园艺作物果实苹果酸代谢与转运及其调控研究进展
陈雷 齐希梁 石彩云 董媛鑫 宋露露 刘聪利 李明

摘 要:番茄、苹果、梨、枣等园艺作物是典型的苹果酸型果实,其果实酸度主要取决于液泡中苹果酸的積累量。苹果酸不仅决定果实的风味品质,还可作为呼吸底物为植物体提供必需的物质和能量,在调节植物细胞渗透势、酸碱平衡、抗逆性等方面起着重要作用。苹果酸代谢途径比较复杂,涉及众多结构催化酶的参与,而苹果酸主要贮存于液泡中,从细胞质向液泡的跨膜运输和储存是复杂的生物学过程,需要多种转运蛋白、质子泵的参与。总结了苹果酸型果实酸度性状遗传研究、转运蛋白及质子泵在苹果酸跨膜转运中的作用,并将转录因子对苹果酸的代谢调控进行了概述,以深入理解苹果酸代谢调控网络,为园艺作物品质育种提供理论基础。
关键词:园艺作物;果实;苹果酸;代谢;质子泵;转运蛋白;调控
中图分类号:S66 文献标志码:A 文章编号:1009-9980(2023)12-2598-12
收稿日期:2023-07-10 接受日期:2023-09-25
基金项目:国家自然科学基金青年基金项目(3210180675);中国农业科学院创新工程专项经费(CAAS-ASTIP-2023-ZFRI)
作者简介:陈雷,男,在读硕士研究生,研究方向为果树遗传育种。E-mail:1416744170@qq.com
*通信作者 Author for correspondence. E-mail:liming06@caas.cn;E-mail:liucongli@caas.cn
Advances in research of malate metabolism and regulation in fruit of horticultural crops
CHEN Lei, QI Xiliang, SHI Caiyun, DONG Yuanxin, SONG Lulu, LIU Congli*, LI Ming*
(Zhengzhou Fruit Research Institute, CAAS, Zhengzhou 450009, Henan, China)
Abstract: Acidity is an important part of the sensory quality of fruit. Malic acid is the main organic acid in ripe fruits of tomatoes, apples, pears, and jujubes. Malic acid not only determines fruit acidity and quality but also has multiple important functions in the plant. Malate is well known as a key intermediate in the tricarboxylic acid (TCA) cycle and is imported into mitochondria as a respiratory substrate. Malate also participates the glyoxalate cycle pathway and is closely related to plant primary metabolism, carbon cycling, and carbohydrate accumulation. Malate plays an important role in regulating the osmotic potential, pH balance, and stress resistance in horticultural crops. Thus, it is of important theoretical significance and practical value for high-quality breeding programs as well as the study of the mechanism underlying malic acid biosynthesis and transport in fruits. Malic acid is synthesized in the cytoplasm, accumulated in the vacuole during the early stages of fruit development, and used as a respiratory substrate during fruit ripening. Malate accumulation is affected by synthesis, transport, and metabolism, and involves the participation of numerous catalytic enzymes. Malate metabolism is a complex biological system influenced not only by genetic factors but also by environmental factors, agronomic practices, and post-harvest treatments. In the cytoplasm of fruit, glycogen is converted to phosphoenolpyruvate (PEP) through the glycolytic pathway. PEP is carboxylated by phosphoenolpyruvate carboxylase (PEPC) to produce oxaloacetate (OAA), which is the first step of malic acid synthesis. Then, malate synthesis is catalyzed by cytosolic NAD-dependent malate dehydrogenase (cyMDH) and cytosolic NADP-dependent malic enzyme (cyME). The cyMDH is a key enzyme involved in malate synthesis and catalyzes the conversion reaction from OAA to malate, while cyME is an important malate-degrading enzyme that catalyzes the conversion of malate to pyruvate in the cytoplasm. In addition, malate accumulation is regulated by transmembrane transport between the vacuole and cytoplasm. The transmembrane transport of malic acid requires not only a proton pump to provide energy but also the assistance of channel proteins or transmembrane transporters. The main vacuolar transporters, such as the tonoplast-localized malate transporter (tDT) and aluminum-activated malate transporter (ALMT), participate in the transmembrane transport and accumulation of malate in the fruit. Among the ALMT family members, ALMT9 is the most widely studied gene. Apple Ma1 gene is a key malate transporter responsible for differences in malic acid content between wild and cultivated fruits. SlALMT9 is considered to be responsible for variation in malate content in the fruit among tomato genotypes. VvALMT9, a homolog of AtALMT9 in grapes, is a vacuolar malate channel that mediates the accumulation of malate and tartrate in the vacuoles of grape berries. Tonoplast proton pumps such as vacuolar-type H+-ATPase (V-ATPase, VHA), vacuolar-type H+-pumping pyrophosphatase (V-PPase, VHP), and P-ATPase (PHA) generate the driving force for vacuolar acidification by transporting protons across the membrane into the vacuole. In petunia flowers, the P-type proton pump genes PhPH1 and PhPH5 interact with each other and form a complex to promote vacuolar acidification. MdPH1 and MdPH5, homologs of PhPH1 and PhPH5 in apples, have been identified and shown to be involved in vacuolar acidification and malate accumulation. Another P-type proton pump gene Ma10 in apples was found to be significantly correlated with malic acid accumulation, explaining about 8% of the variation in fruit acidity phenotypes in natural apple populations. Increasing evidences showed that transcription factors, such as MYB, bHLH, WRKY, and ERF family members, participate in the regulation of malate transporters and proton pumps. In apples, MdMYB1, MdMYB44, and MdMYB73 regulate malate accumulation and vacuolar acidification in fruits by activating or repressing the promoter activities of the malate transporter and proton pump genes. Apart from MYB transcription factors, other transcription factors, such as bHLH and WRKY, are also involved in the regulation of malic acid accumulation and vacuolar acidification. In petunia, AN1 (bHLH transcription factor) can form a complex with AN11-PH4 to positively regulate vacuolar acidification and thus affects pH. In apples, MdbHLH3, a homolog of AN1 regulates malate accumulation in fruit by directly activating the expression of the malate dehydrogenase gene MdcyMDH. MdbHLH3 forms a complex with MdMYB1 to promote pulp anthocyanin and malate accumulation. In tomatoes, SlWRKY42 directly binds to the promoter of SlALMT9, repressing its transcription, and thereby inhibiting malate accumulation in tomato fruit. ZjWRKY7 transcription factor activates the transcription of ZjALMT4 by the W-box region of the high-acidity genotype in sour jujube, thereby promoting malate accumulation, whereas the binding ability was weakened in jujube. This paper summarizes the mechanism of malate accumulation in horticultural crops, such as tomato, apple, pear, and jujube, and provides an overview of the role of transporters, proton pumps, and upstream transcription factors responsible for malate accumulation and vacuolar acidification, which will provide a theoretical basis for quality breeding in horticultural crops.
