柑橘衰退病毒RT-RPA-LFD可视化检测方法的建立及应用
申世凯 曾婷 乔兴华 陈力 任杰群 周彦


摘 要:【目的】柑橘衰退病由柑橘衰退病毒(citrus tristeza virus,CTV)引起,是一種世界性的重要柑橘病害。为实现CTV的田间快速检测,建立一种准确、快速且可视化的检测方法。【方法】以CTV外壳蛋白(CP)的保守区域为靶标,设计3对特异性引物和探针,通过引物筛选,以及优化引物浓度、反应时间和反应温度等条件,建立CTV的反转录-重组酶聚合酶扩增-侧流层析试纸条(RT-RPA-LFD)快速检测方法,明确其灵敏度,并用于田间疑似样品的检测。【结果】建立了CTV的RT-RPA-LFD检测方法:最佳检测引物为RPA-1F/R,对应探针为RPA-P,最佳反应条件为40 ℃,25 min,且与其他5种柑橘病毒无交叉反应。该方法的灵敏度是RT-PCR的100倍,最低可检测到2.12×101 拷贝·μL-1的CTV核酸,与RT-qPCR相当。采用RT-RPA-LFD法在67份田间样品中检测出CTV阳性样品41份,与RT-PCR法检测结果一致。【结论】建立的CTV RT-RPA-LFD法具有操作简单、快速、结果可视等优点,适合基层植保工作者对田间样品开展快速检测。
关键词:柑橘;柑橘衰退病毒;反转录-重组酶聚合酶扩增-侧流层析试纸条;快速检测
中图分类号:S666 文献标志码:A 文章编号:1009-9980(2023)12-2652-09
收稿日期:2023-07-28 接受日期:2023-10-20
基金项目:财政部和农业农村部国家现代农业产业技术体系(CARS-26-05B)
作者简介:申世凯,女,在读硕士研究生,研究方向为植物病理学。E-mail:1091893469@qq.com
*通信作者 Author for correspondence. E-mail:zybook1@163.com
Establishment and application of RT-RPA-LFD visualization assay for rapid detection of citrus tristeza virus
SHEN Shikai1, ZENG Ting1, QIAO Xinghua2, CHEN Li2, REN Jiequn3, ZHOU Yan1*
(1Southwest University, National Citrus Engineering Research Center, Chongqing 400712, China; 2Plant Protection and Fruit Technology Extension Station of Wanzhou District, Chongqing 400712, China; 3Chongqing Three Gorges Academy of Agricultural Sciences, Chongqing 400712, China)
Abstract: 【Objective】 Tristeza caused by citrus tristeza virus (CTV) is one of the most destructive citrus diseases in the world, which is mainly spread by several aphid species and bud-grafting. Severe CTV isolates could cause quick decline of sour orange rootstock, and stem pitting of susceptible cultivars. In recent years, stunted, severe stem pitting and reduced fruit quality were observed in Newhall navel orange and some tangor cultivars, causing severe economic losses in major citrus-growing provinces of China, especially in Hunan, Jiangxi, Yunnan, Sichuan provinces. Prompt and accurate CTV detection in the nursery and field samples is necessary to control CTV. To date, serological techniques, reverse transcription PCR (RT-PCR), RT- real-time PCR (RT-qPCR) and other methods have been used to detect CTV. However, these traditional detection techniques are generally flawed. The purpose of this study was to establish a reliable, accurate, convenient and visual reverse transcription-recombinase polymerase amplification (RT-RPA) combined with lateral flow dipstick (LFD) method for CTV detection. 【Methods】 Three pairs of primers and a specific probe used for CTV detection were designed according to the conservative sequence of the coat protein (CP) gene of CTV isolates (NCBI number MH558665.1, MH558666.1, JX266712.1, JQ911664.1 and JQ061137.1) from China. By detecting CTV-infected citrus samples, primers with the best specificity and amplification efficiency were selected to establish the RT-RPA-LFD for CTV detection. The total RNAs were extracted from 100 mg CTV-infected citrus leaf samples using RNAiso Plus and used for CTV detection. The reverse transcription was performed using a C1000 Thermal Cycler in a 20 μL reaction mix containing 1 μL of Oligo dT Primer, 1 μL of 10 μmol·L-1 dNTP Mixture, 1 μL of RNA template, 4 μL of PrimeScript Buffer, 0.5 μL of RNase Inhibitor, and 1 μL of PrimeScript RTase. The reaction was carried out for 45 min at 42 ℃ and 5 min at 95 ℃. RT-RPA-LFD reaction system was optimized with respect to the