色素上皮衍生因子与胰岛素抵抗的相关性
李翔 杨铭 董茜


【摘要】 色素上皮衍生因子(pigment epithelium-derived factor,PEDF)是具有多种生理活性的糖蛋白,包括抗血管新生、降低血管通透性、抗肿瘤以及神经营养活性。最近研究表明PEDF能够调节糖代谢和脂代谢,与胰岛素抵抗的有一定的联系。本文就PEDF引起胰岛素抵抗这方面的研究做一综述,阐述了PEDF与代谢性疾病的广泛联系,并总结了PEDF引起胰岛素抵抗的可能机制。
【关键词】 PEDF; 代谢; 胰岛素抵抗; 机制
doi:10.14033/j.cnki.cfmr.2017.5.088 文献标识码 A 文章编号 1674-6805(2017)05-0162-03
The Relationships of PEDF and Insulin Resistance/LI Xiang,YANG Ming,DONG Qian.//Chinese and Foreign Medical Research,2017,15(5):162-164
【Abstract】 Pigment epithelium-derived factor(PEDF) is a glycoprotein with a variety of physiological activities including anti-angiogenic,anti-vasopermeability,anti-tumor,and neurotrophic activities.Recent studies have shown that PEDF is a metabolic regulatory protein that plays a casual role in glucose and lipid metabolism.This review focus on researches which regard PEDF as the causes of insulin resistance,describes the extensive contacts between PEDF and metabolic diseases,and summarizes the probable mechanisms adopted by PEDF to induce insulin resistance.
【Key words】 PEDF; Metabolism; Insulin resistance; Mechanisms
First-authors address:Xuzhou Medical University,Xuzhou 221000,China
PEDF是在人視网膜色素上皮细胞的分泌物中发现并纯化的50-KDa糖蛋白。最初认为PEDF是一种生长因子,能够诱导神经元的分化。直到1999年,人们才逐渐发现PEDF具有十分丰富的生理活性,包括抗血管新生[1],抗肿瘤[2],降低血管通透性[3],以及神经营养活性[4]。国内外研究表明色素上皮衍生因子(pigment epithelium-derived factor,PEDF)与胰岛素抵抗的发生有关,而众所周知,胰岛素抵抗是2型糖尿病的发病基础,也是代谢综合征的核心环节。因此,现就PEDF与胰岛素抵抗的关系展开分析,并试图总结PEDF引起胰岛素抵抗的可能机制。
1 PEDF与胰岛素抵抗
目前有多项研究表明PEDF与IR有密切关系。但是PEDF究竟是会导致IR,还是改善IR尚存争议。
Yoshida等[5]研究了PEDF与糖基化终产物(AGE)的相关性。研究显示PEDF能够抑制Rac-1的活性,从而抑制AGE引起的肝细胞IRS-1酪氨酸磷酸化降低,还能够阻断JNK、IKB激酶依赖IRS-1丝氨酸磷酸化,从而ROS、CRP的产生,起到抗炎症、抗氧化应激的作用。因此,PEDF可改善IR。