Key words: Horticultural crops; Fruit; Malate; Metabolism; Proton pump; Transporter protein; Regulation
有機酸是影响园艺作物果实风味品质的重要因素,番茄、苹果、梨、枣等园艺作物属于苹果酸型果实,苹果酸是成熟果实有机酸的主要成分,其果实酸度主要取决于液泡中苹果酸的积累量。苹果酸不但决定着果实的风味品质,同时作为呼吸代谢底物参与到细胞质的糖酵解、线粒体中三羧酸循环(TCA)、乙醛酸循环等过程,为植物体提供能量物质[1-2]。此外,苹果酸对果实花青苷具有共色作用,可以通过增强花青苷的稳定性影响果实色泽形成[3-4]。目前研究表明,液泡膜苹果酸转运蛋白与质子泵对苹果酸跨液泡膜转运起重要作用[5-7]。苹果酸转运蛋白主要负责苹果酸的跨液泡膜转运[8];而质子泵将H+转运到液泡内,促使液泡内外形成较大的pH梯度和电化学梯度,为苹果酸跨液泡膜运输提供动力[9]。笔者结合前人研究,从果实酸度遗传学研究、苹果酸合成降解途径、苹果酸转运蛋白和质子泵类型及功能、转录调控因子等方面进行总结,阐述苹果酸代谢转运机制,对园艺作物优质品种的选育具有重要理论意义与应用价值。
1 植物苹果酸功能
苹果酸主要以苹果酸酯的形式广泛存在植物体中,分布于根、茎、叶、果实等多种组织器官,不但决定果实风味和品质,同时作为呼吸代谢的底物参与细胞质的糖酵解、线粒体中三羧酸循环(TCA)、乙醛酸循环等过程,为植物体提供能量物质。此外,苹果酸还参与植物体内pH平衡、植物细胞渗透势调节等代谢过程[1-2]。植物根系分泌的苹果酸,可以解除铝离子的毒害作用,促进对营养成分的吸收,为根际土壤微生物提供良好的环境[10]。在苹果和枣中研究发现,野生型果实苹果酸含量显著高于栽培型品种,这种现象可能是自然选择的结果,果实中较高的酸度水平能够帮助植物抵御外界不良环境而生存下来[7,10-12]。
2 园艺作物果实苹果酸遗传学水平研究进展
在园艺作物中,果实酸度属于数量性状遗传,受自身和外界等多种因素的影响,其中遗传因素是影响果实酸度的重要因素。以桃、苹果、番茄和杏为代表的苹果酸型果实在有关果实酸度的遗传研究中表明,果实酸度由多个基因协同控制,遗传机制较为复杂。控制桃果实酸度的主效基因位于第5号染色体顶端,又命名为D位点,且低酸为显性性状[13];Wang等[14]结合全基因组关联分析和BSA-seq技术发现第5号染色体上存在调控桃果实有机酸积累的主效基因PpTST1。在苹果中,多数研究者一致认为苹果果实酸含量由一对主效基因(Ma/ma)和其他多基因控制,位于16号染色体顶端的Ma位点是控制苹果成熟果实酸度的主效QTL,其中编码铝诱导的苹果酸转运蛋白的Ma1基因是主效基因,且相对于ma1具有不完全显性特征,显性纯合体Ma1/Ma1为高酸,杂合体Ma1/ma1表现为中酸,在同一基因型内株系间表现出连续性酸度变异,则是多基因控制的结果[5,15-16];除Ma1基因外,在多个遗传群体中均检测到位于8号染色体的另一个主效QTL位点,其中编码P型质子泵的Ma10基因对果实苹果酸的积累起重要作用[17]。Sauvage等[18]利用163份番茄种质中的19种主要代谢物和5995个SNPs进行mGWAS研究,发现第6号染色体的SNP位点与果实苹果酸含量相关。Ye等[6]结合全基因组关联分析和BSA技术进一步证实第6号染色体上存在调控果实苹果酸的主效基因SlALMT9,该基因与苹果Ma1基因具有较高的同源性。Dondini等[19]基于F1群体进行QTL定位,发现杏果实酸度是多基因控制的数量性状,在4、5、6、7、8号染色体上均检测到QTL位点。
综上所述,苹果酸型果实酸度有两种遗传方式:一种是主效基因控制的数量性状,高酸/中酸/低酸由一对主效基因控制,低酸性状的显隐性因树种而异;另一种是多基因控制的数量性状,多数品种杂交后代果实酸度性状表现出连续变异。
3 园艺作物果实苹果酸合成与降解
在果实发育前期,苹果酸在细胞质中合成,在液泡中积累;在果实发育后期,苹果酸从液泡中释放出来,在细胞质中降解。苹果酸代谢途径比较复杂,涉及众多酶参与(图1):叶片经光合作用制造的光合产物,通过韧皮部运输到果实。在果实细胞质中,糖通过糖酵解途径生成磷酸烯醇式丙酮酸(PEP),PEP经磷酸烯醇式丙酮酸羧化酶(PEPC)羧化后生成草酰乙酸(OAA),这是苹果酸合成第一步,OAA在NAD-苹果酸脱氢酶(NAD-cyMDH)的催化下形成苹果酸[20]。部分苹果酸被转运到液泡中储存,形成果实风味品质的重要组成因素。PEP和NAD-cyMDH是苹果酸合成的关键酶[21-22]。对不同酸度类型的桃[23]、苹果[24]、枇杷[25]、杏[26]果实分析发现,PEPC虽然催化苹果酸的合成,但其表达量和酶活性与果实中苹果酸含量没有显著相关性,在苹果愈伤和番茄中超表达MdcyMDH会导致苹果酸含量显著增加,同时诱导苹果酸代谢相关基因上调表达,表明MdcyMDH直接参与苹果酸合成[27]。
在果实成熟后期,部分苹果酸通过跨膜转运从液泡释放出来,在细胞质内降解后重新合成PEP。降解过程关键酶包括磷酸烯醇式丙酮酸羧激酶(PEPCK)和苹果酸酶(NADP-cytME)。一方面,苹果酸可以通过NADP-cytME催化脱羧形成丙酮酸,再由丙酮酸正磷酸盐二激酶(PPDK)反向催化生成PEP;另一方面,苹果酸还可以通过NAD-cyMDH反向转化为OAA,然后在PEPCK的作用下生成PEP。PEP是糖酵解和糖异生作用的中间产物,当果肉细胞内没有足够的葡萄糖进行糖酵解时,PEP可在果糖1,6-二磷酸酶和葡萄糖激酶等的作用下反向合成葡萄糖,实现果实苹果酸向可溶性糖的转变,此转变过程通过糖异生途径来实现[28-30]。
4 园艺作物果实苹果酸转运
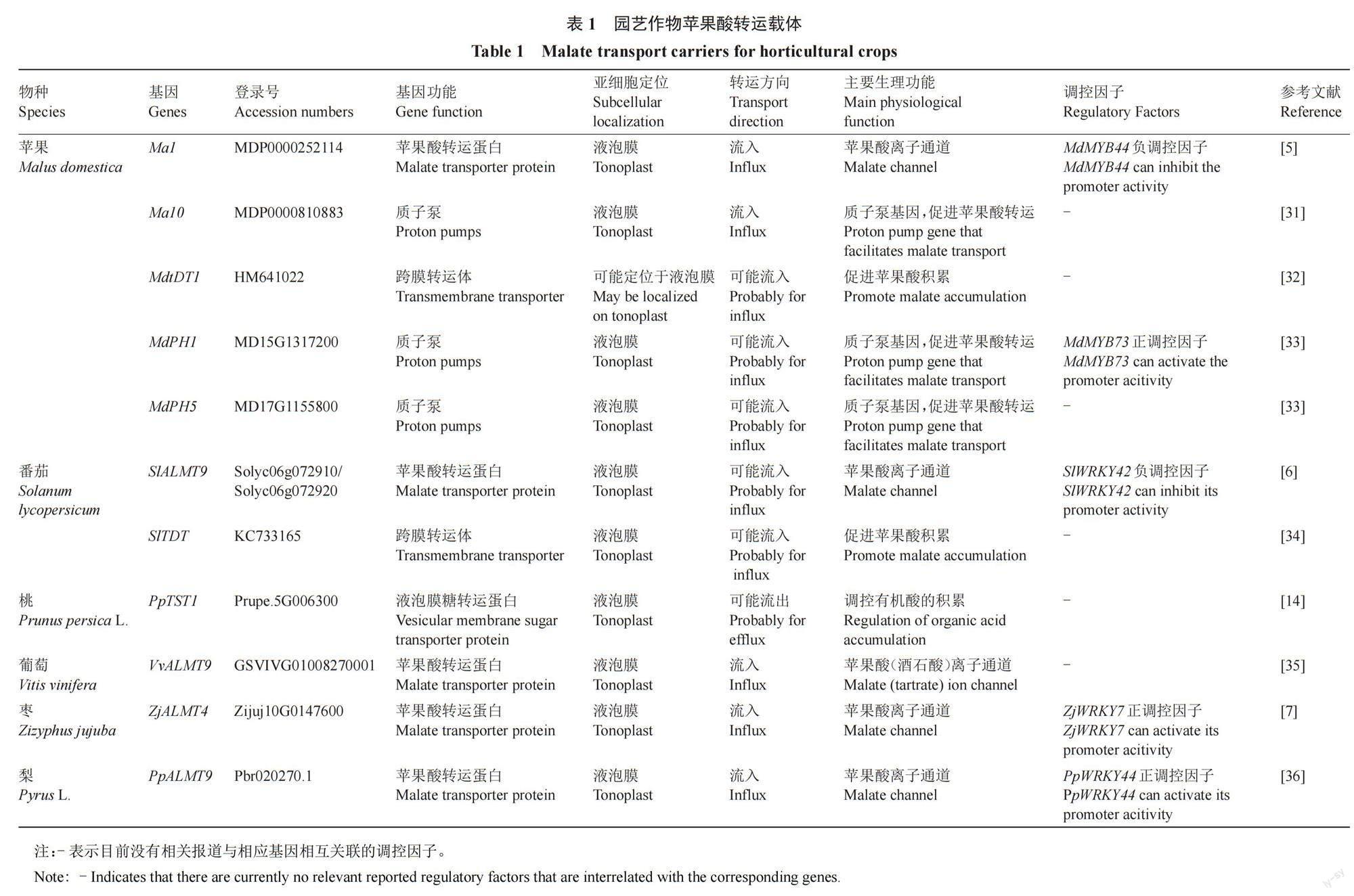
苹果酸主要贮存于液泡中,液泡中的跨膜转运与果实酸度密切相关,迄今为止,控制果实苹果酸含量的关键基因多为苹果酸跨膜转运相关基因。苹果酸的跨膜转运不仅需要质子泵来提供能量,还需要苹果酸转运蛋白和离子通道蛋白的协助,目前研究较多的是液泡膜二羧酸转运蛋白(tonoplast dicarboxylate transporter,tDT/TDT)和铝诱导的苹果酸转运蛋白(aluminum-activated malate transporter,ALMT)(表1)。
4.1 苹果酸转运蛋白
