Droplet microfluidic chip for precise monitoring of dynamic solution changes
Cong Ma, Zehang Gao, Jianlong Zhao, and Shilun Feng
AFFILIATIONS 1 School of Information Science and Technology,ShanghaiTech University,Shanghai 201210,China
2Shanghai Institute of Microsystem and Information Technology,Chinese Academy of Sciences,Shanghai 200050,China
3Center of Materials Science and Optoelectronics Engineering,University of Chinese Academy of Sciences,Beijing 100049,China
4Department of Clinical Laboratory,Third Affiliated Hospital of Guangzhou Medical University,Guangdong 510150,China
ABSTRACT In this work,an automated microfluidi chip that uses negative pressure to sample and analyze solutions with high temporal resolution was developed.The chip has a T-shaped channel for mixing the sample with a fluorescen indicator,a flow-focusin channel for generating droplets in oil,and a long storage channel for incubating and detecting the droplets.By monitoring the fluorescenc intensity of the droplets,the device could detect changes in solution accurately over time.The chip can generate droplets at frequencies of up to 42 Hz with a mixing ratio of 1:1 and a temporal resolution of 3–6 s.It had excellent linearity in detecting fluorescei solution in the concentration range 1–5 μM.This droplet microfluidi chip provides several advantages over traditional methods,including high temporal resolution,stable droplet generation,and faster flo rates.This approach could be applied to monitoring calcium ions with a dynamic range from 102 to 107 nM and a detection limit of 10 nM.
KEYWORDS Microfluidic chip,Droplet sampling,Fluorescence detection,Calcium ion dynamics,Temporal resolution
I.INTRODUCTION
The homeostasis and dynamics of intracellular calcium ions play a crucial role in neural communication in the brain.To better understand brain function,it is essential to accurately monitor changes in its concentration within neurons.Detecting fluorescence using probes such as fluo-4 is commonly used for monitoring calcium ions.However,the high temporal resolution required for monitoring such rapid dynamics remains a challenge.
However,when monitoring biochemical molecules,collecting,manipulating,and conducting a high temporal resolution analysis of nanoliter samplesin vivois still challenging.1Sampling and monitoring rapidly changing biochemical signals in a tiny liquid sample with a high temporal resolution are significan for studying kinetic reactions.2A widely used liquid biopsy method in neuroscience for quantifying the release of neurotransmitters is microdialysis.Its advantages include the small size of the equipment,minor trauma,and long-time continuous monitoring of drug concentrations.3However,the primary concentration of samples collected by microdialysis probes needs to be revised by recovery,which increases the complexity of the experiment and limits the accuracy of the results.It also has a low temporal resolution of up to minutes because of the low perfusion rate and Taylor dispersion.4
One way to reduce the size of monitoring devices is microfabrication.There is less tissue trauma due to the high-resolution sampling microstructure.5Electrochemical probes based on microelectrodes can achieve high spatial resolutions down to 0.003 mm2and time resolutions down to several seconds forin vivomonitoring.6They have a low threshold and can simultaneously detect glutamate below 500 nM and dopamine below 20 nM.7However,they rely on selective enzymatic reactions to detect a specifi molecule,so the devices must be calibrated regularly.8
Droplet microfluidi chips are a widely used and promising technology for precisely manipulating and analyzing small volumes of fluid9typically from nanoliters to microliters.10The solution is encapsulated in oil.These chips are considered to be a powerful tool in biomedical research with advantages such as small independent reactors,high throughput,minimal Taylor diffusion,11less channel surface absorption,and effective mixing.12Droplets can also be ideal containers for biopsy sampling13because they reduce the impact on the targetin vivodue to the small sample volumes.14Isolated samples can be collected at different times.The devices have high temporal15and spatial resolutionsin situorin vivo.16
Many kinds of backend biomolecules have been used for accurate,efficient and high-resolution online detection of droplets,17based on techniques such as fluorescence18chromatography,mass spectrometry,19electrochemistry,and conductivity.20
