A randomized clinical trial assessing the efficacy of single and multiple intralesional collagenase injections for treating contracted scars
Shuangbai Zhou, Poh-Ching Tan, Cheng-An Chiang, Yun Xie, Peiqi Zhang, Qingfeng Li,Kai Liu
Department of Plastic and Reconstructive Surgery, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai 200011, China
Keywords:Contracted scar Collagenase Intralesional injection Functional improvement Clinical trial
A B S T R A C T
1.Introduction
Scar contraction caused by burns, trauma, or surgery can cause significant physical distress.In severe cases, while affecting both physical function and appearance, scar contraction can restrict joint movement,and bone and muscle growth.
Contracted scars are traditionally treated with surgical procedures,such as skin grafts, flap transplantation, and Z-plasty.1These methods can be effective; however, all are accompanied by possible secondary scars and long downtime.In addition to surgical intervention,minimally invasive methods,such as pulsed dye lasers,2carbon dioxide lasers,3and intralesional steroid injection4have also been reported for scar contraction treatment.However,these treatments have limited effects on the release contraction.
Excessive pathological collagen growth is the fundamental cause of scar contraction.Collagenase, an enzyme capable of degrading collagen in human tissues,5has been used to treat diseases caused by excessive collagen production, such as Dupuytren’s disease6–8and Peyronie’s disease.9,10Herein,we present a randomized clinical trial investigating the feasibility of intralesional collagenase injections for the treatment of scar contraction due to trauma or burns.
2.Methods
2.1.Study design
Based on preclinical research, we designed a randomized placebocontrolled clinical trial.According to a pilot study, 16 patients were required per study arm,and the statistical power was>90%(two-sided,alpha=0.025).Assuming an attrition rate of 25%over the course of the study, 60 patients(20 per group)were included.
2.2.Patients
We recruited patients with limited joint movement and physical disfiguration for>1 year between July 2017 and February 2018.Patients considering surgical procedures were excluded.Other exclusion criteria included a history of allergy, diabetes, any energy-based procedure or triamcinolone acetonide injection within the past 3 months, use of steroids and tobacco within the past 6 months, and surgical procedures within the past 12 months.Patient characteristics are shown in Table 1.
2.3.Study oversight
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Shanghai Ninth People’s Hospital (approval no.2016-182-T126).All treatments and follow-ups were performed at the Shanghai Ninth People’s Hospital.This study was registered at www.chictr.org.cn(ChiCTRIIR-16009910).
2.4.Randomization and masking
After the patients signed informed written consent forms, they were randomized into three study groups, the multiple treatment (multiple)group, the single treatment (single) group, and the control group, in a 1:1:1 ratio, using a computer-generated randomization schedule.The patient recruitment team randomized the participants along the schedule, and the conduct team performed the assigned treatment.Investigators and data collectors were blinded during the trial.The trial profiles are listed in Table 1.
2.5.Intervention
Commercial collagenase (Qiaoyuan Biological Pharmaceutical Co.,Shanghai, China) was diluted in sterile saline to a concentration of 300 U/mL.The firm palpable cords/area of the contracted scar was marked as the target.Approximately 15 U/cm2of collagenase was injected directly into the palpable cords using a 30-gauge needle with a multidirectional technique that targeted multiple points and multiple layers, and was multidirectional.6Patients from the multiple group received an injection every four weeks (V1–3, ±5 days).Patients in the single group received collagenase injections at V1 and placebo injections(saline injected)at V2 and V3.In the control group,saline was injected at V1,V2,and V3.
The scars were vigorously massaged immediately after treatment.Patients were asked to stretch and massage the treated scar five times a day for 10 min each time throughout the treatment course.No other scar procedures were performed.Outcomes were assessed at baseline(immediately before treatment),and at 4 and 12 weeks after treatment.
2.6.Primary endpoint
Scar elongation rate was used as the primary endpoint.At each follow-up visit,the maximum stretched length of the scars was recorded.The maximum stretching length of the contracted scar was defined as the scar length at which the joint was at the largest stretching position.Scar length was measured at the middle axis of the scar.Scar elongation rate was calculated as the elongated scar length divided by the measured length at baseline.The primary endpoints were evaluated at 4 and 12 weeks after treatment.
2.7.Secondary endpoint
Self-Evaluation of Scar Contract Relief.Patients were asked to selfevaluate and describe scar changes as “very much improved,”“improved,” “no change,” and “deteriorated,” according to the scale described in Table 1.Secondary endpoints were documented at weeks 4 and 12.
2.8.Safety evaluations
The safety of intralesional collagenase injection was also evaluated.All adverse events at the injection sites were recorded and assessed by the investigators for severity to determine any relationship with the treatment.Safety evaluations were conducted at each visit.