Crowe等[6]的研究结果却与之相反。小鼠短期注射PEDF会导致ERK、JNK磷酸化增加,胰岛素信号通路中IRS1、AKT活性磷酸化位点的磷酸化减少[7]。因此PEDF会诱导产生IR。
本文则综述了支持PEDF引起IR的相关研究进展。
2 PEDF与代谢性疾病
PEDF具有多能性,在不同的组织细胞中表现出不同的功能。如:PEDF在血管抑制信号的作用下发挥了有效的抗炎症作用[8]。在代谢系统中却起到了致炎症的作用[9]。在多种代谢性疾病中(图1),PEDF的循环水平都会升高。
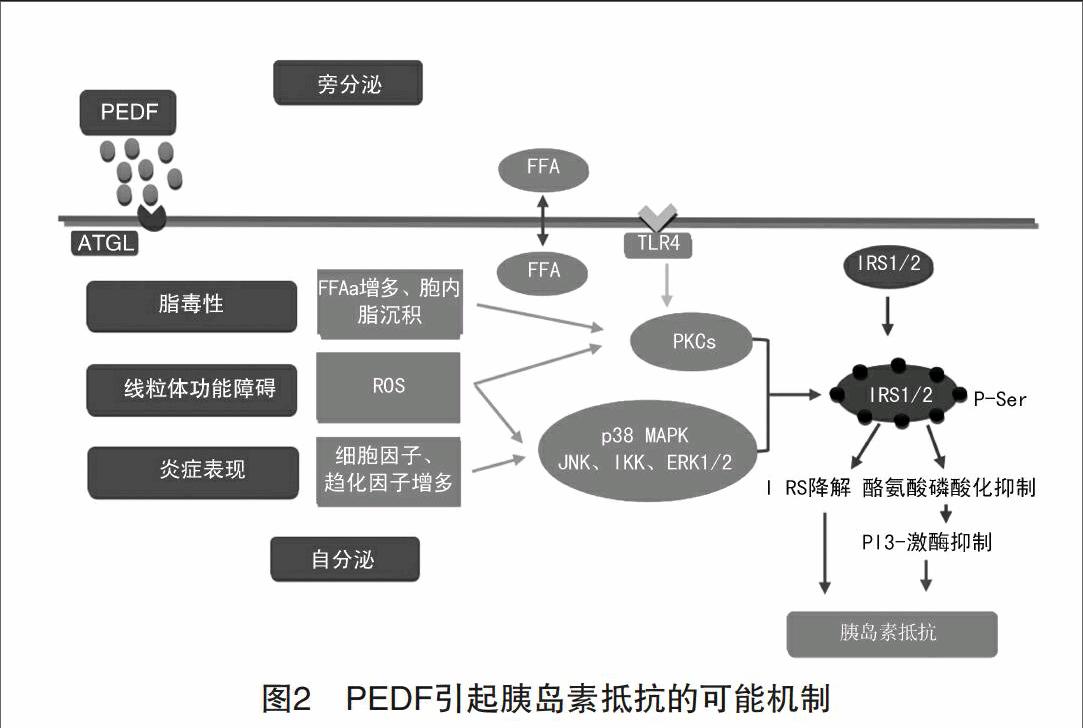
Nakamura等[10]的研究表明,在2型糖尿病患者的血清中,中心性肥胖相关因子(腰围、甘油三酯、肿瘤坏死因子-α)与PEDF密切相关。另外PEDF还与空腹胰岛素水平、HOMA-IR、BMI、脂肪量呈负相关。肝细胞PEDF基因的表达与肥胖者PEDF的水平呈正相关,且与2型糖尿病患者的丙氨酸转移酶、天冬氨酸转移酶的水平(代表肝功能)呈负相关。这表明,PEDF与IR相关,且肝脏是PEDF的主要来源[11]。PEDF与脂肪形成密切相关。最近的研究表明,PEDF可能促成脂肪组织的形成,促成IR和代谢功能紊乱[12]。Crowe等[6]给正常小鼠注射PEDF,检测到胰岛素敏感性降低,且若降低PEDF量,胰岛素敏感性会有所回升。但是PEDF引起IR的机制暂不清楚。给小鼠喂食PEDF引起IR的同时,血清中的TNF以及其他的细胞因子水平会有所上升,因此TNF可能是PEDF引起IR的下游调节分子(图2)。
代谢综合症患者[13],以及多囊卵巢病(PCOS)的患者血清中PEDF水平较高,而且与IR相关[14]。PCOS患者的PEDF水平与CRP密切相关,提示PEDF与PCOS病人慢性炎症的发生有一定的联系[15]。图3总结了目前研究中显示的PEDF参与的代谢。
3 PEDF引起胰島素抵抗的可能机制
3.1 影响脂代谢阻断胰岛素信号转导通路
PEDF与脂肪的分解有关,能够使游离脂肪酸(FFAs)增多。增多的FFAs会抑制磷脂酰肌醇3-激酶(PI3K)的激活,还会导致异位的脂沉积,这两者均会阻断胰岛素信号转导通路[16]。另外,胞内的脂质积累能够激活PKC-β,δ,θ的活性。PKCθ的活性提高会伴随着胰岛素受体底物(IRS)的酪氨酸磷酸化以及葡萄糖转运的减少。
还有研究显示,PEDF与JNK、IKK和ERK1/2的激活有关。JNK能够磷酸化IRS-1中丝氨酸或苏氨酸残基,这种磷酸化作用会干扰胰岛素受体与IRS-1的作用,从而阻止IRS-1的酪氨酸磷酸化[17]。IκKβ与IRS-1组成复合物,与TNFα介导的IR有关[18]。ERK1/2也可能是PEDF的作用靶点[19],能够引起IRS-1的丝氨酸磷酸化,抑制酪氨酸磷酸化,减弱胰岛素信号转导[20]。
3.2 引起线粒体功能障碍
PEDF能够抑制脂肪酸氧化,从而形成一种“脂毒性”的环境。Gao等[21]的研究表明,FFAs能够引起线粒体功能障碍。PEDF抑制脂肪酸的β氧化和线粒体生成,还刺激ATGL生成脂肪酸配体,从而激活过氧化体增殖物激活型受体(PPARs),增加线粒体的脂肪酸氧化[22]。功能紊乱的线粒体能够生成ROS,导致胰岛素抵抗。在胰岛素抵抗的周缘组织中(如骨骼肌、肝脏、脂肪),线粒体数量降低、形态和功能异常。PEDF引起的线粒体功能异常会阻碍胰岛素信号转导,还有损害线粒体生成。
3.3 引起代谢炎症