拟南芥液泡膜二羧酸转运蛋白(AttDT)是最早发现的一类具有苹果酸转运特性的蛋白,定位于液泡膜上,主要参与苹果酸在液泡和细胞质之间的跨膜转运,还参与调节植物细胞pH动态平衡[37]。现已在番茄(SlTDT)[34]、柑橘(CsCit1)[38]、梨(PbrTDT1)[39]等园艺作物中克隆到AttDT的同源基因。在拟南芥中过表达AttDT显著提高了叶片苹果酸含量,但降低了柠檬酸含量[40]。在番茄中同源过表达番茄SlTDT和异源过表达梨PbrTDT1后显著提高了番茄果实苹果酸含量,但降低了柠檬酸含量,说明其与拟南芥AttDT具有相似功能[34,39]。柑橘CsCit1则属于柠檬酸/H+同向转运载体,主要介导柠檬酸从液泡流出[38]。
4.2 苹果酸离子通道蛋白
铝诱导的苹果酸转运蛋白(ALMT)是普遍存在于植物体内的一类阴离子通道蛋白,其部分成员能够参与到苹果酸跨膜转运[6,15,41]。拟南芥ALMT家族被分为3个亚家族,其中ALMTⅡ家族成员是一类位于液泡膜上、具有苹果酸盐转运功能的通道蛋白,已经发现参与苹果酸转运的成员有AtALMT6和AtALMT9[42-43],其中ALMT9是发现最早且被广泛研究的液泡膜苹果酸通道蛋白[44]。在葡萄中,AtALMT9同源基因VvALMT9被证明可以调控果实苹果酸和酒石酸积累[35]。在苹果中,控制果实酸度的主效候选基因Ma1编码ALMT,其编码框尾端单碱基G突变为A时,翻译提前终止,少了84个氨基酸,造成编码的蛋白质不完整,丧失苹果酸转运功能,不利于有机酸积累,导致低酸性状形成[41]。番茄中控制果实酸度的主要候选基因SlALMT9同样编码ALMT蛋白。SlALMT9基因启动子区GTC插入/缺失与自然群体中番茄果实苹果酸含量完全连锁[6]。枣中导致果实苹果酸含量自然变异的主要候选基因ZjALMT4编码ALMT蛋白。ZjALMT4基因启动子区W-box元件中存在SNP位点,在高酸型酸枣中,ZjWRKY7转录因子与ZjALMT4启动子W-box元件相结合,正向调控其转录,促进苹果酸积累;而栽培枣中W-box位点突变后降低ZjWRKY7与之结合的能力,导致苹果酸积累减少。在栽培枣长期驯化过程中低酸突变基因型被选择固定下来[7]。番茄SlALMT9基因、葡萄VvALMT9基因、蘋果Ma1基因与枣ZjALMT4基因序列同源性较高,表明园艺作物果实苹果酸代谢调控具有一定的保守性。
4.3 其他参与苹果酸转运的蛋白
除tDT和ALMT两种苹果酸转运蛋白之外,最近一个编码液泡膜糖转运蛋白的PpTST1基因在桃中被证明与果实酸度有关。前人研究表明,液泡膜糖转运蛋白TST是负责细胞质葡萄糖向液泡的跨膜运输,部分成员还具备蔗糖转运功能[45-46]。我国科学家通过全基因组关联分析确定了控制桃果实非酸/酸含量的关键基因PpTST1,该基因第三个外显子区的单碱基突变被证实与桃果实有机酸含量连锁。在桃和番茄中超量表达PpTST1His导致果实总糖含量增加、有机酸含量减少,同时导致苹果酸转运相关基因下调表达,表明PpTST1具备参与桃果实有机酸和糖积累的双重功能[14]。
4.4 质子泵对苹果酸跨膜转运的作用
质子泵在液泡积累有机酸的过程中起着重要作用。目前植物中与酸度有关的是位于液泡膜上的V型[V-H+-ATPase(VHA)和V-H+-PPase(VHP)]和P型质子泵[P-H+-ATPase(PHA)][47-51]。
4.4.1 V型质子泵 V型质子泵VHA和VHP分别通过水解ATP或PPi产生能量,可将H+从细胞质转运到液泡致使液泡酸化,也能够为次级转运蛋白的跨膜运输提供能量[9,52]。VHA结构较为复杂,是由10多个不同亚基组成的复合物,而VHP仅由单一多肽组成[48,53]。然而,关于VHA和VHP在苹果酸积累方面的研究较少。Hu等[54]在苹果中过表达VHA亚基MdVHA-B1发现果实苹果酸含量升高,盐胁迫处理诱导MdVHA-B1蛋白磷酸化从而驱动苹果酸向液泡运输致使果肉细胞苹果酸含量升高[55]。Yao等[52]发现MdVHP1过表达显著促进转基因苹果愈伤组织与番茄果实中Na和苹果酸盐积累。Krebs等[56]研究表明拟南芥VHA突变株的叶片pH显著高于野生型,同时突变VHA和VHP后突变体叶片pH显著升高,说明在拟南芥中VHA和VHP共同调控着有机酸的积累,值得注意的是,在同时缺乏V-ATPase和V-PPase活性的突变体中,液泡仍保留着酸化能力,这说明可能还有其他因素参与液泡有机酸的积累[57]。
4.4.2 P型质子泵 P型质子泵是另外一类参与质子转运和液泡酸化的质子泵家族,其中P3亚家族主要参与维持液泡内外的pH平衡和提供跨膜运输驱动力[58-59]。位于拟南芥细胞膜上的P型质子泵基因AHA10最先被证实参与液泡形成和酸化过程[60]。在矮牵牛花中,Faraco等[61]证实质子泵基因PhPH1和PhPH5可以相互作用形成复合体,对液泡中有机酸的积累有调控作用,使花瓣呈现不同的颜色;苹果中PhPH1和PhPH5同源基因MdPH1和MdPH5可能参与了液泡酸化和苹果酸积累[33],研究还发现苹果中另外一个P型质子泵基因Ma10表达量与苹果酸的积累显著相关,可解释苹果自然群体果实酸度8%左右的表型变异[31];此外,在柠檬酸型果实柑橘中也发现了类似矮牵牛的酸度调控机制,定位于液泡膜上的P型质子泵基因CitPH1和CitPH5/CsPH8对液泡中酸的积累有调控作用[62-64]。拟南芥AHA10基因、矮牵牛PhPH5基因、苹果Ma10基因与柑橘CsPH8基因序列同源性较高,表明液泡膜上P型质子泵基因在参与有机酸积累调控方面具有一定的保守性。
5 园艺作物果实苹果酸代谢和转运的调控
5.1 转录因子对园艺作物果实苹果酸代谢和转运的调控
越来越多研究表明苹果酸转运蛋白和质子泵相关基因的表达受到MYB、bHLH、WRKY多种转录因子的调控[6,21,33,48,65-68]。
5.1.1 R2R3-MYB转录因子 在苹果中,R2R3-MYB转录因子MdMYB1/10、MdMYB44和MdMYB73通过直接调控液泡膜苹果酸转运蛋白和质子泵基因表达来调控果实苹果酸积累和液泡酸化[33,66-67]。其中MdMYB1/10和MdMYB73是正调控因子,而MdMYB44是负调控因子,它们分别作用于不同的下游基因。MdMYB1直接结合并激活质子泵基因MdVHA-B1、MdVHA-B2、MdVHA-E和MdVHP1表达,促进苹果酸在液泡中积累[66]。MdMYB73直接激活下游质子泵基因MdVHA-A、MdVHP1和苹果酸转运蛋白MdALMT9,从而促进果实液泡的酸化[33]。MdMYB44通过抑制Ma1、MdVHA-A3、MdVHA-D2、Ma10和MaALMT9启动子活性,负调控苹果果实苹果酸积累,MdMYB44启动子区2个遗传变异位点被证实与苹果果实苹果酸含量显著相关[66]。同时,MYB转录因子还可以与WD40蛋白和bHLH转录因子形成MBW蛋白复合体,通过直接结合苹果酸转运蛋白相关基因和液泡型质子泵基因启动子,转录激活或抑制其表达,最终影响苹果酸含量[33,66-67]。
