%–2.7%,1.1%–3.2%,and 1.4%–2.5% higher than of those of PCU13,respectively.This indicated that 50–90 g∙L–1∙d–1of OLRs for PCP13 and PCU13 AD system were all within the optimal range of pH.In general,AN concentration of a stable AD system was 50–1500 mg∙L–1[33].AN concentration of PCP13 and PCU13 were 200–570 and 160–660 mg∙L–1at different OLRs,which did not exceed the range,implying that the AD system was stable.Simultaneously,similar change trends occurred in TAC with pH.TAC of 4235–5660 and 2550–4575 mg∙L–1for PCP13 and PCU13 were within suitable buffering capacity[34].Studies have reported that AD system appeared severely inhibition to methanogen activity when VFAs exceeded 6000 mg∙L–1[35].VFAs concentrations of PCP13 was 536.4–690.3 mg∙L–1at different OLRs conditions,which was 0.2%–16.1%lower than that of PCU13.This result indicated that substrates in the AD system were very well digested,and the AD efficiency of pretreated group was higher than that of the untreated group.More specifically,the concentration of iso-butyric acid of PCP13 and PCU13 were 360.6–373.5 mg∙L–1and 365.0–373.3 mg∙L–1,accounting for 52.2%–68.5%and 47.0%–63.6%for total VFAs(TVFAs)in the AD system.Acetic acid concentration accounted for 8.8%–13.0% and 5.2%–10.7% of TVFA.Acetic and butyric acids are more easily converted to methane by methanogens [36].TVFAs/TAC is a more reliable parameter for evaluating system stability than VFAs and TA[37].According to literature,the system can operate stably without the risk of acidification when TVFAs/TAC <0.4,however,when TVFAs/TAC >0.8,the system collapses due to the accumulation of organic acids [16].The TVFAs/TAC of PCP13 and PCU13 were 0.10–0.14 and 0.15–0.24,which was below 0.4,indicating that the AD system is stable. 3.3.1.Bacterial and archaeal communities To further investigate AD differences at different OLRs,the microbial community structures of different OLRs samples were analyzed (Fig.4).For bacteria,12 phyla were detected for PCP13 and PCU13 at OLRs of 50 and 90 g∙L–1∙d–1(TS) (Fig.4(a)).The dominant bacteria phyla were Firmicutes,Bacteroidetes,Synergistetes,and Proteobacteria,which accounted for 22.2%–84.0%,8.7%–49.1%,1.6%–5.8%,and 1.0%–5.4% for PCP13_50,PCU13_50,PCP13_90,and PCU13_90.At the phylum level,the relative abundance of bacteria did not differ between the pretreatment and untreated groups under the same OLRs conditions,however,there were significant differences between different OLRs.The relative abundance of Firmicutes for different experimental groups at OLR of 90 g∙L–1∙d–1were 73.6%–70.8% lower than those of 90 g∙L–1∙d–1.Firmicutes are a typical acid-forming bacteria that are widespread in AD systems.They can utilize organics to produce organic acids,and maintain system stability [38,39].Bacteroidetes can degrade the macromolecular organic matter of saccharides,chitin,and proteins,and produce organic acidsviathe conversion of macromolecules into monosaccharides [40,41].The relative abundance of Bacteroidetes for different experimental groups at OLR of 50 g∙L–1∙d–1were 74.2%–82.3%lower than those of 90 g∙L–1∙d–1,which was consistent with the positive effect on DBP and DMY at the higher OLR of 90 g∙L–1∙d–1. Fig.4. Bacterial and archaeal communities at phylum and genus levels. At the genus level,bacteria species diversity in PCP13 and PCU13 at 90 g∙L–1∙d–1was higher than that at 50 g∙L–1∙d–1(Fig.4(b)).The relative abundance ofClostridium_sensu_stricto_1,DMER64,andBacteroideswere the dominant genera.The relative abundance ofClostridium_sensu_stricto_1andTerrisporobacterwere 49.0%,45.5%,and 12.5%,11.9% for PCU13_50 and PCP13_50,and its decreased to 2.0%,5.1% and 0.9%,1.8% when OLR increased from 50 g∙L–1∙d–1to 90 g∙L–1∙d–1,respectively.Clostridium_sensu_stricto_1andTerrisporobacterare hydrolytic acidified bacteria that generate VFAs and hydrogen [42,43].The relative abundances ofDER64and norank_f_Bacteroidales_UCG_001were 17.0%and 15.4%at PCP13_90,however,Bacteroideshad the highest relative abundance of 22.3%in PCU13_90.This indicated that these bacteria were more easily enriched under high-load conditions.Meanwhile,pretreatment also affected the bacterial composition,resulting in changes in microbial diversity.This phenomenon was similar to the co-digestion of CS and CM in CSTR at different OLRs and pretreatment conditions [16]. Fig.5. Co-occurrence network and correlation between AD performance parameters and microbial community. In OLRs of 50 g∙L–1∙d–1and 90 g∙L–1∙d–1AD system,Euryarchaeota and Crenarchaeota were the major archaeal communities of the two phyla(Fig.4(c)).The relative abundance was 91.8%–99.0% and 1.0%–7.3% for Euryarchaeota and Crenarchaeota at the phylum level,respectively.The abundance of Euryarchaeota was above 90%in the four archaea samples.Methanosaeta,Methanobacterium,andMethanospirillumwere also observed at the genus levels (Fig.4(d)).The relative abundances ofMethanosaetawere 74.0%,89.6%,32.5%,and 56.4%for PCU13_50,PCP13_50,PCU13_90,and PCp13_90,respectively.Methanosaetais a methanogenic species that utilizes only acetate[44].Methanosaetashowed the highest