代谢紊乱也被认为是一种长期低水平的炎症状态。PEDF通过直接或间接地方式引起代谢炎症(图2)。PEDF引起的FFAs增多,会激活Toll样受体介导的炎症信号,从而激活IκKβ和JNK。于是多种细胞因子被激活,包括TNFα、IL-1β、IL-6[23]。在人脐静脉内皮细胞(HUVECs)实验中,PEDF能增加p38 MAPK和JNK的磷酸化,降低AKT活性[24]。AKT活性的降低可以激活MAPK2[25]。MAPK2的激活,又可以激活p38 MAPK。因此PEDF可以引发,维持,并推进激酶介导的丝氨酸/苏氨酸磷酸化级联反应,抑制胰岛素在外周组织的信号转导(表1)。
参考文献
[1] Dawson D W,Volpert O V,Gillis P,et al.Pigment epithelium-derived factor:a potent inhibitor of angiogenesis[J].Science,1999,285(5425):245-248.
[2] Abe R,Fujita Y,Yamagishi S,et al.Pigment epithelium-derived factor prevents melanoma growth via angiogenesis inhibition[J].Current Pharmaceutical Design,2008,14(36):3802-3809.
[3] Pollina E A,Legesse-Miller A,Haley E,et al.Regulating the angiogenic balance in tissues: a potential role for the proliferative state of fibroblasts[J].Cell Cycle,2008,7(13):2056-2070.
[4] Becerra S P,Sagasti A,Spinella P,et al.Pigment Epithelium-derived Factor Behaves Like a Noninhibitory Serpin Neurotrophic Activity Does Not Require the Serpin reactive Loop[J].Journal of Biological Chemistry,1995,270(43):25992-25999.
[5] Yoshida T,Yamagishi S,Nakamura K,et al.Pigment epithelium-derived factor (PEDF) ameliorates advanced glycation end product (AGE)-induced hepatic insulin resistance in vitro by suppressing Rac-1 activation[J].Hormone and Metabolic Research,2008,40(9):620-625.
[6] Crowe S,Wu L E,Economou C,et al.Pigment epithelium-derived factor contributes to insulin resistance in obesity[J].Cell metabolism,2009,10(1):40-47.
[7] Borg M L,Andrews Z B,Duh E J,et al.Pigment Epithelium-Derived Factor Regulates Lipid Metabolism via Adipose Triglyceride Lipase[J].Diabetes,2011,60(5):1458-1466.