primer concentration (1, 2.5, 5, 10, 20, and 50 nmol·L-1), reaction time (5, 10, 15, 20, 25, 30, 35 and 40 min), and reaction gradient temperature (10, 15, 20, 25, 30, 35, 40, 45 and 50 ℃). For visual detection, LFD strips from the AmplifyRP × RT Discovery Kit were added to the RT-RPA products. The reactions should be allowed to incubate for no more than 30 min. The two visual bands of the test and control lines suggested that the tested sample was CTV-positive, and only one band on the control line indicated a negative result. The optimized reaction conditions were determined through the colour density of the test line. The specificity of the established RT-RPA-LFD was evaluated by detecting the samples infected with CTV, citrus yellow vein clearing virus (CYVCV), Citrus tatter leaf virus (CTLV), citrus exocortis viroid (CEVd), citrus psorosis virus (CPV), citrus chlorotic dwarf-associated virus (CCDaV), and the virus-free citrus plants, respectively. To evaluate the detection range of the optimized RT-RPA-LFD, eight CTV genotypes and eleven CTV isolates from different countries were used. A series of 10-fold dilutions (2.12×106-2.12×10-1 copies·μL-1) of CTV samples were used to test the sensitivity of the RT-RPA-LFD assay, and the sensitivity was compared with the conventional RT-PCR and RT-qPCR. Furthermore, the leaves of 67 CTV-suspected different tangor cultivar samples were randomly collected from Chongqing, Sichuan and Guangxi provinces, and used for RT-PCR and RT-RPA-LFD detection. 【Results】 A RT-RPA-LFD assay for rapid visual detection of CTV was established, with primer pairs RPA-1F (5-CTTGCTGGCGTCCCTTGTTTCTGTTCTTGTCTT-3) and RPA-1R (5-ATTCTGTTTCCTT TCCTAGCCGGGCTTCTTCAC-3), and RPA-P probe (5-GGCGAAAAATCTTTTCGTCTACT TGGTTTTCACTCGCGAAG GCA-3). It could specifically amplify the target fragment of CTV with a size of 156 bp. The optimal reaction conditions for the determination of RT-RPA-LFD assay were determined as 10 μmol·L-1 primer concentration, 25 min reaction time and 40 ℃ incubation temperature. This method has high specificity to CTV, and no test line was observed when total nucleic acid extracts from CTLV, CYVCV, CEVd, CPV, CCDaV, or healthy citrus plants were tested. This method could also detect different genotypes and origin of CTV. In the sensitivity detection, 2.12×101 copies·μL-1 was the lowest detection sensitivity of RT-RPA-LFD and RT-qPCR. The limit of detection of RT-PCR was 2.12×103 copies·μL-1, indicating that the RT-RPA-LFD method would be 100 times more sensitive than RT-PCR, which was consistent with that of RT-qPCR. Furthermore, the RT-RPA-LFD detection of CTV required shorter detection time (approximately 30 min) than RT-PCR and RT-qPCR. Among 67 citrus samples randomly collected from the field, CTV was detected from 41 samples using RT-RPA-LFD and RT-PCR assay showed the same results. These results suggested that the RT-RPA-LFD method would be suitable for CTV detection in the field. 【Conclusion】 In this study, a visual RT-RPA-LFD method for CTV detection was developed and the optimal reaction conditions for the RT-RPA-LFD assay were determined. The new RT-RPA-LFD method would be more effective and sensitive for the precise quantification of CTV than RT-PCR. It could be applied to on-site rapid detection for the plant protection and quarantine station.