5.1.2 bHLH和WRKY转录因子 近年,除了MYB转录因子外,bHLH和WRKY转录因子在有机酸代谢中的功能也被发掘[6-7,36,62,69-70]。在矮牵牛中,AN1(bHLH转录因子)可以与AN11-PH4形成复合体正向调节液泡酸化,从而影响pH[47,71]。在苹果中,AN1的同源基因MdbHLH3可以直接激活苹果酸脱氢酶基因MdcyMDH表达,促进果实苹果酸积累,也可以与MdMYB1形成复合体,促进果肉花青素和苹果酸积累[21,67]。在柑橘中,AN1的同源基因CitAN1可以与CitPH4形成复合体直接激活P型质子泵基因CitPH1和CitPH5表达,CitAN1基因突变会导致柑橘果实酸度降低[62]。在拟南芥中,AtWRKY46转录因子通过负调控AtALMT1基因表达,调控苹果酸跨膜转运[72]。在矮牵牛中,编码WRKY的PH3基因能够被AN11-AN1-PH4复合物诱导转录,通过形成PH3-AN11-AN1-PH4复合物,诱导P型质子泵基因PhPH5转录从而控制液泡酸度[60]。在番茄中,SlWRKY42转录因子通过结合W-box元件來负调控SlALMT9表达,抑制番茄果实苹果酸积累[6]。在枣中,ZjWRKY7转录因子通过结合W-box元件正调控ZjALMT4表达,促进酸枣果实苹果酸积累,而栽培枣中ZjWRKY7与ZjALMT4的结合能力较弱[7]。
5.2 外界环境和栽培条件对园艺作物果实苹果酸的调控
园艺作物果实苹果酸的积累受很多因素的影响,包括温度、水分、光照、矿物营养及土壤盐分胁迫等[20,73]。温度是影响果实苹果酸积累和代谢的关键因素,其对园艺作物果实苹果酸含量的影响因树种而异,在桃、苹果等果实发育或者贮藏期间环境温度升高导致果实苹果酸含量降低,而草莓果实发育过程暴露在较高温度下果实苹果酸含量升高[74-77];在葡萄和猕猴桃中研究发现,温度对果实苹果酸积累的影响因发育时期而异,果实发育前期暴露在较高温度下果实苹果酸含量升高,但在果实发育后期暴露在较高温度下果实苹果酸含量减少[78-80]。水分是影响果实苹果酸代谢的另一关键因子。研究表明,在大多数情况下,果实发育过程中水分供应量与成熟果实苹果酸含量呈负相关[81]。在苹果、葡萄等果实发育过程中,适度干旱胁迫会提高果实中可溶性糖和苹果酸含量[2,82-83]。光照度与苹果酸积累关系密切,在蓝莓、葡萄、苹果等研究中发现,光周期延长或光照增强会降低果实苹果酸含量,如苹果成熟时树冠上部和外围的果实酸度较低[84-86],葡萄套袋后造成的弱光胁迫抑制苹果酸降解,导致果实苹果酸含量增加[87]。适当增施Ca、P、K肥可降低果实苹果酸含量,而微量元素如铁、铜的缺乏同样也能够使果实酸度升高[88-89]。
综上所述,园艺作物果实苹果酸代谢是一个复杂的过程,外部环境条件对果实苹果酸含量的影响错综复杂,因此,园艺作物果实苹果酸的含量受自身遗传因素、环境条件和栽培条件的共同影响。
6 总结与展望
通过数十年遗传学研究和多组学技术的应用,目前在以番茄、苹果、梨、枣等为代表的苹果酸型果实园艺作物中研究发现,果实酸含量是一种数量性状,受多种因素(自身和外界)的影响,而遗传因素是影响果实酸度的重要因素,其中位于液泡膜上的苹果酸转运蛋白与质子泵对苹果酸跨液泡膜转运起到重要作用,苹果酸转运蛋白和质子泵相关基因的转录水平受到多种转录因子的调控。综合已有的研究内容,未来研究领域重点可以集中在以下几个方面:(1)利用正向遗传学与反向遗传学研究相结合的技术手段开展果实苹果酸代谢基因挖掘及功能分析;(2)开发果实酸度性状相关分子标记,用于分子标记辅助育种;(3)挖掘调控苹果酸代谢的转录因子,解析苹果酸代谢机制;(4)研究环境因子(如温度、水分、光照等)对苹果酸代谢和转运的调控机制。
参考文献 References:
[1] FERNIE A R,CARRARI F,SWEETLOVE L J. Respiratory metabolism:Glycolysis,the TCA cycle and mitochondrial electron transport[J]. Current Opinion in Plant Biology,2004,7(3):254-261.
[2] SWEETMAN C,DELUC L G,CRAMER G R,FORD C M,SOOLE K L. Regulation of malate metabolism in grape berry and other developing fruits[J]. Phytochemistry,2009,70(11/12):1329-1344.
[3] CAI D B,LI X S,CHEN J L,JIANG X W,MA X Q,SUN J X,TIAN L M,VIDYARTHI S K,XU J W,PAN Z L,BAI W B. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors[J]. Food Chemistry,2022,366:130611.
[4] LV X R,LI L L,LU X M,WANG W X,SUN J F,LIU Y Q,MU J L,MA Q Y,WANG J. Effects of organic acids on color intensification,thermodynamics,and copigmentation interactions with anthocyanins[J]. Food Chemistry,2022,396:133691.
[5] MA B Q,LIAO L,ZHENG H Y,CHEN J,WU B H,OGUTU C,LI S H,KORBAN S S,HAN Y P. Genes encoding aluminum-activated malate transporter II and their association with fruit acidity in apple[J]. The Plant Genome,2015,8(3):1-14.
