Effects of laser energy on the surface quality and properties of electrodeposited zinc-nickel-molybdenum coatings
Tao Ni,Zhaoyang Zhang, Yucheng Wu,and Shuai Yang
AFFILIATIONS Laser Technology Institute,School of Mechanical Engineering,Jiangsu University,Zhenjiang 212013,China
ABSTRACT As a substitute for toxic cadmium coatings in the aerospace industry,zinc-nickel coatings have excellent application prospects,and their properties can be improved by adding molybdenum.In this study,laser-assisted electrodeposition is used to improve the surface quality and properties of Zn–Ni–Mo coatings,with investigation of how laser energy in the range of 0–21.1 μJ affects their element content,surface morphology,crystal phase,microhardness,residual internal stress,and corrosion resistance.The laser irradiation accelerates the electrodeposition,refine the grain size,improves the hydrogen adsorption,and reduces the residual tensile stress,and a laser energy of 15.4 μJ gives the highest Ni and Mo contents and the lowest Zn content,as well as the optimum surface morphology,microhardness,residual internal stress,and corrosion resistance of the coating.
KEYWORDS Electrodeposition,Laser energy,Zn-Ni-Mo,Surface quality,Properties
I.INTRODUCTION
Zinc and zinc-based alloy coatings are used widely to protect metal substrates from corrosion and are alternatives to toxic cadmium coatings in the aerospace industry.1Zn–Ni binary alloy coatings are more important than pure zinc coatings because of their improved corrosion resistance and mechanical properties,2,3and research has shown that adding molybdenum can improve the corrosion resistance,wear resistance,and hardness even further.4,5In general,Mo cannot be electrodeposited alone in aqueous solution,but it can be co-deposited with some iron group elements(Fe,Ni,Co)in a process known as induced co-deposition,6–8and some scholars have found that Zn and Mo can also induce co-deposition in citrate solution.9,10
There have been previous studies of adding Mo to Zn-based alloy coatings.Szczygiel and Laszczynska used citrate-solution electrodeposition systems to prepare Zn–Ni–Mo coatings,findin that their corrosion resistance was improved by the presence of passivation layers rich in molybdenum compounds.11Costaet al.studied the effects of current density and forced convection on Zn–Fe–Mo alloy coatings,findin that high current density reduced the electrodeposition efficienc and that forced convection increased the deposition amount of Mo.12Winiarskiet al.investigated the effect of molybdate concentration on Zn–Ni–Mo coatings,findin that excessive Mo content caused many holes in the coating,resulting in poor adhesion between coating and substrate and so lower overall strength.13
Laser irradiation can assist in electrodeposition processing to give coatings with better performance.14Zhuet al.found that the laser thermal effect can slightly increase the current density and accelerate the electrodeposition.15Wuet al.used laser-assisted electrodeposition (LAED) to prepare amorphous nickel-based alloys,findin that laser action can change the crystal state,refin the grain size,reduce the residual internal stress of the coating,and improve corrosion and wear properties.16,17Daiet al.used pulsed LAED for a Cu-Al2O3composite coating,findin that it caused impact force and micro-region agitation in the solution,thereby reducing the agglomeration of nanoparticles.18Yanget al.found that laser action can affect crystal growth,making it easier for some phases to grow.19
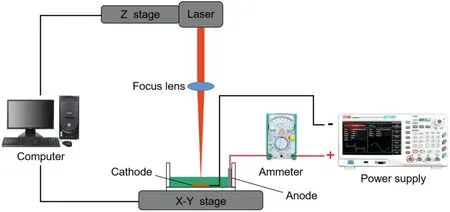
FIG.1.Schematic of laser-assisted electrodeposition(LAED)processing system.