2.9.Statistical analysis
Continuous variables are presented as mean (standard deviation),unless otherwise noted.Categorical variables were presented as counts and percentages.Chi-square and Student’s t-tests were used to compare groups and ANOVA was used for three-group comparisons.The endpoint analysis was performed on an intention-to-treat basis.Fisher’s exact test was used to evaluate group associations at different time points when the expected cell counts were low.Safety analysis was conducted for all patients who underwent randomization and treatment.A repeatedmeasures linear model was used to identify changes in continuous variables over time for each group.The per-protocol population consisted of patients who strictly adhered to the study protocol and completed the planned 12-week follow-up period.The primary outcome was analyzed using a per-protocol approach.Statistical significance was set at P<0.05.SPSS Statistics (version 16.0)was used for all the analyses.
3.Results
3.1.Patient characteristics
Of the 71 patients assessed for eligibility between July 2017 and February 2018,11 were excluded.The remaining 60 patients underwent randomization.The scar locations included the neck, perioral area,hands, feet, and lateral thigh.Ultimately, 19 patients in the multiple group,17 in the single group,and 17 in the control group were included in the per-protocol analysis of the primary outcome(Fig.1).The baseline demographic and disease characteristics were not significantly different between the groups(Table 2).
3.2.Primary outcome
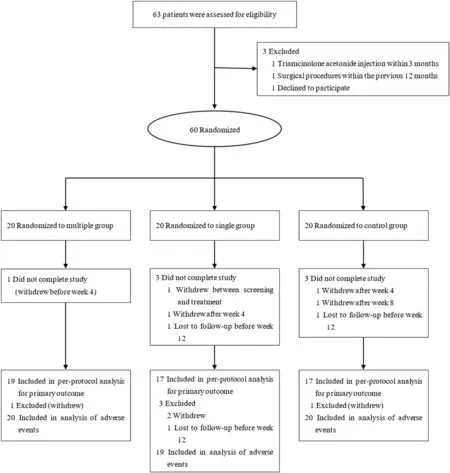
Fig.1.Clinical trial profile.
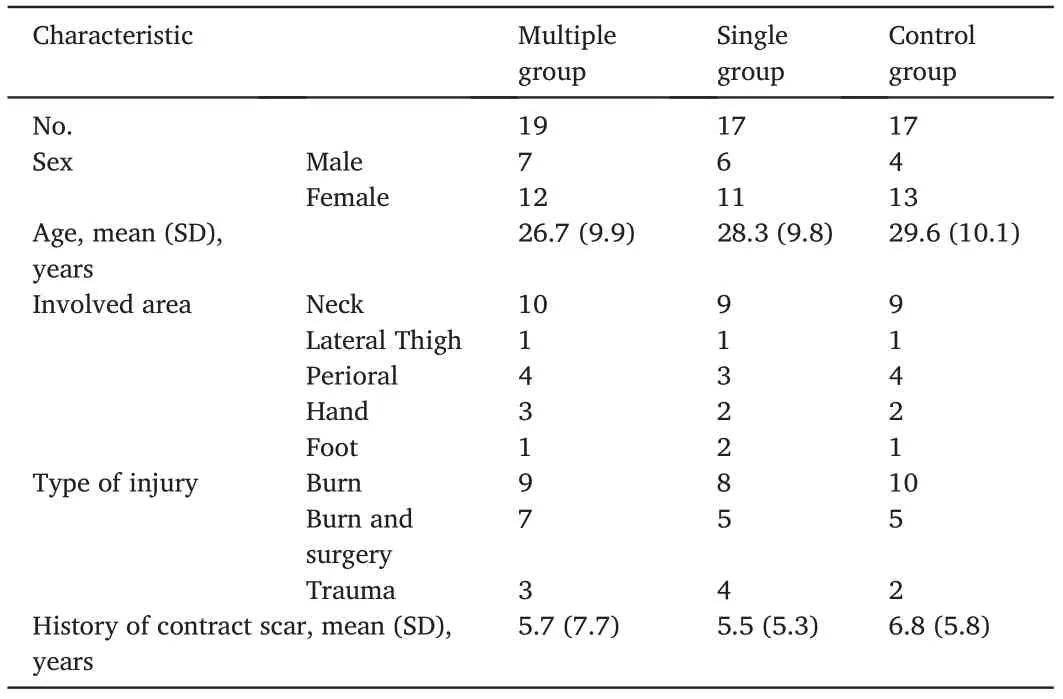
Table 2 Baseline characteristics.
The primary outcome showed that collagenase injection affected the elongation of the contracted scars.Patients in both the multiple and single groups showed elongation 4 weeks after one treatment session.The mean improvement at 4 weeks was 14.79(11.60%) in the multiple group and 16.56 (9.71%) in the single group, which was significantly improved compared with that in the control group (mean difference of 8.95 (95% CI, 1.88–16.10), P=0.016 and 10.72 (95% CI, 4.10–17.33),P=0.002, respectively).The results from the multiple and single groups showed no significant differences at 4 weeks(P=0.624).At 12 weeks,the elongation of the multiple group increased to 26.83(15.79%),which was significantly higher than that of the single group (16.36%; mean difference of 10.46 (95% CI, 0.93–20.00), P=0.032).Repeated treatments resulted in better outcomes than single treatments(Fig.2).