[6] YE J,WANG X,HU T X,ZHANG F X,WANG B,LI C X,YANG T X,LI H X,LU Y E,GIOVANNONI J J,ZHANG Y Y,YE Z B. An InDel in the promoter of Al-ACTIVATED MALATE TRANSPORTER9 selected during tomato domestication determines fruit malate contents and aluminum tolerance[J]. The Plant Cell,2017,29(9):2249-2268.
[7] ZHANG C M,GENG Y Q,LIU H X,WU M J,BI J X,WANG Z T,DONG X C,LI X G. Low-acidity ALUMINUM-DEPENDENT MALATE TRANSPORTER4 genotype determines malate content in cultivated jujube[J]. Plant Physiology,2023,191(1):414-427.
[8] SHIRATAKE K,MARTINOIA E. Transporters in fruit vacuoles[J]. Plant Biotechnology,2007,24(1):127-133.
[9] MARTINOIA E,MAESHIMA M,NEUHAUS H E. Vacuolar transporters and their essential role in plant metabolism[J]. Journal of Experimental Botany,2007,58(1):83-102.
[10] FERNIE A R,MARTINOIA E. Malate. Jack of all trades or master of a few?[J]. Phytochemistry,2009,70(7):828-832.
[11] 馬百全. 苹果资源果实糖酸性状评估及酸度性状的候选基因关联分析[D]. 武汉:中国科学院武汉植物园,2016.
MA Baiquan. Comparative assessment of sugar and acid characteristics and candidate gene assocaiton analysis for acidity in apple germplasm[D]. Wuhan:Wuhan Botanical Garden,Chinese Academy of Sciences,2016.
[12] 刘孟军,王玖瑞. 新中国果树科学研究70年:枣[J]. 果树学报,2019,36(10):1369-1381.
LIU Mengjun,WANG Jiurui. Fruit scientific research in New China in the past 70 years:Chinese jujube[J]. Journal of Fruit Science,2019,36(10):1369-1381.
[13] CAO K,LI Y,DENG C H,GARDINER S E,ZHU G R,FANG W C,CHEN C W,WANG X W,WANG L R. Comparative population genomics identified genomic regions and candidate genes associated with fruit domestication traits in peach[J]. Plant Biotechnology Journal,2019,17(10):1954-1970.
[14] WANG Q,CAO K,CHENG L L,LI Y,GUO J,YANG X W,WANG J,KHAN I A,ZHU G R,FANG W C,CHEN C W,WANG X W,WU J L,XU Q,WANG L R. Multi-omics approaches identify a key gene,PpTST1,for organic acid accumulation in peach[J]. Horticulture Research,2022,9:uhac026.
[15] BAI Y,DOUGHERTY L,LI M J,FAZIO G,CHENG L L,XU K N. A natural mutation-led truncation in one of the two aluminum-activated malate transporter-like genes at the Ma locus is associated with low fruit acidity in apple[J]. Molecular Genetics and Genomics,2012,287(8):663-678.
[16] JIA D J,SHEN F,WANG Y,WU T,XU X F,ZHANG X Z,HAN Z H. Apple fruit acidity is genetically diversified by natural variations in three hierarchical epistatic genes:MdSAUR37,MdPP2CH and MdALMTII[J]. The Plant Journal,2018,95(3):427-443.
[17] MA B Q,ZHAO S,WU B H,WANG D M,PENG Q,OWITI A,FANG T,LIAO L,OGUTU C,KORBAN S S,LI S H,HAN Y P. Construction of a high density linkage map and its application in the identification of QTLs for soluble sugar and organic acid components in apple[J]. Tree Genetics & Genomes,2016,12(1):1-10.