Researchers have explored the use of droplet microfluidi chips for monitoring dynamic changes in solutions in various fields12For example,one application is measuring chemical kinetics.Droplet microfluidi chips have been used to study the kinetics of chemical reactions in real time with high precision.21Another application is in environmental monitoring,as microfluidi chips can detect changes in the concentration of pollutants in water over time.Additionally,droplet microfluidi chips have shown promise in diagnostics,because they can detect biomarkers in bodily fluid and provide results in minutes.22
Researchers have recently focused on developing droplet microfluidi chips for monitoring dynamic changes in solutions in real time.23The continuous monitoring of a solution requires the integration of sensors and other components into a microfluidi chip.23For example,researchers have developed droplet microflu idic chips that can monitor changes in pH and temperature during reactions under controlled conditions.Other researchers have developed microfluidi chips that can monitor changes in the concentration of analytes over time,which may be a powerful tool for environmental monitoring and biomedical research.24
Because of these advantages of droplet microfluidics many researchers have made progress in bioscience applications.In Song’s work,nanoliter droplets served as reaction tanks when measuring the millisecond kinetic parameters of an enzymatic reaction.25They were able to determine reaction times precisely by measuring the transport distance.However,regrettably,injecting pretreated samples into a channel and generating droplets is inappropriate forin vivocollection.So,Feng developed a microfluidi needle for sampling and delivering chemical signals by segmented flows26The method produced droplets with good volumes and frequency consistency when determining the concentration of hydrogen peroxide.A push-pull perfusion microprobe was developed by Van den Brinket al.It can generate droplets for sampling and online detection with high chemical and spatial resolutions and a temporal resolution of 3 s.6It is a useful tool for fundamental neuroscience research,but the temporal resolution of 3 s still needs improvement because the neurotransmitter release process takes only milliseconds.Liet al.developed an automated droplet generation and analysis device based on a push-up valve with high resolution when sampling and measuring hormone secretion.27
Overall,the use of droplet microfluidi chips for monitoring dynamic changes in solutions has the potential to revolutionize a wide range of fields from drug discovery and environmental monitoring to point-of-care diagnostics.28,29As researchers continue developing and refinin this technology,we expect to see many exciting new applications.
II.DESIGN
This work explores the potential of droplet microfluidi chips for monitoring applications.The workflo of our device is illustrated in Fig.1.In summary,a negative pressure pump pulls the flui through the chip.The fluorescenc is recorded on video using a microscope and used for flo analysis.
The device utilizes negative pressure to transport liquid samples into a T-shaped channel where the detection reagent is added.A flow-focusin structure is then used to generate sub-nanoliter droplets.These are mixed in the sample incubation area before being transported to the detection area below the microscope.This approach enables real-time monitoring of rapidly changing molecules and is an efficien online fluorescenc monitoring platform.
III.MATERIALS
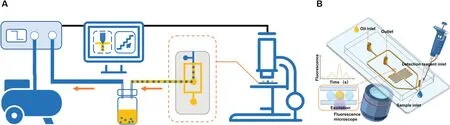
FIG.1.Droplet sampling and detection system:(a)Pneumatic control module,chip,and microscope.(B)Sampling and detecting droplets in the chip.
The materials used in this study included a negative photoresist(SU-8 3050,MicroChem,Newton,MA) and 4-inch silicon wafers(MCL Electronic Materials).Lithography was performed using a maskless aligner(MicroWriter ML3,Durham Magneto Optics Ltd.).Polydimethylsiloxane(PDMS;Sylgard 184)was used as the material for the microfluidi chip.Fluorescent calcium indicator (Fluo-4,Thermo Fisher)and fluorescei dye(Sigma-Aldrich)were prepared in different concentrations.A plate reader was used for detection(Victor 2 V Multilabel Counter,PerkinElmer).The oil used in droplet formation was homogenized with surfactants using an ultrasonic bath(Selecta Ultrasonic).A 10 mM solution of CaCl2in 20 ml of deionized water was prepared as the calcium ion source and lower concentrations were prepared using serial dilution.