Key words: Citrus; Citrus tristeza virus (CTV); Reverse transcription-recombinase polymerase amplification-lateral flow dipstick test strip; Rapid detection
柑橘衰退病是危害柑橘產业的重要病害之一,其病原为柑橘衰退病毒(citrus tristeza virus,CTV),主要通过感病接穗、苗木和多种蚜虫进行传播,广泛分布于世界各柑橘产区[1]。CTV是长线性病毒科(Closteroviridae)长线性病毒属(Closterovirus)的正义单链RNA病毒,基因组全长19.8 kb,含12个开放阅读框(ORF),可编码两个外壳蛋白(CP和CPm),其中CP在CTV基因组中高度保守[2]。CTV在田间存在复杂的株系分化现象,除导致酸橙及其作砧木植株的快速死亡外,还导致葡萄柚、梾檬和部分甜橙、柚类、杂柑等敏感品种的茎陷点症状,以及酸橙、尤力克柠檬和葡萄柚实生苗的矮缩、黄化,CTV弱毒株在植株上不会产生严重的症状[3]。根据CTV的生物学特性及其基因组变异,CTV被分为T36、VT、T30、T3、RB、T68、HA16-5和S1等多个基因型,且其基因型的种类还在不断增加[4-5]。
我国由于长期使用枳、香橙、酸柚等抗速衰型衰退病砧木,茎陷点型衰退病是我国衰退病危害的主要类型[6-7]。近年来,由于茎陷点型衰退病随苗木流通,其发生范围不断扩大,已在湖南、江西、云南等柑橘主产区造成了严重的危害[8-10]。目前采用无毒繁殖材料是防治CTV最有效的手段,而这依赖于高效快速的检测方法。
目前常用血清学[11]、RT-PCR[12]以及RT-qPCR[13-14]等方法检测CTV。这些技术虽然灵敏度高、特异性强,但难以实现田间现场检测。因此为满足果园、苗圃现场快速检测的需要,亟待研发一种准确、快捷、简便的CTV检测方法。重组酶聚合酶扩增(Recombinase polymerase amplification,RPA)模拟T4噬菌体核酸复制机制,在体外实现恒温扩增[15]。通过结合侧流层析试纸条(lateral flow dipstick,LFD),从而实现了检测结果的可视化。由于RT-RPA-LFD检测技术具有快速、灵敏和简便的优点,尤其适用于普通工作人员开展田间检测,目前已成功应用于柑橘碎叶病毒(citrus tatter leaf virus,CTLV)、樱桃病毒A(cherry virus A,CVA)、李矮缩病毒(prune dwarf virus,PDV)、李痘病毒(plum pox virus,PPV)等多种植物病毒的检测[16-19]。笔者在本研究中以CTV保守的CP基因为靶标设计特异性引物和探针,建立、优化了CTV的RT-RPA-LFD检测技术,为CTV的快速检测提供了新的选择。
1 材料和方法
1.1 试验材料
单一感染CTV、柑橘黄脉病毒(citrus yellow vein clearing virus,CYVCV)、柑橘碎叶病毒、柑橘裂皮病类病毒(CEVd)、柑橘鳞皮病毒(citrus psorosis virus,CPV)和柑橘褪绿矮缩病毒(citrus chlorotic dwarf-associated virus,CCDaV)的病株,无病毒柑橘植株;核酸浓度为2.12×106 拷贝·μL-1的CTV阳性样品。以上材料均为西南大学柑桔研究所保存提供。
1.2 主要试剂