[18] SAUVAGE C,SEGURA V,BAUCHET G,STEVENS R,DO P T,NIKOLOSKI Z,FERNIE A R,CAUSSE M. Genome-wide association in tomato reveals 44 candidate loci for fruit metabolic traits[J]. Plant Physiology,2014,165(3):1120-1132.
[19] DONDINI L,DOMENICHINI C,DONG Y H,GENNARI F,BASSI D,FOSCHI S,LAMA M,ADAMI M,DE FRANCESCHI P,CERVELLATI C,BERGONZONI L,ALESSANDRI S,TARTARINI S. Quantitative trait loci mapping and identification of candidate genes linked to fruit acidity in apricot (Prunus armeniaca L.)[J]. Frontiers in Plant Science,2022,13:838370.
[20] ETIENNE A,G?NARD M,LOBIT P,MBEGUI?-A-MB?GUI? D,BUGAUD C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells[J]. Journal of Experimental Botany,2013,64(6):1451-1469.
[21] YU J Q,GU K D,SUN C H,ZHANG Q Y,WANG J H,MA F F,YOU C X,HU D G,HAO Y J. The apple bHLH transcription factor MdbHLH3 functions in determining the fruit carbohydrates and malate[J]. Plant Biotechnology Journal,2021,19(2):285-299.
[22] BER?TER J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation[J]. Journal of Plant Physiology,2004,161(9):1011-1029.
[23] MOING A,ROTHAN C,SVANELLA L,JUST D,DIAKOU P,RAYMOND P,GAUDILL?RE J P,MONET R. Role of phosphoenolpyruvate carboxylase in organic acid accumulation during peach fruit development[J]. Physiologia Plantarum,2000,108(1):1-10.
[24] YAO Y X,LI M,LIU Z,YOU C X,WANG D M,ZHAI H,HAO Y J. Molecular cloning of three malic acid related genes MdPEPC,MdVHA-A,MdcyME and their expression analysis in apple fruits[J]. Scientia Horticulturae,2009,122(3):404-408.
[25] CHEN F X,LIU X H,CHEN L S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya japonica Lindl.) cultivars differing in fruit acidity[J]. Food Chemistry,2009,114(2):657-664.
[26] 陳美霞,赵从凯,陈学森,郝会军,张宪省. 杏果实发育过程中有机酸积累与相关代谢酶的关系[J]. 果树学报,2009,26(4):471-474.
CHEN Meixia,ZHAO Congkai,CHEN Xuesen,HAO Huijun,ZHANG Xiansheng. Relationship between accumulation of organic acid and organic acid-metabolizing enzymes during apricot fruit development[J]. Journal of Fruit Science,2009,26(4):471-474
[27] YAO Y X,LI M,ZHAI H,YOU C X,HAO Y J. Isolation and characterization of an apple cytosolic malate dehydrogenase gene reveal its function in malate synthesis[J]. Journal of Plant Physiology,2011,168(5):474-480.
[28] WU W F,CHEN F X. Malate transportation and accumulation in fruit cell[J]. Endocytobiosis and Cell Research,2016,27(2):107-112.
[29] FAIT A,HANHINEVA K,BELEGGIA R,DAI N,ROGACHEV I,NIKIFOROVA V J,FERNIE A R,AHARONI A. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development[J]. Plant Physiology,2008,148(2):730-750.
[30] KATZ E,BOO K H,KIM H Y,EIGENHEER R A,PHINNEY B S,SHULAEV V,NEGRE-ZAKHAROV F,SADKA A,BLUMWALD E. Label-free shotgun proteomics and metabolite analysis reveal a significant metabolic shift during citrus fruit development[J]. Journal of Experimental Botany,2011,62(15):5367-5384.
[31] MA B Q,LIAO L,FANG T,PENG Q,OGUTU C,ZHOU H,MA F W,HAN Y P. A Ma10 gene encoding P-type ATPase is involved in fruit organic acid accumulation in apple[J]. Plant Biotechnology Journal,2019,17(3):674-686.
[32] 张燕子. 不同苹果糖酸组成及苹果酸转运体功能研究[D]. 杨凌:西北农林科技大学,2010.
ZHANG Yanzi. Carbohydrates and organic acids composition of different apple genotypes & the role of malate transporter[D]. Yangling:Northwest A & F University,2010.
[33] HU D G,LI Y Y,ZHANG Q Y,LI M,SUN C H,YU J Q,HAO Y J. The R2R3-MYB transcription factor MdMYB73 is involved in malate accumulation and vacuolar acidification in apple[J]. The Plant Journal,2017,91(3):443-454.
[34] LIU R L,LI B Q,QIN G Z,ZHANG Z Q,TIAN S P. Identification and functional characterization of a tonoplast dicarboxylate transporter in tomato (Solanum lycopersicum)[J]. Frontiers in Plant Science,2017,8:186.
[35] DE ANGELI A,BAETZ U,FRANCISCO R,ZHANG J B,CHAVES M M,REGALADO A. The vacuolar channel VvALMT9 mediates malate and tartrate accumulation in berries of Vitis vinifera[J]. Planta,2013,238(2):283-291.
[36] ALABD A,CHENG H Y,AHMAD M,WU X Y,PENG L,WANG L,YANG S L,BAI S L,NI J B,TENG Y W. Abre-binding factor 3-wrky dna-binding protein 44 module promotes salinity-induced malate accumulation in pear[J]. Plant Physiology,2023,192(3):1982-1996.
[37] HURTH M A,SUH S J,KRETZSCHMAR T,GEIS T,BREGANTE M,GAMBALE F,MARTINOIA E,NEUHAUS H E. Impaired pH homeostasis in Arabidopsis lacking the vacuolar dicarboxylate transporter and analysis of carboxylic acid transport across the tonoplast[J]. Plant Physiology,2005,137(3):901-910.
[38] SHIMADA T,NAKANO R,SHULAEV V,SADKA A,BLUMWALD E. Vacuolar citrate/H+ symporter of citrus juice cells[J]. Planta,2006,224(2):472-480.
[39] 許林林. 梨液泡膜上PbrALMT9,PbrTDT1和PbrVHA-c4基因调控有机酸积累的功能研究[D]. 南京:南京农业大学,2018.
XU Linlin. Functional analysis of tonoplast-localized genes,PbrALMT9,PbrTDT1 and PbrVHA-c4,regulate the accumulation of organic acids in pear[D]. Nanjing:Nanjing Agricultural University,2018.
[40] FREI B,EISENACH C,MARTINOIA E,HUSSEIN S,CHEN X Z,ARRIVAULT S,NEUHAUS H E. Purification and functional characterization of the vacuolar malate transporter tDT from Arabidopsis[J]. Journal of Biological Chemistry,2018,293(11):4180-4190.
[41] LI C L,DOUGHERTY L,COLUCCIO A E,MENG D,EL-SHARKAWY I,BOREJSZA-WYSOCKA E,LIANG D,PI?EROS M A,XU K N,CHENG L L. Apple ALMT9 requires a conserved C-terminal domain for malate transport underlying fruit acidity[J]. Plant Physiology,2020,182(2):992-1006.