3.3.Self-evaluation

Fig.2.The outcome of scar length, elongation rate, and patient satisfaction.(A) The maximal stretched scar length from three groups at baseline, 4 weeks, and 12 weeks.(B)The elongation of scars in the multiple and single groups showed significant improvement compared to that in the control group, both at 4 weeks and 12 weeks.The multiple group showed better improvement than the single group at 12 weeks.(C)The self-evaluation result at 4 weeks.(D)The self-evaluation result at 12 weeks.The outcome indicated higher satisfaction in the multiple group than in the other two groups.
At the 4-week visit,eight patients(42.1%)in the multiple group and nine(52.9%)in the single group indicated that their scar had“very much improved;”both groups showed more improvement than in the control group(2,11.76%,vs multiple group,P=0.014;vs single group,P=0.012)(Figs.3–5).At the 12-week visit, more patients reported “very much improved” (15, 78.9%) in the multiple group than in the control group(P=0.003).There were nine patients who felt“very much improved”and five who felt “improved” in the single group, but the results were not prominent in the control group(P=0.195) (Fig.2).
The results also showed that in the control group, there were four“very much improved” cases and seven “improved” cases at 12 weeks,which may be related to physical stretch.None of the patients complained of a worsening scar contracture.
According to patient feedback and physician observations, the scar texture softened after collagenase treatment.Additionally, the scar flattened and redness was alleviated.
3.4.Safety evaluation
All patients reported mild-to-moderate pain for several hours after treatment.All patients felt that the pain was tolerable and did not require pain relievers.Two patients with perioral scars, one from the multiple group and one from the single group,reported post-treatment tenderness and swelling that resolved within 3 days.No severe adverse effects such as ulceration,pigmentation,or necrosis were observed.
4.Discussion
Collagenase is an enzyme capable of degrading native collagen,and is produced by various microorganisms and animal cells.This enzyme was first isolated in 19535and was later widely used to digest tissues and isolate cells.11In addition to its laboratory uses,collagenase has recently been used in tissue contracture treatments,such as those for Dupuytren’s disease6–8and Peyronie’s disease.9,10
Scar contraction is caused by excessive and compact extracellular matrix that restricts strain.MRI has demonstrated that collagenase chemically dissolved the Dupuytren’s cord, but the neurovascular bundles were not affected.12In addition to dissolving the extracellular matrix(ECM),collagenase can suppress cell proliferation13and the expression of alpha-smooth muscle actin,TGF-β1,fibronectin,and desmin.14Based on these reports, we hypothesized that the intralesional injection of collagenase might be a feasible therapy for improving physical dysfunction caused by contracted scars.In our preclinical experiments,we found that collagenase effectively dissolved the ECM in hypertrophic scars.The structure of the dermis was loosened, and the density of type I and III collagen was reduced.
The results of this trial showed that intralesional collagenase injection effectively elongated contracted scars with either one or multiple treatments.The average elongation at 12 weeks was 16.36%after one treatment and 26.83%after three treatments.While analyzing the outcome by scar location,we found that collagenase treatment had a better outcome when the scar was located where physical stretching could be applied,such as on the finger,palm,and neck.
Repeated collagenase treatment is used to treat Peyronie’s disease.15In this trial, we found that at 12 weeks, patients in the multiple groups reported more scar release than those in the single group.The results indicated that serial treatments could achieve more satisfactory outcomes, and even a single treatment could achieve sustained effects in elongating contracted scars.Serial injections of collagenase can dissolve the ECM more thoroughly and stabilize scars.
We also observed that patients in the control group reported improvement, indicating that massage, even when not combined with collagenase injection, was beneficial against contraction.Rehabilitation is important to resist early scar contractions.Our results demonstrate that physical exercise helps relieve the tenderness of scars.16
The results also showed that outcomes were limited when scarring occurred at a young age.The deformity not only involved dermal contracted cords but also impacted the development of the skin, muscle,joints, and even bone.These dysplastic tissues limit the outcomes of collagenase treatment.