However,previous studies tended to be of the performance of cathodic coatings,rarely considering the disappearance of protective effects after coating damage.Therefore,in the study reported herein,LAED was selected to prepare Zn–Ni–Mo anodic coating to provide longer-lasting protection to the substrate,with a composite LAED processing technique used to prepare Zn–Ni–Mo coatings by means of laser-reciprocating scanning.How the laser energy affected the surface quality,element content,phase,microhardness,residual stress,and corrosion resistance of the coatings was analyzed,the presence of Mo in the coatings was analyzed,and the sacrificia protective effect of the Zn–Ni–Mo coatings was verified
II.EXPERIMENTAL WORK
Figure 1 shows a schematic of the LAED processing system comprising a laser processing system and an electrodeposition system.The laser processing system was a picosecond one(PX1001I-A;Edge Wave,Germany)comprising a picosecond-pulse laser generator,an optical system,a workbench (X-Y-Z),a computer control system,and a water cooling system;the laser processing system provided laser energy in the range of 8.6–70 μJ.Meanwhile,a pulsed power supply was used for electrodeposition processing(UTG4082A;HuaQing,Shenzhen).The specifi parameters of the LAED process are given in Table I.
In these experiments,citric acid was used as the organic complexing agent for the sulfate solution,whose specifi composition is given in Table II.ZnSO4·7H2O,NiSO4·6H2O,and Na2MoO4·2H2O provided respectively the Zn2+,Ni2+,and MoO42-required for the experiments,with H3BO3acting as a pH buffer to stabilize the pH value and C12H25SO4Na acting as a surfactant to reduce the plating defects and the cathode surface tension.Finally,C7H4NO3S was used to reduce the plating stress.
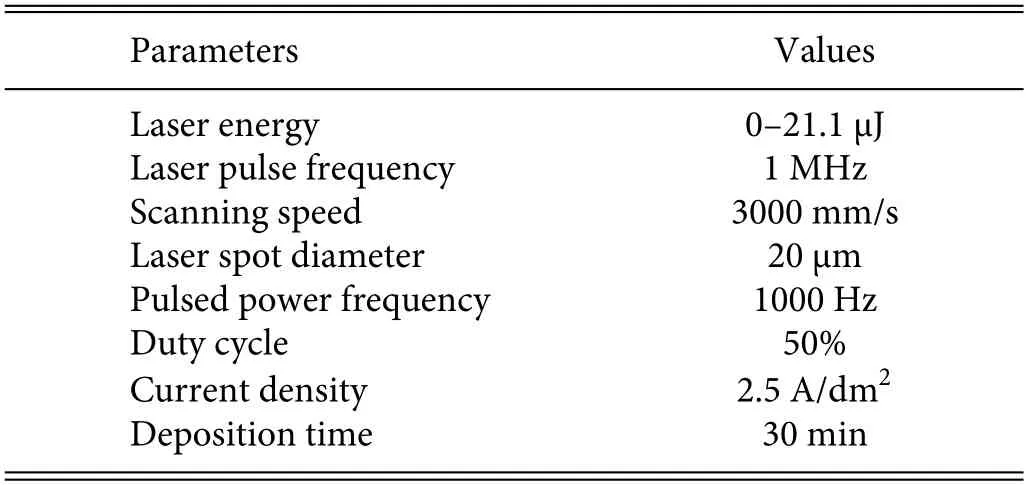
TABLE I.Main parameters of LAED processing.
The electrodeposited anode plate comprised a Zn plate and a Ni plate(60×40×2 mm3),and the cathode substrate was a Cu plate(10×10×1.5 mm3).Before an experiment,the copper plate was polished with metallographic sandpaper (1000,1500,and 2000 mesh)to remove the surface oxide fil and impurities,then polished with diamond particles (0.5,0.25 μm),and then placed in alcohol and cleaned ultrasonically for 10 min.The deposition tank (150×120×100 mm3)contained 200 ml of electroplating solution,and when LAED was used,the focal point was kept 0.5 mm above the substrate;only the laser energy was changed (zero,8.6,12.1,15.4,18.2,and 21.1 μJ),with the other parameters remaining constant.
An SEM(S-3400;Hitachi,Japan)and an accompanying energydispersive spectrometer(EDS)were used to observe the morphologies of the coating surfaces and analyze their element contents.The phase structures and grain sizes of the coatings were determined by means of XRD (D8 Advance;Bruker,Germany),with XPS (AXIS SUPRA;Shimadzu,Japan)used to characterize the surface elements and their chemical states.