[42] MEYER S,SCHOLZ-STARKE J,DE ANGELI A,KOVERMANN P,BURLA B,GAMBALE F,MARTINOIA E. Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation[J]. The Plant Journal,2011,67(2):247-257.
[43] KOVERMANN P,MEYER S,H?RTENSTEINER S,PICCO C,SCHOLZ-STARKE J,RAVERA S,LEE Y,MARTINOIA E. The Arabidopsis vacuolar malate channel is a member of the ALMT family[J]. The Plant Journal,2007,52(6):1169-1180.
[44] DE ANGELI A,ZHANG J B,MEYER S,MARTINOIA E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis[J]. Nature Communications,2013,4:1804.
[45] SCHULZ A,BEYHL D,MARTEN I,WORMIT A,NEUHAUS E,POSCHET G,B?TTNER M,SCHNEIDER S,SAUER N,HEDRICH R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2[J]. The Plant Journal,2011,68(1):129-136.
[46] CHENG R,CHENG Y S,L? J H,CHEN J Q,WANG Y Z,ZHANG S L,ZHANG H P. The gene PbTMT4 from pear (Pyrus bretschneideri) mediates vacuolar sugar transport and strongly affects sugar accumulation in fruit[J]. Physiologia Plantarum,2018,164(3):307-319.
[47] VERWEIJ W,SPELT C,DI SANSEBASTIANO G P,VERMEER J,REALE L,FERRANTI F,KOES R,QUATTROCCHIO F. An H+ P-ATPase on the tonoplast determines vacuolar pH and flower colour[J]. Nature Cell Biology,2008,10(12):1456-1462.
[48] HUANG X Y,WANG C K,ZHAO Y W,SUN C H,HU D G. Mechanisms and regulation of organic acid accumulation in plant vacuoles[J]. Horticulture Research,2021,8(1):227.
[49] GAO M,ZHAO H Y,ZHENG L T,ZHANG L H,PENG Y J,MA W F,TIAN R,YUAN Y Y,MA F W,LI M J,MA B Q. Overexpression of apple Ma12,a mitochondrial pyrophosphatase pump gene,leads to malic acid accumulation and the upregulation of malate dehydrogenase in tomato and apple calli[J]. Horticulture Research,2022,9:uhab053.
[50] MARTINOIA E. Vacuolar transporters - companions on a longtime journey[J]. Plant Physiology,2018,176(2):1384-1407.
[51] 石彩云,劉丽,魏志峰,高登涛,刘永忠. 园艺植物质子泵及其对有机酸积累调控的研究进展[J]. 园艺学报,2022,49(12):2611-2621.
SHI Caiyun,LIU Li,WEI Zhifeng,GAO Dengtao,LIU Yongzhong. Research progress of proton pumps and their regulation in organic acid accumulation in horticultural plants[J]. Acta Horticulturae Sinica,2022,49(12):2611-2621.
[52] YAO Y X,DONG Q L,YOU C X,ZHAI H,HAO Y J. Expression analysis and functional characterization of apple MdVHP1 gene reveals its involvement in Na+,malate and soluble sugar accumulation[J]. Plant Physiology and Biochemistry,2011,49(10):1201-1208.
[53] REA P A,KIM Y,SARAFIAN V,POOLE R J,DAVIES J M,SANDERS D. Vacuolar H+-translocating pyrophosphatases:A new category of ion translocase[J]. Trends in Biochemical Sciences,1992,17(9):348-353.
[54] HU D G,SUN M H,SUN C H,LIU X,ZHANG Q Y,ZHAO J,HAO Y J. Conserved vacuolar H+-ATPase subunit B1 improves salt stress tolerance in apple calli and tomato plants[J]. Scientia Horticulturae,2015,197:107-116.
[55] HU D G,SUN C H,SUN M H,HAO Y J. MdSOS2L1 phosphorylates MdVHA-B1 to modulate malate accumulation in response to salinity in apple[J]. Plant Cell Reports,2016,35(3):705-718.
[56] KREBS M,BEYHL D,G?RLICH E,AL-RASHEID K A S,MARTEN I,STIERHOF Y D,HEDRICH R,SCHUMACHER K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation[J]. Proceedings of the National Academy of Sciences of the United States of America,2010,107(7):3251-3256.
[57] KRIEGEL A,ANDR?S Z,MEDZIHRADSZKY A,KR?GER F,SCHOLL S,DELANG S,PATIR-NEBIOGLU M G,GUTE G,YANG H B,MURPHY A S,PEER W A,PFEIFFER A,KREBS M,LOHMANN J U,SCHUMACHER K. Job sharing in the endomembrane system:Vacuolar acidification requires the combined activity of V-ATPase and V-PPase[J]. The Plant Cell,2015,27(12):3383-3396.
[58] ZHANG Y X,LI Q H,XU L L,QIAO X,LIU C X,ZHANG S L. Comparative analysis of the P-type ATPase gene family in seven Rosaceae species and an expression analysis in pear (Pyrus bretschneideri Rehd.)[J]. Genomics,2020,112(3):2550-2563.
[59] LI Y B,PROVENZANO S,BLIEK M,SPELT C,APPELHAGEN I,DE FARIA L M,VERWEIJ W,SCHUBERT A,SAGASSER M,SEIDEL T,WEISSHAAR B,KOES R,QUATTROCCHIO F. Evolution of tonoplast P-ATPase transporters involved in vacuolar acidification[J]. New Phytologist,2016,211(3):1092-1107.
[60] VERWEIJ W,SPELT C E,BLIEK M,DE VRIES M,WIT N,FARACO M,KOES R,QUATTROCCHIO F M. Functionally similar WRKY proteins regulate vacuolar acidification in Petunia and hair development in Arabidopsis[J]. The Plant Cell,2016,28(3):786-803.
[61] FARACO M,SPELT C,BLIEK M,VERWEIJ W,HOSHINO A,ESPEN L,PRINSI B,JAARSMA R,TARHAN E,DE BOER A H,DI SANSEBASTIANO G P,KOES R,QUATTROCCHIO F M. Hyperacidification of vacuoles by the combined action of two different P-ATPases in the tonoplast determines flower color[J]. Cell Reports,2014,6(1):32-43.
[62] STRAZZER P,SPELT C E,LI S J,BLIEK M,FEDERICI C T,ROOSE M L,KOES R,QUATTROCCHIO F M. Hyperacidification of Citrus fruits by a vacuolar proton-pumping P-ATPase complex[J]. Nature Communications,2019,10:744.
[63] SHI C Y,HUSSAIN S B,YANG H,BAI Y X,KHAN M A,LIU Y Z. CsPH8,a P-type proton pump gene,plays a key role in the diversity of citric acid accumulation in citrus fruits[J]. Plant Science,2019,289:110288.
[64] SHI C Y,SONG R Q,HU X M,LIU X,JIN L F,LIU Y Z. Citrus PH5-like H(+)-ATPase genes:Identification and transcript analysis to investigate their possible relationship with citrate accumulation in fruits[J]. Frontiers in Plant Science,2015,6:135.