RPA扩增试剂盒购自美国Agdia公司;PlantGen DNA Kit购自中国康为世纪;RNAiso Plus试剂盒购自宝生物工程(大连)有限公司;All-In-One 5× RT MasterMix,2 × Taq Master Mix购自诺唯赞公司。
1.3 总核酸提取和cDNA合成
使用PlantGen DNA Kit和RNAiso Plus提取总核酸,并于-80 ℃冰箱保存备用。将1 μL Oligo dT Primer,1 μL dNTP Mixture(10 μmol·L-1),1 μL总RNA模板,7 μL ddH2O混合后,65 ℃ 5 min;冰上冷却后加入4 μL PrimeScript Buffer,0.5 μL RNase Inhibitor(40 U·μL-1),1 μL PrimeScript RTase(200 U·μL-1,TaKaRa),加ddH2O至总体积为20 μL。42 ℃ 45 min,95 ℃ 5 min。
1.4 RT-RPA-LFD的引物和探针设计
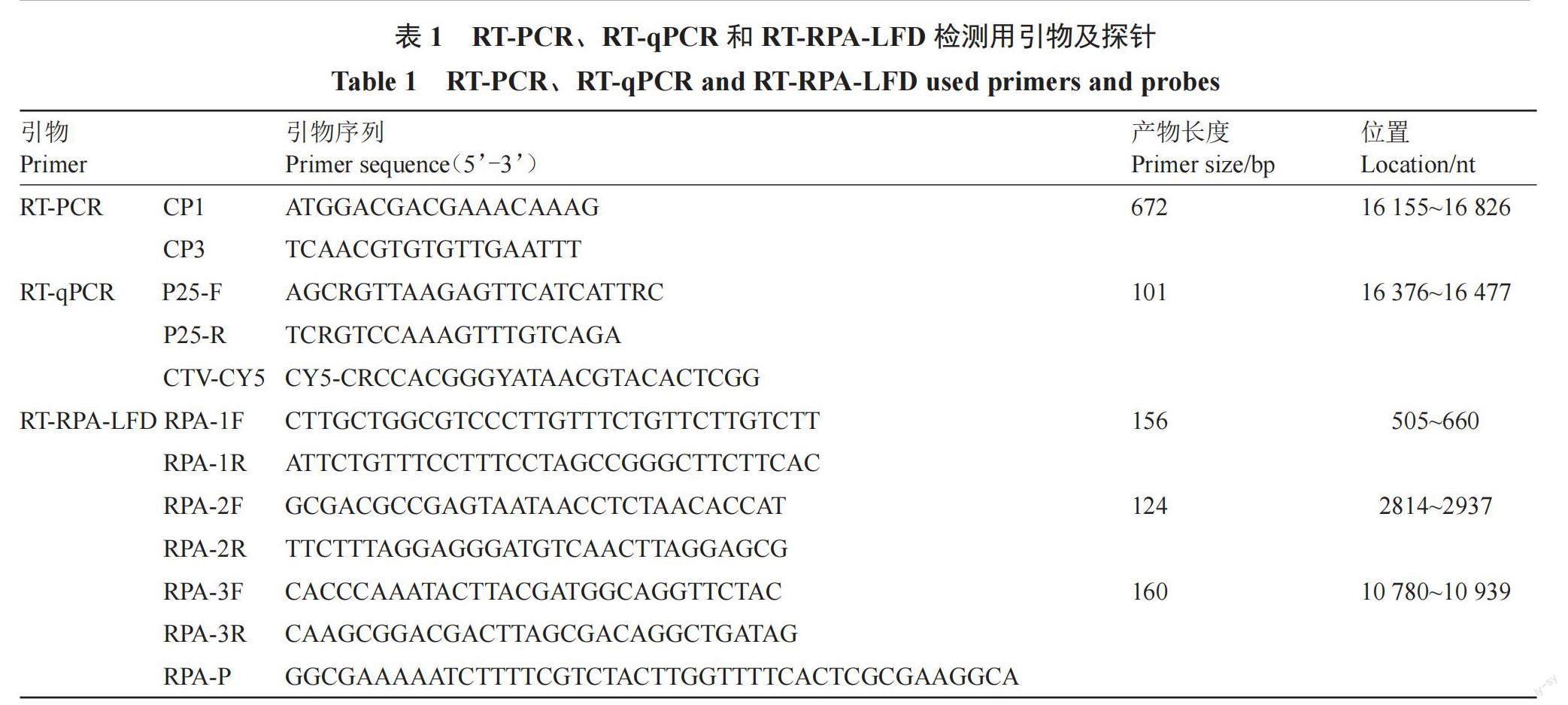
比对分析NCBI中已报道的5个中国茎陷点型CTV CP基因序列(GenBank:MH558665.1、MH558666.1、JX266712.1、JQ911664.1和JQ061137.1),以及Amplify Discovery Kits中引物和探针设计要求,使用Primer Premier 5.0设计特异性引物和探针(表1),所用引物和探针均由北京擎科生物技术有限公司合成。
1.5 RT-RPA-LFD反应体系的建立及优化
使用AmplifyRP × RT Discovery Kit进行RPA扩增反应。反应体系包括5.9 μL Rehydration Buffer,0.42 μL RPA-F/R(设6个浓度梯度:1.0、2.5、5.0、10.0、20.0、50.0 μmol·L-1)、10 μmol·L-1 RPA-P 0.12 μL、1 μL cDNA、1.64 μL ddH2O。反应液与固体反应物混匀后加入0.5 μL 280 mmol·L-1 MgOAc进行孵育(分别设置8个反应时间5、10、15、20、25、30、35、40 min和9个温度梯度10、15、20、25、30、35、40、45、50 ℃)。孵育结束后,放入试纸条,室温放置10~20 min后观察结果。以质控线(Control line)和测试线(Test line)显示清晰,判断结果为阳性;质控线显示清晰,测试线无条带时,结果为阴性;质控线未出现条带时,结果无效。
1.6 特异性分析
以单一感染CTV、CYVCV、CTLV、CEVd、CPV和CCDaV阳性样品,以及无病毒柑橘样品的核酸为模板,使用建立的RT-RPA-LFD体系进行检测,评价其特异性。此外,为了验证该方法是否可检测不同基因型或来源的CTV毒株,按上述方法对T36、T30、VT、T3(由美国佛罗里达大学柑橘研究与教育中心William Dawson教授赠送)、S1、RB、L1、M1基因型毒株,以及来自澳大利亚的PB61,PB135(由巴西Centrode Citricultrua Sylvio Moreira 研究所Marcos A Machado博士赠送),来自巴西的PerIAC(由澳大利亚EMAI实验室Patricia Barkley研究员赠送),来自巴基斯坦的CT-Pak1,以及来自中国不同产区的CT3、CT9、CT14、CT15、CT30、CT31和CT68毒株(表2)进行检测。