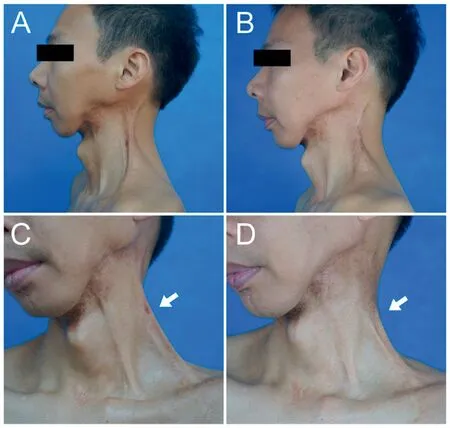
Fig.3.Effect of multiple collagenase treatment for contracted neck scar in a 38-year-old male patient from the multiple treatment group.The patient suffered from a postsurgical neck scar for five years.(A,C)Movement of the left cervical region was limited by a contracted scar.A distinct tension cord (white arrow)was observed when the patient turned his head to the right.(B,D)Photos of the patients after 1 year.The contracted scar cords were softened and elongated.The patient felt a released neck and improved movement.

Fig.4.Effect of multiple treatments for a contracted upper lid scar in a 37-year-old male patient due to trauma from the multiple treatment group.(A)Baseline.(B)12 weeks.(C) 24 weeks.The contracted scar was released, and the shape of the upper lip was more symmetrical.
In this study, we evaluated the safety of collagenase.No severe adverse events were reported.The most common adverse event was posttreatment pain, which was universally relieved within several hours.Neither tendon rupture nor disruption of normal tissues was observed in our clinical trial.Laceration secondary to collagenase treatment, which was once reported in Dupuytren’s disease,was not observed in this study.Moreover,the application of collagenase did not aggravate scarring.
This treatment is suitable for patients seeking an alternative to surgery.With serial collagenase injections, patients can achieve scar relief and better function.This method will change the traditional treatment strategy for contractile scarring through a minimally invasive method to effectively prolong scarring and shorten recovery time.
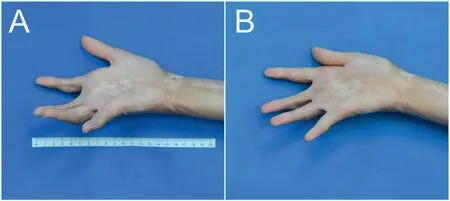
Fig.5.The outcome of a single treatment for postburn palm contracted scar in a 20-year-old female from the single treatment group.(A) Before treatment.Flexion contracture of index, middle and ring fingers.(B) Twelve weeks after treatment.Joint extension was improved in all three fingers.
The limitations of our study include its small sample size,and larger multicenter trials are warranted.While the safety of collagenase in fibrotic diseases has been verified in clinical trials,long-term follow-up of our cohort is needed to assess its safety and long-term results.
5.Conclusion
Our study demonstrated that the intralesional injection of collagenase is an effective and minimally invasive method with limited downtime and complications.Unlike surgical methods that remove most of the scar tissue, intralesional collagenase injections allow patients to resume normal daily activities almost immediately.We believe that this method is a promising alternative treatment option for patients with scar contraction.
Ethics approval and consent to participate
The study received ethical approval from the Ethics Committee at the Shanghai Ninth People’s Hospital (approval no.2016-182-T126).All participants provided written informed consent prior to study enrolment.
Consent for publication
The patients gave written informed consent to publish the data contained within this study.
Authors’ contributions
Zhou S:Conceptualization,Methodology,Resources,Writing-Original Draft.Tan P: Investigation, Formal analysis.Chiang A: Visualization,Writing-Original Draft.Xie Y:Resources,Investigation.Zhang P:Investigation.Li Q:Supervision,Writing-Review&Editing.Liu K:Conceptualization,Supervision,Writing-Review and Editing.
Declaration of competing interests
Li Q is an editorial board member for Chinese Journal of Plastic and Reconstructive Surgery and was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos.81501678, 81971848, and 82272287), Clinical Research Plan of Shanghai Hospital Development Center (grant nos.SHDC2020CR1019B and SHDC2020CR4029), Shanghai Municipal Key Clinical Specialty (grant no.shslczdzk00901), and Innovative Research Team of High-Level Local University in Shanghai (grant no.SSMUZDCX20180700).
 Chinese Journal of Plastic and Reconstructive Surgery2023年3期
Chinese Journal of Plastic and Reconstructive Surgery2023年3期
- Chinese Journal of Plastic and Reconstructive Surgery的其它文章
- Application of a jigsaw puzzle flap based on free-style perforator to repair large scalp defects after tumor resection: A case series
- Eight-year follow-up on postoperative improvement protocol in extensive metoidioplasty transgenders: A case series
- Absolute ethanol embolization for treatment of peripheral arteriovenous malformations
- Efficacy of tibial transverse transport combined with platelet-rich plasma versus platelet-rich plasma alone in the treatment of diabetic foot ulcers: A meta-analysis
- Efficacy, effectiveness, and safety of combination laser and tranexamic acid treatment for melasma: A meta-analysis
- Integrated percutaneous sclerotherapy and surgical intervention for giant cutaneomucosal venous malformation from TIE2 mutation: A case report