[65] JIA D J,WU P,SHEN F,LI W,ZHENG X D,WANG Y Z,YUAN Y B,ZHANG X Z,HAN Z H. Genetic variation in the promoter of an R2R3-MYB transcription factor determines fruit malate content in apple (Malus domestica Borkh.)[J]. Plant Physiology,2021,186(1):549-568.
[66] HU D G,SUN C H,MA Q J,YOU C X,CHENG L L,HAO Y J. MdMYB1 regulates anthocyanin and malate accumulation by directly facilitating their transport into vacuoles in apples[J]. Plant Physiology,2016,170(3):1315-1330.
[67] ZHANG L H,MA B Q,WANG C Z,CHEN X Y,RUAN Y L,YUAN Y Y,MA F W,LI M J. MdWRKY126 modulates malate accumulation in apple fruit by regulating cytosolic malate dehydrogenase (MdMDH5)[J]. Plant Physiology,2022,188(4):2059-2072.
[68] XIONG T T,TAN Q Q,LI S S,MAZARS C,GALAUD J P,ZHU X Y. Interactions between calcium and ABA signaling pathways in the regulation of fruit ripening[J]. Journal of Plant Physiology,2021,256:153309.
[69] AMATO A,CAVALLINI E,WALKER A R,PEZZOTTI M,BLIEK M,QUATTROCCHIO F,KOES R,RUPERTI B,BERTINI E,ZENONI S,TORNIELLI G B. The MYB5-driven MBW complex recruits a WRKY factor to enhance the expression of targets involved in vacuolar hyper-acidification and trafficking in grapevine[J]. The Plant Journal,2019,99(6):1220-1241.
[70] AMATO A,CAVALLINI E,ZENONI S,FINEZZO L,BEGHELDO M,RUPERTI B,TORNIELLI G B. A grapevine TTG2-like WRKY transcription factor is involved in regulating vacuolar transport and flavonoid biosynthesis[J]. Frontiers in Plant Science,2017,7:1979.
[71] SPELT C,QUATTROCCHIO F,MOL J,KOES R. ANTHOCYANIN1 of petunia controls pigment synthesis,vacuolar pH,and seed coat development by genetically distinct mechanisms[J]. The Plant Cell,2002,14(9):2121-2135.
[72] DING Z J,YAN J Y,XU X Y,LI G X,ZHENG S J. WRKY46 functions as a transcriptional repressor of ALMT1,regulating aluminum-induced malate secretion in Arabidopsis[J]. The Plant Journal,2013,76(5):825-835.
[73] CEUSTERS J,BORLAND A M,DE PROFT M P. Drought adaptation in plants with crassulacean acid metabolism involves the flexible use of different storage carbohydrate pools[J]. Plant Signaling & Behavior,2009,4(3):212-214.
[74] LOBIT P,GENARD M,SOING P,HABIB R. Modelling malic acid accumulation in fruits:Relationships with organic acids,potassium,and temperature[J]. Journal of Experimental Botany,2006,57(6):1471-1483.
[75] KWEON H J,KANG I K,KIM M J,LEE J,MOON Y S,CHOI C,CHOI D G,WATKINS C B. Fruit maturity,controlled atmosphere delays and storage temperature affect fruit quality and incidence of storage disorders of ‘Fuji apples[J]. Scientia Horticulturae,2013,157:60-64.
[76] OLMEDO P,ZEPEDA B,DELGADO-RIOSECO J,LEIVA C,MORENO A A,SAGREDO K,BLANCO-HERRERA F,PEDRESCHI R,INFANTE R,MENESES C,CAMPOS-VARGAS R. Metabolite profiling reveals the effect of cold storage on primary metabolism in nectarine varieties with contrasting mealiness[J]. Plants,2023,12(4):766.
[77] WANG S Y,CAMP M J. Temperatures after bloom affect plant growth and fruit quality of strawberry[J]. Scientia Horticulturae,2000,85(3):183-199.
[78] RICHARDSON A C,MARSH K B,BOLDINGH H L,PICKERING A H,BULLEY S M,FREARSON N J,FERGUSON A R,THORNBER S E,BOLITHO K M,MACRAE E A. High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit[J]. Plant,Cell & Environment,2004,27(4):423-435.
[79] LAKSO A N,KLIEWER W M. The influence of temperature on malic acid metabolism in grape berries. II. Temperature responses of net dark CO2 fixation and malic acid pools[J]. American Journal of Enology and Viticulture,1978,29(3):145-149.
[80] SWEETMAN C,SADRAS V O,HANCOCK R D,SOOLE K L,FORD C M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit[J]. Journal of Experimental Botany,2014,65(20):5975-5988.
[81] WU B H,G?NARD M,LESCOURRET F,GOMEZ L,LI S H. Influence of assimilate and water supply on seasonal variation of acids in peach (cv. Suncrest)[J]. Journal of the Science of Food and Agriculture,2002,82(15):1829-1836.
[82] MA W F,LI Y B,NAI G J,LIANG G P,MA Z H,CHEN B H,MAO J. Changes and response mechanism of sugar and organic acids in fruits under water deficit stress[J]. PeerJ,2022,10:e13691.
[83] WANG Y J,LIU L,WANG Y,TAO H X,FAN J L,ZHAO Z Y,GUO Y P. Effects of soil water stress on fruit yield,quality and their relationship with sugar metabolism in ‘Gala apple[J]. Scientia Horticulturae,2019,258:108753.
[84] SIM I,SUH D H,SINGH D,DO S G,MOON K H,LEE J H,KU K M,LEE C H. Unraveling metabolic variation for blueberry and chokeberry cultivars harvested from different geo-climatic regions in Korea[J]. Journal of Agricultural and Food Chemistry,2017,65(41):9031-9040.
[85] RESHEF N,WALBAUM N,AGAM N,FAIT A. Sunlight modulates fruit metabolic profile and shapes the spatial pattern of compound accumulation within the grape cluster[J]. Frontiers in Plant Science,2017,8:70.
[86] 張振英,宋来庆,刘美英,赵玲玲,唐岩,孙燕霞,姜中武. 郁闭果园不同部位光照条件对烟富3号苹果果实品质的影响[J]. 山东农业科学,2013,45(9):42-44.
ZHANG Zhenying,SONG Laiqing,LIU Meiying,ZHAO Lingling,TANG Yan,SUN Yanxia,JIANG Zhongwu. Analysis on apple fruit quality of Yanfu 3 under different light conditions in closing orchard[J]. Shandong Agricultural Sciences,2013,45(9):42-44.
[87] DEBOLT S,RISTIC R,ILAND P G,FORD C M. Altered light interception reduces grape berry weight and modulates organic acid biosynthesis during development[J]. HortScience,2008,43(3):957-961.
[88] BAI Q,SHEN Y Y,HUANG Y. Advances in mineral nutrition transport and signal transduction in Rosaceae fruit quality and postharvest storage[J]. Frontiers in Plant Science,2021,12:620018.
[89] ZHANG W,ZHANG X,WANG Y F,ZHANG N S,GUO Y P,REN X L,ZHAO Z Y. Potassium fertilization arrests malate accumulation and alters soluble sugar metabolism in apple fruit[J]. Biology Open,2018,7(12):bio024745.