A microhardness tester (FM-ARS 900;New Pool,China) was used to measure the microhardness of the coatings with a load of 100 g for 15 s.An x-ray stress tester (X-350A;Aisite,China) was used to measure the residual internal stress of the coatings;the measurement method was the tilting fixed Ψ method,with a scanning angle 2θof 0.10°,a counting time of 0.50 s,a voltage of 22.0 kV,and a current of 6.0 mA.
Full-immersion corrosion tests were performed in 3.5% NaCl solution,and to reduce errors,an electronic balance (HC1004;HuaChao,China) was used to measure the mass repeatedly.Tafel curves were obtained using an electrochemical workstation(CHI660E;ChenHua,China) in the 3.5% NaCl solution;the reference electrode was a saturated calomel electrode,the auxiliary electrode was a platinum electrode,and the open-circuit voltage was measured before a test as a reference.For each Tafel curve,the scanning rate was 0.001 V/s,the scanning range was from-1.4 to-0.6 V,and the corrosion potential (Ecorr) and corrosion current density(Icorr)were obtained by analyzing the Tafel curve.
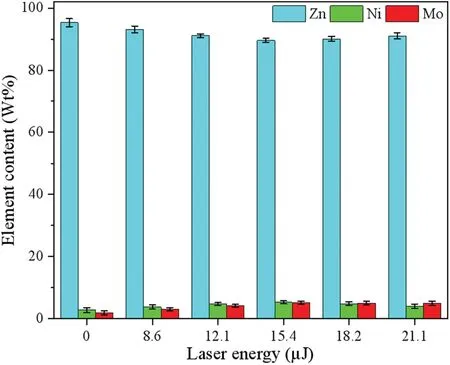
FIG.2.Element content of Zn-Ni-Mo coatings under different laser energy.
III.RESULTS AND DISCUSSION
A.Effect of laser energy on elemental composition
In citrate (Cit-) electroplating solution,metal ions exist as complex ions,and the main reactions for Zn2+,Ni2+,andare
where Me is the metal element Zn or Ni.20
The element content of the Zn–Ni–Mo coatings under different laser energy is shown in Fig.2.The molybdenum content is lowest(1.9%)without laser irradiation,then with increasing laser energy it increases to its maximum value(5.12%)at 15.4 μJ and then decreases slightly.
Because Mo is deposited via induced co-deposition,this is affected mainly by the diffusion rate of composite ions in the solution.19The temperature of the laser-irradiated region increases,and the solution flow because of temperature difference,resulting in micro-stirring,which inhibits the polarization concentration and contributes to the continuous process of electrodeposition.21In addition,with an increase in the solution temperature,the solution viscosity decreases,the diffusion rate of ions increases,the thickness of the diffusion layer decreases,and the complex ions on the cathode surface are quickly supplemented.16,19
When the laser energy is 21.1 μJ,the Mo content decreases slightly,which is possibly because of the accelerated deposition rate.Hydrogen precipitates violently on the cathode surface,and the resulting hydrogen bubbles interfere with the laser effect,resulting in energy loss.Also,the high laser energy leads to local boiling of the solution,which destroys the normal electrodeposition process and invalidates the auxiliary effect of the laser on electrodeposition.15,17
Under the present experimental conditions,the electrodeposition of Zn–Ni exhibits anomalous co-deposition.The reduction potentials of Zn and Ni are -0.762 and -0.257 V,respectively,and because of the anomalous co-deposition,Zn2+and Ni2+vary with opposite trends: with increasing laser energy,the Ni content increases while the Zn content decreases.When the laser energy is 15.4 μJ,the Ni content reaches its highest value and the Zn content reaches its lowest value,and when the laser energy exceeds 15.4 μJ,the Ni content decreases and the Zn content increases slightly.
Electrodeposition of Ni2+in the solution is affected mainly by the activation energy of the chemical reactions.22It can be inferred that when the laser energy is 15.4 μJ,the temperature of the solution in the laser-irradiated area increases and the activation energy required for the electrodeposition reaction decreases;the deposition rate also increases to a certain extent,and reduction of Ni2+is easier.When the laser energy is too high(21.1 μJ),the deposition is accelerated,the hydrogen evolution reaction is intensified and a large amount of H+precipitation causes more OH-and Zn2+to react and form Zn(OH)2,23,24which is deposited on the surface and so inhibits the deposition of Ni2+,leading to decreased nickel content.