IV.FABRICATION
The lengths of each channel were determined from fluidi resistance calculations so that the droplets were generated with an appropriate separation in the oil.The fluidi resistance match,which determines the flo rate among the sample,reagent,and oil,was calculated as follows:
whereL,W,andHare the length,width,and height of each section of a channel andμis the flui viscosity.Here the viscosity of the mineral oil and water used in the experiment were 25 and 1 mPa s,respectively.
This microfluidi chip was fabricated by a soft lithography process,as shown in Fig.2.The chip consists of a glass slide and a PDMS block.It has three inlets for the sample,reagent,and oil,which are produced by punching holes into the ends of the channels.A pipette tip can be plugged into each of these holes.
The process flo is illustrated in Fig.2(a).Figure 2(b)further demonstrates the microchannel fabrication and punching process as well as the hierarchy of the chip.Soft lithography was used to fabricate the microfluidi chip from PDMS as follows.A mixture of PDMS was poured into a mold made from the photoresist.It was then heated and cured to cross-link it with the sample and finall stripped off.The PDMS and glass were treated with oxygen plasma and placed together so that covalent bonds formed between the PDMS and the glass sheet.The PDMS was then heated in an oven at 110°C for 12 h to restore its hydrophobicity so that stable droplets could be generated.
V.EXPERIMENTS
A.Device demonstration with the dye solution
A vacuum pump with a negative pressure valve was connected to the chip outlet and used to drive the flow The mixing process at the front of the chip is depicted in Fig.3.First,mineral oil is injected into the chip and dye solution is injected into the reagent channel.Negative pressure of-10 kPa then extracted the air in the chip.Since the pressure of the reagent channel is less than that of the sampling channel,the reagent flowe into the droplet generation point.The transparent sample flowe into the channel where it immediately mixed with the dye solution and the mixture separated into droplets.Then the negative pressure was increased to-15 kPa to drive the sample reagent to discharge the oil and dye solution into the sampling channel.If the liquid flo of the two aqueous phases is stable,droplets naturally form which continue to mix or react during their transport along the channel.The droplets were mixed entirely with the reagent solution during transportation and detected by fluorescenc microscopy as they flowe through the channel.
The channel size controlled the size and speed of the droplets.The length of the droplets could be fixe by adjusting the pressure valve.Thus,the process in the chip is stable.The chip can be seen as an analog-to-digital converter because it transforms the continuous concentration of the solution into discrete droplets arranged in sequence at different positions in the channel.This experiment verifie the ability of the chip to collect and mix samples with a reagent and form droplets.
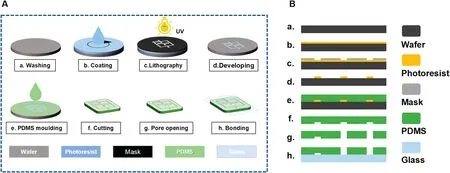
FIG.2.Fabrication.(a)Manufacture process with soft lithography and binding.(b)Side views showing layers of the chip during fabrication.
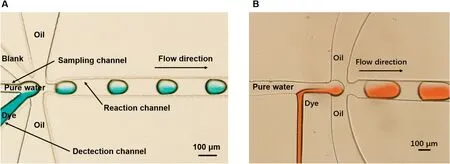
FIG.3.Structure for sampling and mixing before droplet generation:(a)mixer with two reagent channels and(b)mixer with one reagent channel.
The statistical results indicate that droplets were generated with a frequency of 42 ± 2 Hz under a -15 kPa negative pressure.The droplet length was consistent with an average value of 223±11 μm in a 200 μm width channel,indicating that the volume of each droplet was about 4.19 nl and that the sample flo rare was 88 nl/s.These results suggest that a microfluidi droplet chip can effectively monitor the dynamic changes of a solution.