1.7 RT-RPA-LFD检测灵敏性分析
将CTV阳性样品RNA按10倍梯度稀释得到2.12×10-1~2.12×106拷贝·μL-1稀释液作为模板,按照所建立的RT-RPA-LFD,以及Gillings等[12]和Yokomi等[13]的方法进行RT-PCR和RT-qPCR平行检测,比较3种方法的灵敏度。15 μL PCR反应体系:cDNA模板1.0 μL,PrimeScript I step Enzyme Mix 0.5 μL,2×I Step Buffer 7.5 μL,CP1/CP3(10 μmol·L-1)0.3 μL。反应程序:45 ℃ 30 s;95 ℃ 2 min;94 ℃ 30 s,55 ℃ 30 s,72 ℃ 1 min,36个循环;72 ℃ 5 min。25 μL RT-qPCR反应体系:2 × RT-PCR reaction mix for probe 12.5 μL,P25-F/P25-R(10 μmol·L-1)0.5 μL,CTV-CY5(10 μmol·L-1) 0.2 μL,RNA模板2 μL,iScript reverse transcriptase for one-step RT-PCR 0.5 μL。反应程序:55 ℃,2 min;95 ℃,5 min;95 ℃ 15 s,59 ℃ 30 s,40个循环。引物序列见1.4。
1.8 田间样品检测
将67份田间样品按照1.3的方法提取总核酸后,分别采取RT-PCR和优化后的RT-RPA-LFD反应体系进行检测,比较检测效果。
2 结果与分析
2.1 引物筛选
以CTV阳性样品的总核酸为模板,分别使用设计的3对引物进行扩增。结果显示,RPA-1F/R扩增条带单一、明亮。所扩增产物与CTV毒株CT11A(JQ911664.1)相应序列的相似性为100%。引物RPA-2F/R无扩增条带、RPA-3F/R存在非特异性扩增(图1)。
2.2 RT-RPA-LFD引物浓度优化
当引物浓度为1~10 μmol·L-1时,测试线的颜色随引物浓度的增加逐渐变深。当引物浓度高于10 μmol·L-1时,测试线无明显变化(图2)。因此选择10 μmol·L-1作为RT-RPA-LFD反应最适的引物浓度。
2.3 反应时间及温度优化
检测结果表明,在推荐温度37 ℃下反应超过20 min后,试纸条均出现清晰的测试线,且反应超过25 min后,测试线颜色不再加深(图3-A)。反应时间为25 min,反应温度10~40 ℃时,测试线颜色逐渐加深;40~45 ℃时其颜色无明显变化,温度高于50 ℃时,测试线不清晰(图3-B)。综上,确定引物浓度10 μmol·L-1,反应时间25 min,反应温度40 ℃为最佳反应条件。
2.4 RT-RT-RPA-LFD特异性检测
利用优化后的反应体系检测分别感染了CTV、CYVCV、CTLV、CEV、CPV、CCDaV的样品,以及无病毒柑橘样品。结果显示,仅感染CTV样品的检测结果呈阳性,其余样品的检测结果均為阴性,且能检测出来自不同国家的19个CTV毒株。表明该反应体系与其他主要柑橘病毒无交叉反应,特异性强,且适用于对不同基因型或来源CTV毒株的检测。
2.5 RT-RPA-LFD灵敏性检测
将已知浓度的CTV阳性样品按10倍梯度稀释得到2.12×10-1~2.12×106拷贝·μL-1的稀释液作为模板,进行RT-PCR、RT-qPCR和RT-RPA-LFD检测(图4)。结果表明,RT-qPCR和RT-RPA-LFD均能检测出2.12×101拷贝·μL-1稀释液中的CTV,而RT-PCR仅检测出2.12×103拷贝·μL-1稀释液中的CTV。以上结果表明RT-RPA-LFD检测法与RT-qPCR相当,且比RT-PCR的灵敏度提高了100倍。