B.Effect of laser energy on coating surface quality
Figure 3 shows SEM images of Zn–Ni–Mo coating surfaces prepared under different laser energy.Without laser irradiation,the coating surface is uneven with massive particle clusters,pores,and other defects,as shown in Fig.3(a).With increasing laser energy,as shown in Figs.3(b)and 3(c),the surface quality improves gradually but defects such as particle clusters and pores remain evident,and the coating surface structure is still uneven albeit smoother than before.When the laser energy reaches 15.4 μJ,as shown in Fig.3(d),there are very few surface defects and the grains are fin and compact.
The laser thermal effect increases the temperature of the solution in the irradiated area,accelerates the diffusion rate of ions,reduces the activation energy of the chemical reactions,and promotes the progress of the reaction.25When the solution temperature increases,the cathode over-potential increases and the nucleation rate of metal nuclei exceeds the growth rate,resulting in fine and more-regular grains.21Moreover,when the temperature of the laser irradiation area increases,turbulence occurs there,resulting in timely micro-stirring that acts to prevent bubble adsorption on the coating surface and reduce surface defects.18Also,part of the heat energy of the laser is absorbed by metal ions that then deposit on the coating surface,thereby subjecting it to mechanical force.The associated energy goes into forming compressive stress,which partially offsets the tensile stress and improves both the stress state of the coating17and the surface quality.
However,when the laser energy is increased to 18.2 μJ,the uniformity of the Zn–Ni–Mo coating is reduced,and defects such as pores reappear.As shown in Fig.3(f),when the laser energy is 21.1 μJ,the surface quality of the coating decreases further,and clustering becomes more serious.When the laser energy is too high,the temperature in the irradiation area rises too rapidly,and the reaction produces many bubbles whose timely removal the laser action makes difficult Also,as the laser passes through the solution,cavitation and plasma are generated that obstruct the optical path,thereby reducing the laser effect and causing below-expectation plating quality.19

FIG.3.SEM images (1000×) of Zn-Ni-Mo coating surfaces under different laser energy.
C.Effect of laser energy on phase of coating
Figure 4 shows XRD profile of the coatings under different laser energy.Each coating comprises the following phases:pure zinc(or solid solution η with the same structure),substrate copper,and possibly γ(intermetallic compound Ni5Zn21).9,13Without laser irradiation,the diffraction intensities of the zinc (or η) peaks (002),(100),and(101)are relatively low,indicating that the crystal structure that they represent is not obvious,while those of the copper substrate peaks(200)and(220)are relatively high.With increasing laser energy,the diffraction intensities of the(002),(100),and(101)peaks increase gradually,while those of the base peaks decrease,and when the laser energy is 15.4 μJ,the diffraction intensities of the three peaks reach their maximum values.Generally,if the Zn content in the coating decreases,so do the diffraction intensities of the related peaks,so we speculate that the zinc phase of the coating is not pure zinc but rather a solid solution η with the same structure;laser irradiation causes more Ni2+and MoO42-in the solution to join the reaction and form a solid solution η.The decreasing diffraction intensities of the base peaks may be because the laser action increases the reaction rate,thickens the coating,and reduces the detected diffraction signal.
Using the software JADE(Materials Data,Inc.),we found that when the laser energy is zero,8.6,12.1,15.4,18.2,and 21.1 μJ,the average grain size is 52,48,42,36,32,and 34 nm,respectively.First,according to the Nernst equation,with increasing temperature,the cathodic overvoltage of the reaction increases,and the nucleation rate of metal ions increases and facilitates the formation of smaller crystal nuclei.15Second,increasing laser energy leads to increased current density and lattice distortion,causing lattice defects such as dislocations,26and as these accumulate,small angular grain boundaries appears,leading to grain size refinement Therefore,the Zn–Ni–Mo coatings deposited by LAED contain uniform and fin particles.