Two kinds of solutions come into contact when they reach the T-junction in the microfluidi droplet chip.They were efficientl mixed during droplet transportation,resulting in consistent and accurate immunoassay results,as shown in Fig.3.The mixing ratio was determined through fluorescenc imaging of droplets containing fluorescei mixed with a buffer.Characterization of 33 droplets of each concentration revealed that the fluorescen intensity of the droplets was 48.05±0.22%in the reference experiment,indicating a mixing ratio of 1:1.08.The mixing precision was high,with a relative standard deviation of less than 0.5%.This consistent mixing ratio for the sample solution and probe solution within each droplet is crucial for accurate droplet immunoassays.Thus,this chip is a reliable platform for monitoring dynamic changes in a solution.
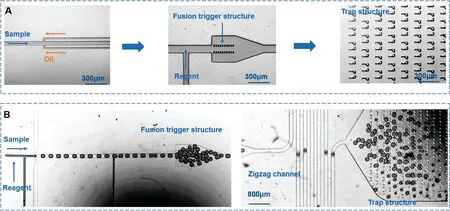
FIG.4.Droplet generation,mixing,and capture structures:(a)chip A and(b)chip B.
A mixer with two reagent channels can be used for more complex reactions with different combinations of reagents.However,when there are two reagent channels,it is difficul to ensure that both phase reagents are stably mixed with the sample at the same time.Thus,the stability of the chip is reduced,making the subsequent analysis more difficult Therefore,a feasible approach is to pre-mix multiple reagents off-chip and then mix them with the sample in a single reagent channel.
Our aim is to put as much of the droplet generation and mixing structure as possible at the back end of the chip.This would reduce the size of the front of the chip,allowing it to be developed into a probe forin vivodetection in the future.Moreover,we want to keep the test reagents some distance from the sampling point to avoid mutual contamination.This would prevent the fusion or interference of droplets in the reaction channel after mixing.Because detecting fluorescenc needs time to incubate,long exposures are needed to collect the weak fluorescenc signals.
Thus,we designed two microstructures for generating,mixing,and capturing droplets(Fig.4).The droplet generation structure of chip A is shaped like a needle tip.This structure is followed by a fusion trigger structure and a trapping structure.Chip B has reagent inlets before and after the droplet generation structure.This is followed by a fusion trigger structure,a zigzag incubation channel,and a different type of trapping structure.However,our experiments found that,although it was easy to determine the volume of the mixed reagent and clarify the specifi reaction time point for the structure in which the detection reagent was mixed into a droplet after the droplet had been produced,30the mixing was unstable and not all droplets were mixed properly.
The most significan difficult for the droplet capture structure is ensuring the sequential arrangement of droplets,which this structure cannot accomplish.Thus,we adopted a bypass structure.However,the bypass structure requires that the channel is accurately designed and manufactured and also needs a high driving pressure.Therefore,we finall found that the best structure is the continuous zigzag microchannel,which has a simple design and a high area utilization rate.29Most importantly,it generated the best sequential arrangement of droplets,so we chose this structure.
B.Resolution measurements by fluorescent dye
We tested the temporal and concentration resolutions of the chip by monitoring a rapidly changing fluorescen dye solution.To do this,we manually adjusted the solution within the reservoir.We tested the assay performance by quickly changing the solution concentration from 1 to 5 μM using a pipette at the sample inlet.A high-concentration dye solution was added to the existing lowconcentration solution so that each subsequent droplet had a higher concentration.
We recorded a video of the droplets flowin through the downstream chip channel under the objective lens of a fluorescenc microscope camera.However,the high flo speed of the droplets limited the exposure time,necessitating a higher fluorescenc intensity than for static objects.We resolved this problem by improving the intensity of the excitation LED light source(100%),maximizing the camera amplificatio (iso1600),using the highest magnificatio possible(10×),and merging a 4×4 pixel array into 1 pixel.