2.6 RT-RPA-LFD田间样品检测
田间样品的检测结果显示,对随机选取的67份田间样品进行RT-PCR和RT-RPA-LFD检测,其结果一致,均能从沃柑、红美人、W·默科特等杂柑品种中检测出41份CTV阳性样品,检出率为61.19%,经进一步验证,其中包括T36、T30、VT、T3、T68、RB等多种基因型毒株,表明建立的RT-RPA-LFD检测方法稳定可靠(表3,图5)。
3 讨 论
近年来,随着我国柑橘产业的迅猛发展,柑橘衰退病随苗木和蚜虫传播的速度加快,造成其在我国的发生区域不断扩大,损失加剧[8-10]。因此准确、灵敏、便捷的病害检测技术对于监测和防治柑橘衰退病具有重要意义。目前,CTV检测中常用的血清学方法在检测柚类等柑橘类型时检出率较低[20],且基于RT-PCR的检测方法往往依赖于多种特殊仪器设备,检测过程复杂,专业性强。此外,虽然环介导等温扩增技术(LAMP)灵敏度高,且操作较为简便,但其引物设计复杂,且容易出现假阳性[21]。
笔者在本研究中根据CTV CP基因的保守区域设计引物和探针,并通过优化引物浓度、反应温度和时间,建立、优化了CTV的RT-RPA-LFD检测方法,其操作简便、特异性强。与常规RT-PCR法相比,灵敏度提高了100倍,与RT-qPCR法相当。在检测田间样品时,RT-RPA-LFD检测方法与常规RT-PCR法的检测结果相同,反应时间缩短了1 h,且不需要PCR仪、凝胶成像仪等复杂仪器。由于检测通过试纸条呈现,因此更加直观、简捷,可以快速准确地检测田间样品,有助于及时清除病株,从而最大限度地降低CTV传播扩散的风险。此外,因为RT-RPA-LFD反应在单一管中进行,部分反应组分以冻干粉的形式保存,使得检测过程不易发生污染。笔者在本研究中针对目前我国柑橘产业中较被追捧的多个杂柑品种进行检测时发现,沃柑、红美人、W·默科特等品种中CTV的检出率较高,因此今后在引种上述品种时需要加大CTV的检测力度。虽然Crannell等[22]的报道仅靠体温就能完成RT-RPA-LFD反应,但在本研究中其反应仍受温度限制,今后可进一步优化反应体系,降低反应温度,实现在常温下进行检测。
4 结 论
CTV RT-RPA-LFD法特异性强、操作简便、灵敏度高,适用于低浓度样品检测,且部分反应组分以冻干粉的形式保存,不易发生污染。此外,该检测方法较RT-PCR法反应时间缩短了1 h,且不需要PCR仪、凝胶成像仪等复杂仪器,因此尤其适用于基层植保人员开展田间大规模CTV检测。
参考文献 References:
[1] ROISTACHER C N,MORENO P. The worldwide threat from destructive isolates of citrus tristeza virus-A review[C]//International Organization of Citrus Virologists (IOCV). International Organization of Citrus Virologists Conference Proceedings (1957-2010). California:University of California-Riverside,1991:7-19.