D.XPS analysis of plating surface elements
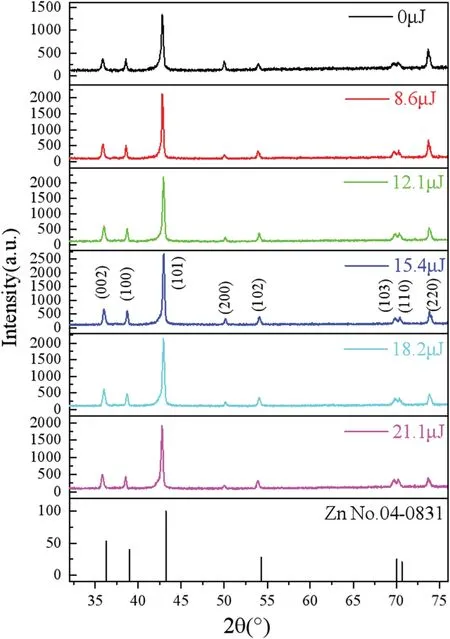
FIG.4.XRD spectra under different laser energy.
Because the XRD pattern contains no peaks related to Mo,we speculate that Mo is completely dissolved in other phases constituting the coating.6,13,27To study the form in which Mo exists,we subjected one of the Zn–Ni–Mo coatings to XPS analysis to determine its surface elemental state,and the XPS spectra of the coating with a laser energy of 15.4 μJ are shown in Fig.5.Figure 5(a)shows the full XPS spectrum of the coating,in which the presence of Mo can be observed.The Mo 3d spectrum[Fig.5(c)]is deconvolved into three bimodal structures corresponding to Mo4+,Mo6+,and intermediate Mo5+,28and the intensities of the peaks show that compared with Mo4+,there are more valence states of Mo6+in the coating,which may be because of the oxidation of the coating surface with air to form MoO3after a period of time.

FIG.5.XPS spectra of coating with laser energy of 15.4 μJ.
The XPS spectrum of Ni 2p[Fig.5(b)]shows two peaks at the binding energies of Ni 2p3/2and Ni 2p1/2at 856 and 872 eV,29respectively,which are typical Ni(OH)2peaks,and the Ni 2p signal can be deconvolved into Ni2+and satellite structures.Because Fig.5(b)shows no obvious peaks of Ni and NiO,we speculate that Ni in the coating exists mainly in the form of Ni(OH)2.
The XPS spectrum of O 1s [Fig.5(d)] exhibits a peak at a binding energy of 531 eV,and this peak is deconvolved into two major parts corresponding to the OH-and metal oxide components.6From the convolution results,the intensities of the peaks of these two parts are close,so we speculate that so is the existence of metal oxides and metal hydroxides in the coating.
E.Effects of laser energy on hardness and residual stress
Figure 6(a) shows that the microhardness of the Zn–Ni–Mo coatings increases with laser energy increasing from zero to 15.4 μJ and then decreases gradually.The microhardness is affected by the element content and coating structure;15as mentioned above,with increasing laser energy,the Mo and Ni contents of the coating increase,and compared to Zn,Mo and Ni have higher hardness values,so the hardness of the coating increases.Moreover,the laser thermal effect increases the temperature of the solution,so the cathodic overvoltage of the reaction increases,which causes the metal nucleation rate to exceed the growth rate,and so the deposition rate increases.21Therefore,the grain size of the coating is fine and the structure is more compact,which also increases the microhardness of the coating.

FIG.7.Weight loss at different corrosion times in NaCl(3.5%).
Figure 6(b) shows how the laser energy influence the surface residual stress.With no laser involvement,the residual tensile stress on the coating surface is 248.5 MPa,but this value decreases with increasing laser energy.Because some of the thermal energy of the laser is absorbed by reducing ions that then deposit on the coating,the laser subjects the coating surface to mechanical force,and the associated energy goes into forming compressive stress that partially offsets the tensile stress to reduce the residual tensile stress of the coating.19However,when the laser energy is 18.2 and 21.1 μJ,the residual stress worsens;when the laser energy is too high,the amount of hydrogen evolution at the cathode increases,and the laser action makes timely bubble removal difficult17When the local temperature of the solution is too high,the laser–electrodeposition composite is damaged,thereby weakening the laser coating effect.