The video was analyzed with the software IMAGEJ,which extracted the fluorescenc intensity over time for a selected line across the channel.We then processed the data using MATLAB to extract the top edge of the point clusters and smooth the discrete points into a plane curve.Figure 5(a) shows the mean gray values varying rapidly with time,indicating the ability of the chip to sample and detect chemical changes.The droplet microfluidi chip showed high temporal and chemical resolutions,enabling the rapid detection of changes in fluorescenc intensity when a higher concentration solution was added to an existing solution.The small dead volume and high-frequency of generating droplets contributed to the high resolution of the chip.The drop observed following a peak was due to dilution.The chip has an excellent temporal resolution of 3–6 s and a chemical resolution of 1 μM for fluorescen dye at a droplet flo frequency of 42 Hz.These finding demonstrate the ability of the droplet microfluidi chip to detect concentration changes that take more than 3 s.This remarkable result surpasses the capabilities of existing similar devices and needles.

FIG.5.(a)Normalized fluorescence intensity plotted against time,showing the rapid changes in the concentration of the fluorescent dye.Each peak represents a droplet as the concentration was increased.(b)Linear relation between normalized fluorescence intensity and concentration.Error bars indicate standard deviations for at least three measurements.
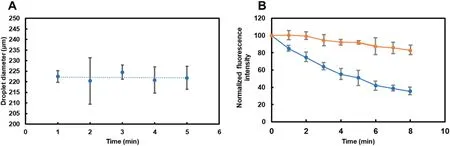
FIG.6.Characterization of droplets.(a)Droplet diameter measured over 4 min.Each point represents an average for at least three droplets.(b)Decay of fluorescence during continuous exposure(blue)and after an interval exposure(orange).
Moreover,we measured the fluorescenc intensity for different concentrations of the dye solution using a 384-well plate and a plate reader.As illustrated in Fig.5(b),the fluorescenc intensity of the droplets had an excellent linear relation with the concentration.We obtained similar results when detecting droplets generated by the chip and recorded on video.We verifie these results after storing the droplets in the channel for 5 min.These results confir that the chip can convert high-speed flo detection into static detection using the space-for-time method,as dynamic changes are recorded in the discrete droplets.
We measured the diameter of the droplets after mixing within 5 min to judge the sampling and the volume after mixing,and found that the change in the diameter of the droplets after mixing was about 5% in the range of 222 μm,indicating that the difference between the volume of each droplet in this sampling and the mixing method was small,laying the foundation for stable sampling and detection,and avoiding the influenc of droplet volume change on detection [Fig.6(a)].The decay of fluorescenc was compared for two methods: long-lasting continuous exposure and short-time-interval exposure (10 ms) in each minute,,which simulates multi-detection for flowin droplets[Fig.6(b)].Pictures were taken at the same time intervals over 8 min under the two conditions.Continuous exposure led to a 50% decay in flu orescence,whereas the interval exposure resulted in only a 10%decay.Each flowin droplet was exposed for less than a second.This suggests that for multi-detection applications,the decay of the fluorescenc for flowin droplets is reduced by~40 percentage points.This findin highlights the potential of using flowin droplets to reduce interference and could improve the accuracy and reliability of back-end fluorescenc measurements of a collected sample.
C.Calcium detection
1. Detecting calcium with a plate reader

FIG.7.Calcium ion detection.(a)Bar plots of fluorescence intensity versus calcium concentration.(b)Linear relation between normalized fluorescence intensity and log of concentration.Error bars indicate standard deviations for at least three measurements.
We mixed 10 μM of fluo-4 a fluorescen calcium indicator,with CaCl2solution in a one-to-nine ratio in 96-well plates.As measured by a plate reader,the fluorescenc intensity had an excellent loglinear relation with CaCl2concentration in solutions ranging from 100 nM to 10 mM(Fig.7).Thus,we chose this concentration range for the experiments with our chip.
2. Detecting calcium in the microfluidic chip with a microscope
For future work,we will determine the linear range and threshold for detecting calcium ions with our device.As above,the liquid sample will be introduced into the sampling channels,where the sample will mix with a reagent to generate fluorescen material.It will then enter a flow-focusin structure with oil so that the aqueous phase will separate into droplets.We plan to mix different concentrations of calcium ions with detection reagents outside the chip,then detect the concentration inside the chip to obtain a standard curve for detecting calcium ions.Thus,we will realize in-chip mixed detection for different concentrations of calcium ions in the bulk reservoir.We hope this method can be used to detect calcium ions in real samples,and we plan to extend this method to more targets.