[2] FOLIMONOVA S Y,FOLIMONOV A S,TATINENI S,DAWSON W O. Citrus tristeza virus:survival at the edge of the movement continuum[J]. Journal of Virology,2008,82(13):6546-6556.
[3] BAR-JOSEPH M,MARCUS R,LEE R F. The continuous challenge of Citrus tristeza virus control[J]. Annual Review of Phytopathology,1989,27:291-316.
[4] HARPER S J. Citrus tristeza virus:Evolution of complex and varied genotypic groups[J]. Frontiers in Microbiology,2013,4:93.
[5] WANG J,ZHOU T Y,SHEN P,ZHANG S,CAO M J,ZHOU Y,LI Z A. Complete genome sequences of two novel genotypes of Citrus tristeza virus infecting Poncirus trifoliata in China[J]. Journal of Plant Pathology,2020,102(3):903-907.
[6] ZHOU C Y,ZHAO X Y,JIANG Y H. Boat-shaped leaf symptoms of Satsuma mandarin associated with Citrus tristeza virus (CTV) [C]//International Organization of Citrus Virologists (IOCV). International Organization of Citrus Virologists Conference Proceedings (1957-2010). California:University of California-Riverside,1996:154-157.
[7] 周彥,周常勇,李中安,王雪峰,刘科宏. 利用弱毒株交叉保护技术防治甜橙茎陷点型衰退病[J]. 中国农业科学,2008,41(12):4085-4091.
ZHOU Yan,ZHOU Changyong,LI Zhongan,WANG Xuefeng,LIU Kehong. Mild strains cross protection against stem-pitting tristeza of sweet orange[J]. Scientia Agricultura Sinica,2008,41(12):4085-4091.
[8] XIAO C,YAO R X,LI F,DAI S M,LICCIARDELLO G,CATARA A,GENTILE A,DENG Z N. Population structure and diversity of citrus tristeza virus (CTV) isolates in Hunan Province,China[J]. Archives of Virology,2017,162(2):409-423.
[9] 易龙,赖晓桦,卢占军,钟八莲. 江西柑橘主产区柑橘衰退病毒分离株组群分析[J]. 植物保护,2012,38(4):112-114.
YI Long,LAI Xiaohua,LU Zhanjun,ZHONG Balian. Analysis of CP/HinfⅠRFLP groups of Citrus tristeza virus isolates in Jiangxi[J]. Plant Protection,2012,38(4):112-114.
[10] QIN Y Y,LIU Y J,ZHAO J F,HAJERI S,WANG J J,YE X,ZHOU Y. Molecular and biological characterization of a novel Citrus tristeza virus isolate that causes severe symptoms in Citrus junos cv. Ziyangxiangcheng[J]. Archives of Virology,2023,168(2):59.
[11] GARNSEY S M,PERMAR T A,CAMBRA M,HENDERSON C T. Direct tissue blot immunoassay (DTBIA) for detection of Citrus tristeza virus (CTV)[C]//International Organization of Citrus Virologists (IOCV). International Organization of Citrus Virologists Conference Proceedings (1957-2010). California:University of California-Riverside,1993:39-50.