[8] Zhang S X,Wang J J,Gao G,et al.Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor[J].The FASEB journal,2006,20(2):323-325.
[9] Chen C,Tso A W K,Law L S C,et al.Plasma level of pigment epithelium-derived factor is independently associated with the development of the metabolic syndrome in Chinese men:a 10-year prospective study[J].The Journal of Clinical Endocrinology & Metabolism,2010,95(11): 5074-5081.
[10] Nakamura K,Yamagishi S,Adachi H,et al.Serum levels of pigment epithelium‐derived factor(PEDF) are positively associated with visceral adiposity in Japanese patients with type 2 diabetes[J].Diabetes/Metabolism Research and Reviews,2009,25(1):52-56.
[11] Moreno-Navarrete J M,Touskova V,Sabater M,et al.Liver,but not adipose tissue PEDF gene expression is associated with insulin resistance[J].International Journal of Obesity,2013,37(9):1230-1237.
[12] Borg M L,Andrews Z B,Duh E J,et al.Pigment Epithelium–Derived Factor Regulates Lipid Metabolism via Adipose Triglyceride Lipase[J].Diabetes,2011,60(5):1458-1466.
[13] Yamagishi S,Adachi H,Abe A,et al.Elevated serum levels of pigment epithelium-derived factor in the metabolic syndrome[J].The Journal of Clinical Endocrinology & Metabolism,2006,91(6):2447-2450.
[14] Yang S,Li Q,Zhong L,et al.Serum pigment epithelium-derived factor is elevated in women with polycystic ovary syndrome and correlates with insulin resistance[J].The Journal of Clinical Endocrinology & Metabolism,2011,96(3):831-836.
[15] Cheng Q,Xia W,Yang S,et al.Association of serum pigment epithelium-derived factor with high-sensitivity C-reactive protein in women with polycystic ovary syndrome[J].Journal of Endocrinological Investigation,2013,36(8):632-635.
[16] Kratchmarova I,Kalume D E,Blagoev B,et al.A proteomic approach for identification of secreted proteins during the differentiation of 3T3-L1 preadipocytes to adipocytes[J].Molecular & Cellular Proteomics,2002,1(3):213-222.
[17] Aguirre V,Werner E D,Giraud J,et al.Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action[J].Journal of Biological Chemistry,2002,277(2):1531-1537.
[18] Nakamori Y,Emoto M,Fukuda N,et al.Myosin motor Myo1c and its receptor NEMO/IKK-γ promote TNF-α–induced serine 307 phosphorylation of IRS-1[J].The Journal of Cell Biology,2006,173(5):665-671.
[19] Wang M,Wang J J,Li J,et al.Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ERK pathway in 3T3-L1 preadipocytes[J].American Journal of Physiology-Endocrinology and Metabolism,2009,297(6):E1378-E1387.
[20] Arimura T,Miura S, Sugihara M,et al.Association between plasma levels of pigment epithelium-derived factor and renal dysfunction in patients with coronary artery disease[J].Cardiol J,2011,18(5):515-520.
[21] Gao C L,Zhu C,Zhao Y P,et al.Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes[J].Molecular and Cellular Endocrinology,2010,320(1):25-33.
[22] Gaetano C,Colussi C,Capogrossi M C.PEDF,PPAR-δ,p53:Deadly circuits arise when worlds collide[J].Cardiovascular Research,2007,76(2):195-196.
[23] Kim F,Pham M,Luttrell I,et al.Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity[J].Circulation Research,2007,100(11):1589-1596.
[24] Chen L,Zhang S S M,Barnstable C J,et al.PEDF induces apoptosis in human endothelial cells by activating p38 MAP kinase dependent cleavage of multiple caspases[J].Biochemical and Biophysical Research Communications,2006,348(4):1288-1295.
[25] Gratton J P,Morales-Ruiz M,Kureishi Y,et al.Akt down-regulation of p38 signaling provides a novel mechanism of vascular endothelial growth factor-mediated cytoprotection in endothelial cells[J].Journal of Biological Chemistry,2001,276(32):30359-30365.
(收稿日期:2016-10-21)