F.Effect of laser energy on corrosion resistance
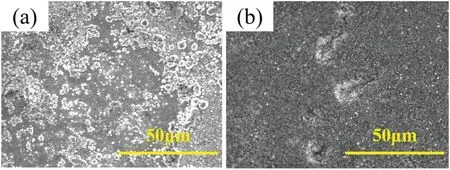
FIG.8.Surface morphologies of coatings after 5 h of NaCl(3.5%)corrosion:(a)without laser irradiation;(b)with laser energy of 15.4 μJ.
Figure 7 shows the weight loss of the Zn–Ni–Mo coatings obtained at different laser energies and corrosion times (120,240,360,and 480 h)in 3.5%NaCl;the lower the weight loss,the lower the corrosion rate of the coating.As shown in Fig.7,the corrosion rate is highest for the coating without laser assistance and then decreases gradually for laser energies of 8.6–15.4 μJ;however,when the laser energy is 21.1 μJ,the corrosion rate increases significantl and the corrosion resistance deteriorates.Also,with increasing corrosion time,the number of coating defects increases and the coating quality decreases.
After 5 h of corrosion,the corrosion morphologies of the coatings prepared without laser irradiation and with a laser energy of 15.4 μJ are shown in Fig.8.The coating prepared without laser irradiation exhibits large areas of corrosion [Fig.8(a)],whereas that prepared with a laser energy of 15.4 μJ exhibits only a few pits caused by corrosion [Fig.8(b)].Therefore,introducing laser irradiation improves the corrosion resistance of the coating.
To verify further the influenc of laser power on the corrosion resistance of Zn–Ni–Mo coatings,the polarization curves of those at different laser energies(zero,8.6,15.4,and 21.1 μJ)were measured by an electrochemical workstation.The test temperature was room temperature(25°C)and the corrosion solution was 3.5%NaCl.The test results are presented in Fig.9 and Table III.
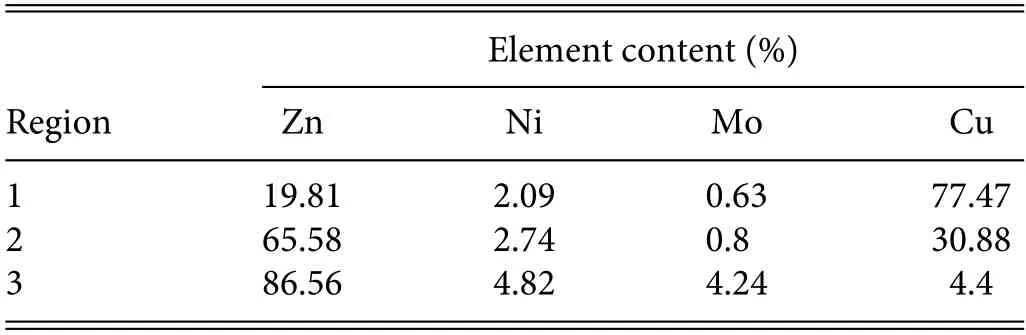
TABLE IV.Element content in different positions of coating after corrosion.
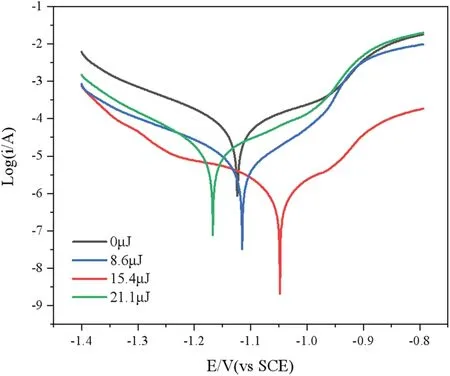
FIG.9.Tafel curves in NaCl(3.5%)under different laser energy.
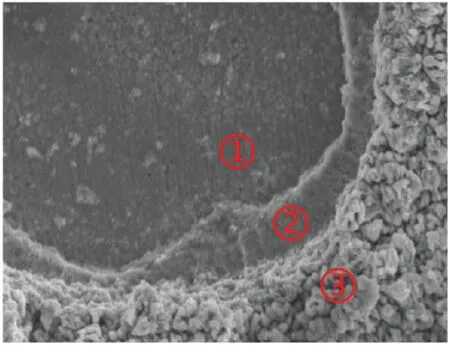
FIG.10.Surface morphology of coating after corrosion.