[12] GILLINGS M,BROADBENT P,INDSTO J,LEE R. Characterisation of isolates and strains of citrus tristeza closterovirus using restriction analysis of the coat protein gene amplified by the polymerase chain reaction[J]. Journal of Virological Methods,1993,44(2/3):305-317.
[13] YOKOMI R K,SAPONARI M,SIEBURTH P J. Rapid differentiation and identification of potential severe strains of Citrus tristeza virus by real-time reverse transcription-polymerase chain reaction assays[J]. Phytopathology,2010,100(4):319-327.
[14] SAPONARI M,MANJUNATH K,YOKOMI R K. Quantitative detection of Citrus tristeza virus in Citrus and aphids by real-time reverse transcription-PCR (TaqMan?)[J]. Journal of Virological Methods,2008,147(1):43-53.
[15] BABU B,OCHOA-CORONA F M,PARET M L. Recombinase polymerase amplification applied to plant virus detection and potential implications[J]. Analytical Biochemistry,2018,546:72-77.
[16] ZENG T,CHEN X L,LIAO P,GAO H X,ZHENG C R,HUANGFU M Y,ZHOU Y. Development of transcription recombinase polymerase based isothermal amplification coupled with lateral flow immunochromatographic assay for visual detection of citrus tatter leaf virus[J]. Journal of Virological Methods,2022,309:114593.
[17] 陳玲,段续伟,张开春,张晓明,王晶,闫国华,周宇. 基于重组酶聚合酶扩增(RPA)技术的樱桃病毒A(CVA)的检测方法[J]. 园艺学报,2020,47(2):390-398.
CHEN Ling,DUAN Xuwei,ZHANG Kaichun,ZHANG Xiaoming,WANG Jing,YAN Guohua,ZHOU Yu. A method for the detection of cherry virus A (CVA) based on recombinase polymerase amplification (RPA) technique[J]. Acta Horticulturae Sinica,2020,47(2):390-398.
[18] 陈玲,闫国华,张晓明,周宇,王晶,段续伟,李彦林,张开春. 李矮缩病毒重组酶聚合酶扩增—侧流层析试纸条检测方法的建立[J]. 园艺学报,2021,48(1):183-192.
CHEN Ling,YAN Guohua,ZHANG Xiaoming,ZHOU Yu,WANG Jing,DUAN Xuwei,LI Yanlin,ZHANG Kaichun. Establishment of recombinase polymerase amplification combined with lateral flow dipstick for detection of prune dwarf virus[J]. Acta Horticulturae Sinica,2021,48(1):183-192.
[19] ZHANG S L,RAVELONANDRO M,RUSSELL P,MCOWEN N,BRIARD P,BOHANNON S,VRIENT A. Rapid diagnostic detection of plum pox virus in Prunus plants by isothermal AmplifyRP? using reverse transcription-recombinase polymerase amplification[J]. Journal of Virological Methods,2014,207:114-120.
[20] 劉科宏,周彦,王雪峰,唐科志,周常勇. 柑橘衰退病毒在3种寄主不同组织中分布的研究初报[J]. 西北农林科技大学学报(自然科学版),2005,33(S1):109-110.
LIU Kehong,ZHOU Yan,WANG Xuefeng,TANG Kezhi,ZHOU Changyong. Distribution of Citrus tristeza virus among three hosts in different tissues[J]. Journal of Northwest A & F University (Natural Science Edition),2005,33(S1):109-110.
[21] WARGHANE A,MISRA P,BHOSE S,BISWAS K K,SHARMA A K,REDDY M K,GHOSH D K. Development of a simple and rapid reverse transcription-loop mediated isothermal amplification (RT-LAMP) assay for sensitive detection of Citrus tristeza virus[J]. Journal of Virological Methods,2017,250:6-10.
[22] CRANNELL Z A,ROHRMAN B,RICHARDS-KORTUM R. Equipment-free incubation of recombinase polymerase amplification reactions using body heat[J]. PLoS One,2014,9(11):e112146.