VI.CONCLUSIONS
In this study,a droplet microfluidi chip was developed.Droplets are generated and driven under negative pressure to meet the requirements of futurein vivosampling applications.This approach improves the detection resolution,which is limited by Taylor dispersion,by encapsulating samples in discrete droplets.The chip could extract sub-nanoliter samples from the front end and mix them with a detecting reagent to produce droplets that a fluo rescence microscope can detect.It can accurately measure changes in concentration with a high temporal resolution.This study investigated the relations between sampling frequency,applied pressure,and mixing ratio and tested the capability of the chip as a sampling device.We demonstrated its linear range by sampling a series of diluted fluorescei solutions from 102to 107nM.The droplets were generated at a frequency of 42 Hz under-15 kPa with a mixing ratio of 1:1.The temporal resolution was 3–6 s.Our results demonstrate the feasibility of using a droplet microfluidi chip for dynamically sampling the concentration of a solution by detecting fluorescence Its advantages over microdialysis probes include high temporal resolution,smaller sampling volume,and controllable mixing ratio.
We established that the detection limit of fluo-4 a calcium ion indicator,was 10 nM,thus laying a foundation for detecting low concentrationsin vivo.Our future work will focus on practical applications,such as monitoring the concentration of calcium ions released by cells in a culture medium and the secretion of other small molecules using fluorescence-base probes.We will assess its capability to dynamically measure changes in the calcium concentration of a solution by mixing the sample with fluo-onchip.Moreover,we are trying to use this chip with an implantable silicon-based microneedle,as we mentioned in our previous report.31
Overall,the droplet microfluidi chip developed in this study is a promising platform for high-resolution dynamic monitoring of changes in the concentration of a solution.
VII.DISCUSSION
Despite its advantages,this microfluidi chip still has several limitations.First,the dynamic range is limited to the 255 quantitative levels measured by the 8-bit camera,so that it can saturate during constant exposure if the fluorescen intensity increases significantly.The dynamic range could be expanded four times using a 10-bit camera or by normalizing the fluorescenc intensity for different exposure parameters,such as using a long exposure time for lowerconcentration solutions and a shorter time for higher-concentration solutions.Second,the low sensitivity of the microscope camera limits the detection frequency compared to a photomultiplier tube.A higher detection sensitivity would require a shorter exposure time and allow the flo rate to be increased.Third,the large size of the chip currently limits its use forin vivosampling.However,a specially designed cutting process could be used to create a sharp needle tip,which could reduce trauma but may also cause a lack of rigidity during implantation.The platform may,instead,be fabricated with a silicon-based needle,as described in the literature.
ACKNOWLEDGMENTS
We acknowledge support from the equipment research and development projects of the Chinese Academy of Sciences,“On-chip integrated optical biochemical detection key technology research and development team,”E11YTB1001.We also thank our research platform: the State Key Lab of Transducer Technology at Shanghai Institute of Microsystem and Information Technology,Chinese Academy of Sciences.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflict to disclose.
Author Contributions
C.M.and Z.G.are co-firs authors of the article.
The firs author,Cong Ma,was responsible for designing,fabricating,and testing the microfluidi chip,for making measurements,and for writing this paper.Zehang Gao helped with experiments and revised the fina manuscript.Corresponding authors Jianlong Zhao and Feng Shilun provided the experimental environment and helped write the manuscript.
DATA AVAILABILITY
The data that support the finding of this study are available within the article.
- 纳米技术与精密工程的其它文章
- Status of research on non-conventionaltechnology assisted single-point diamond turning
- Research trends in methods for controlling macro-micro motion platforms
- Characteristics of the pressure profile in the accelerator on the RF negative ion source at ASIPP
- Oxidation mechanism of high-volume fraction SiCp/Al composite under laser irradiationand subsequent machining
- An optical tweezer-based microdroplet imaging technology
- Effects of laser energy on the surface quality and properties of electrodeposited zinc-nickel-molybdenum coatings