When the laser energy is 15.4 μJ,the corrosion potential of the coating is the most positive,and the self-corrosion current density is 2.115×10-6A cm-2,which indicates the best corrosion resistance.The element content and surface quality of the coating have a signifi cant impact its corrosion resistance.When the laser energy is 15.4 μJ,the contents of Ni and Mo in the coating are the highest,and compared to Zn,these two elements exhibit better corrosion resistance;Mo may form a passivation layer and prevent contact between the corrosion solution and the coating.11Meanwhile,adding Mo distorts the crystal lattice,hinders the formation of grain boundaries,and refine the nucleus,thus reducing corrosion defects.Also,because of the auxiliary effect of the laser,the surface defects of the coating are reduced,and the contact area of the corrosion solution is small,so the corrosion current is small and the corrosion resistance is excellent.
However,when the laser energy is too high,the reaction rate is increased,large amounts of hydrogen are generated at the cathode,the laser action cannot drive away bubbles quickly,the surface defects increase,and the structure is not uniformly compact.Also,the high local temperature of the solution may destroy the electrodeposition process,render the combination of the laser and electrodeposition ineffective,and worsen the corrosion resistance of the coatings.
G.Anodic protection of Zn-Ni-Mo coatings
The surface morphology of the coating after long-term corrosion immersion is shown in Fig.10.EDS analysis of the element composition was performed at the three sites shown,and the specifi element contents are given in Table IV.As can be seen,the Cu content in region 1 is the highest,reaching 77.47%.It can be inferred that the corrosion solution contacted the substrate,but no corrosion of the substrate is observed.The corrosion area expands gradually outward,forming a transition area at region 2,and the coating elements are less affected by corrosion in region 3.When corrosion occurs,because of the negative corrosion potential of the coating itself,it is used as a sacrificia anode to corrode firs and protect the substrate from corrosion.30This is the corrosion protection effect of the anode coating on the substrate.As given in Table III,the corrosion potential of the Zn–Ni–Mo coating is very negative,and it is more negative than those of most common metals and their alloys.Therefore,the Zn–Ni–Mo coating can be used as a sacrificia anode to protect the substrate from corrosion.
IV.CONCLUSIONS
In this study,Zn–Ni–Mo coatings were prepared using LAED processing technology,and the effects of laser energy on the coatings were investigated,including their element content,surface quality,physical phase,residual internal stress,microhardness,and corrosion resistance.
The Zn,Ni,and Mo contents in the coatings varied because of the temperature rise caused by laser irradiation.With increasing laser energy,the Zn content decreased whereas the Ni and Mo contents increased.When the laser energy was 15.4 μJ,the Zn content reached a minimum of 89.6%,and the Ni and Mo contents reached maxima of 5.28%and 5.12%,respectively.However,when the laser energy was too high,the composite laser–electrodeposition processing was destroyed,and the Zn content increased while the Ni and Mo contents decreased.
Laser irradiation can affect crystal growth,facilitating the growth of the zinc or solid solution η phases,with the Mo and Ni in the coating existing mainly in the form of Mo oxides and Ni(OH)2.Laser treatment can also improve the surface quality,microhardness,residual stress,and corrosion resistance of the coatings.When the laser energy was 15.4 μJ,the coating hardness was 140.7 Hv,the residual tensile stress was only 43.6 MPa,the corrosion potential was the most positive,the corrosion current density was the least,and the corrosion resistance of the Zn–Ni–Mo coating was the best.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant Nos.51905226,52075227,and 52105449),the Natural Science Foundation of Jiangsu Province(Grant No.BK20210755),and the Postdoctoral Foundation of Jiangsu Province(Grant No.2021K264B).
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflict to disclose.
DATA AVAILABILITY
The data that support the finding of this study are available within the article.
- 纳米技术与精密工程的其它文章
- Status of research on non-conventionaltechnology assisted single-point diamond turning
- Research trends in methods for controlling macro-micro motion platforms
- Droplet microfluidic chip for precise monitoring of dynamic solution changes
- Characteristics of the pressure profile in the accelerator on the RF negative ion source at ASIPP
- Oxidation mechanism of high-volume fraction SiCp/Al composite under laser irradiationand subsequent machining
- An optical tweezer-based microdroplet imaging technology