relative abundance,indicating that acetic acid was the main metabolic pathway in CSTR.This was similar to the findings of co-digestion using cattle manure and liquid fraction of digestate pretreated CS [45].The second highest relative abundance of archaea wasMethanobacterium,which were 18.5%,8.2%,27.2%,and 24.4% for PCU13_50,PCP13_50,PCU13_90,and PCp13_90,respectively.The relative abundance ofMethanobacteriumin AD system of 90 g∙L–1∙d–1was higher than that of 50 g∙L–1∙d–1.Methanobacteriumare hydrogenotrophic methanogens and can use H2/CO2conversion to methane [37].This indicated that methane yield was primarily derived from acetic acid and H2/CO2during hydrolysis and acidification process.Thus,the methane yield of the pretreatment group was higher than that of the untreated group because the combined action ofMethanosaetaandMethanobacteriumconverted the hydrolyzed acidification products into methane at higher OLRs. 3.3.2.Co-occurrence network and correlation between AD parameters and microbial community The co-occurrence and interaction patterns of the bacterial communities at the genus level are shown in Fig.5(a).The operational taxonomic unit (OTU) represents a species sequence at 97% sequence identity.A total of 189 OTUs positive and 47 OTUs negative cases were surveyed among significant correlated 236 OTUs.A positive feature represents the possibility of extensive interactions between bacterial assemblages.At the genus level,the cluster tightly combined in the different samples.There were 37.0%,31.6%,16.4%,and 15.0% OTUs associated with PCP13_90,PCP13_50,PCU13_90,and PCU13_50,respectively.There were thirty-five OTUs species related to all four samples,accounting for 9.9% of the total OTUs species.Degree centrality were 0.73,0.71,0.67,and 0.61 for PCP13_90,PCP13_50,PCU13_90,and PCU13_50,respectively,indicating that higher OLRs and pretreatment had positive effects on microbial abundance and diversity,which can also promote AD efficiency.Therefore,the OTUs identified by network analysis were significant in enhancing AD efficiency [46]. To further clarify the correlation between AD performance and microbial community structure under different OLRs,Pearson correlation analysis(PCA)was performed at the genus level using the online Major Bio Cloud Platform (Fig.5(b)).Clostridium_sensu_stricto_1(R=0.990**),Terrisporobacter(R=0.992**),Romboutsia(R=0.996**),andTuricibacter(R=0.985*) were strongly and positively correlated with AN.Conversely,Brooklawnia(R=–0.999***) andMethanomassiliicoccus(R=–0.951*) were negatively correlated with AN.Norank_f__Bacteroidales_UCG-001(R=-0.785) and norank_f__Synergistaceae(R=–0.978*) were negatively correlated with TVFAs.Ruminiclostridium(R=0.668),Turicibacter(R=0.435),andMethanosaeta(R=0.715) were positively correlated with DMY-VS.These bacteria and archaea accounted for a large proportion in the high OLR system,which indicated that the high load system was relatively stable.This further suggested that OLR was an important factor affecting the AD process,it can affect methane production through VFAs. SMR of co-digestion and pretreatment can improve biodegradability of the substrate,reduce AD time,and improve the efficiency of the AD process.The highest CMY of 428.5 ml∙g–1(VS) was obtained for PCP13.T90was reduced by 9.4%–12.5%.The maximum IRMY of 378.1% was obtained for PCP13 in the first 5 days.DMY-V of PCP13 was 397.9–668.8 ml∙g–1∙L–1∙d–1(VS) for 50–90 g∙L–1∙d–1(TS).DMY-VS of PCP13 was 11.4%–18.5% higher than that of PCU13.Clostridium_sensu_stricto_1,DMER64,BacteroidesandMethanosaeta,Methanobacterium,Methanospirillum had higher relative abundance at the genus level.OLRs are an important factor affecting the process of AD and can affect methane production through VFAs. Data Availability The data that has been used is confidential. Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. Acknowledgements The authors are grateful to the fund supports from the Fundamental Research Funds for the Central Universities (JD2326).2.5.Microbial community analysis
2.6.Statistical analysis
3.Results and Discussion
3.1.AD performances of the batch experiment



3.2.AD performances in the CSTR system
3.3.Microbial characteristics


4.Conclusions
 Chinese Journal of Chemical Engineering2023年10期
Chinese Journal of Chemical Engineering2023年10期
- Chinese Journal of Chemical Engineering的其它文章
- High catalytic performance of CuCe/Ti for CO oxidation and the role of TiO2
- Experimental and numerical studies of Ca(OH)2/CaO dehydration process in a fixed-bed reactor for thermochemical energy storage
- Volumetric and ultrasonic properties of thiamine hydrochloride drug in aqueous solutions of choline-based deep eutectic solvents at different temperatures
- Synthesis of zeolite A and zeolite X from electrolytic manganese residue,its characterization and performance for the removal of Cd2+ from wastewater
- Metal-organic framework-derived Co-C catalyst for the selective hydrogenation of cinnamaldehyde to cinnamic alcohol
- A pseudo transient nonequilibrium method for rigorous simulation of multicomponent separation columns
