略阳乌鸡褐壳蛋壳颜色量化体系的建立和遗传基础分析
陈球,黄晶晶,王哲鹏
略阳乌鸡褐壳蛋壳颜色量化体系的建立和遗传基础分析
陈球,黄晶晶,王哲鹏
西北农林科技大学动物科技学院,陕西杨凌 712100
【背景】褐壳蛋壳颜色与蛋壳强度、蛋内抗菌肽含量、血斑肉斑和孵化率有密切联系,是影响蛋品质和销售的一个重要的指标。然而,一些地方鸡因选育程度低,存在蛋色偏浅、均匀度差等不足,给鸡蛋的销售和品牌的打造造成不利影响。【目的】探讨以略阳乌鸡为对象,建立能灵敏度量蛋壳颜色变异的量化体系,估计品种特异性蛋壳颜色的遗传力,筛选参与蛋壳颜色调控的候选基因,为略阳乌鸡褐壳蛋壳颜色选育奠定理论基础。【方法】在略阳乌鸡蛋用系62个半同胞家系中选取841只母鸡,每只鸡收集3枚蛋,用L*a*b色度体系量化蛋壳颜色。在贝叶斯框架下,用马尔可夫链蒙特卡罗(Markov Chain Monte Carlo, MCMC)算法计算各色度指标的后验遗传力。用逆γ分布指定育种值和残差方差的先验分布。执行130 000迭代,弃去前30 000次迭代结果,按间隔100存储迭代结果,获得各方差组分的后验分布和各指标的后验遗传力。选取产浅褐壳、褐壳、浅绿壳的母鸡各8只,每只鸡收集3枚蛋用于蛋壳色素含量的测定。用原卟啉和胆绿素标准品建立浓度与吸光度值的回归关系,分别在670 nm和412 nm处测定胆绿素和原卟啉的吸光度,用回归法求得待测样本两种蛋壳色素的浓度。采集褐壳(n=8)和浅褐壳(n=8)母鸡的蛋壳腺,用qPCR法检测和在蛋壳腺中的表达量。【结果】在3个色度指标中,a值递减的变化规律与褐色向绿色调过渡的视觉观感吻合度最高。L值和b值能够反映褐壳颜色深浅的变化,但在区分褐色和绿色调上存在较大误差。L值和b值分布呈现出较高的集中度,50%的样本的L值和b值集中分布于73.3—79.1和13.1—18.2之间,而a值分布更为分散,50%的样本分布于1.4—7.1之间。与上述分布特征保持一致,a值的变异系数88.2%要高于L值5.7%和b值24.0%。遗传力估计结果显示,a值主要受遗传效应调控(h2=0.77),而L值(h2=0.46)和b值(h2=0.37)受环境效应影响更大。在蛋壳色素和蛋壳颜色之间,原卟啉浓度与3个色度指标均存在强相关关系(L:=-0.86,a:=0.73, b:=0.88),但胆绿素浓度仅与a值存在强负相关(=-0.73)。和是两个催化原卟啉前体物合成的酶。表达结果显示,在褐壳鸡中的表达水平是浅褐壳的1.5倍(<0.05),但的表达量无显著差异(>0.05)。【结论】原卟啉是影响略阳乌鸡蛋壳颜色的关键色素,而是与蛋壳原卟啉含量和褐壳颜色显著关联的候选基因。a值是量化略阳乌鸡蛋壳颜色准确度和灵敏度最高的指标,且具有较高的遗传力。对a值向上选择,有望在提高褐壳颜色深度和均匀度方面取得较大选择反应。
略阳乌鸡;蛋壳颜色;L*a*b色度空间;遗传力;;
0 引言
【研究意义】褐壳蛋因蛋壳质量好、蛋内抗菌蛋白含量高、孵化率高,在我国禽蛋市场中占主导地位[1-4]。但是,一些地方鸡由于选育程度低,蛋壳颜色存在较大的变异,给鸡蛋的销售和品牌的创立造成极为不利的影响。在阐明蛋壳颜色遗传调节效应的基础上,建立科学的育种方案,培育蛋色均一、色泽美观的褐壳品种,对地方鸡资源的开发利用具有重要意义。【前人研究进展】在野生鸟类中,蛋壳颜色是变异十分丰富的一个性状[5]。它在伪装[6]、阻挡太阳辐射[7]、调节蛋温[8]、增加蛋壳强度[9]和吸引雄鸟参与孵化[10]等方面发挥了重要的作用。在家禽中,由于人工选择,蛋壳颜色被固定为白壳、粉壳、褐壳、绿壳几种颜色。蛋壳颜色是由沉积于蛋壳中的色素引起。原卟啉Ⅸ和胆绿素Ⅸ是两种主要的蛋壳色素,前者负责红褐的蛋壳着色,后者负责蓝绿的蛋壳着色[11]。这些色素在蛋形成的最后阶段由蛋壳腺分泌到蛋壳上,引起各种颜色、各种斑纹的蛋壳着色[11-12]。虽然年龄、药物、疾病、应激、营养等非遗传因素对蛋壳颜色均可产生不同程度的影响[1,13],但蛋壳颜色仍属于主要受遗传效应调控的性状。鸡和鸭的绿壳表型均被证实是由色素转运基因突变引起,属于单基因控制的显性性状[14-15]。但是,褐壳的遗传机制目前尚不清楚。通过QTL定位,研究人员在鸡的1、2、4和11号染色体上发现4个QTL与蛋壳颜色有关[16-18]。蛋壳腺表达研究发现,原卟啉前体物合成基因、和转运基因、、在深褐壳和浅褐壳间均存在显著表达差异[19-20]。但是,Zheng等[21]在比较白壳、粉壳和褐壳鸡和的表达量时却并未发现显著差异。在用尼卡巴嗪构建的褐壳蛋褪色模型中,、、的表达量与蛋壳颜色变化关系也未得到证实,只有的差异表达的结果得到重复[22]。除了蛋壳腺差异表达基因外,肝脏差异表达基因与蛋壳原卟啉合成和褐壳着色也有密切联系[23]。鉴于QTL定位和表达研究难以锁定主效基因,研究人员通过估计蛋壳颜色的遗传力对遗传效应的总体作用进行了定量评估。在用L*a*b体系量化蛋壳颜色后,研究人员在横斑洛克、洛岛红及白来航与东乡绿壳鸡的杂交群体中获得了较高的色度指标遗传力估计结果(L:0.64—0.74,a:0.42—0.64,b:0.55—0.6)[24-25],Cavero等在罗曼褐中发现褐壳表型为中等遗传力(0.33—0.46)性状[26],但Kamanli在横斑洛克中估计的色度指标遗传力仅为0.2左右[27]。这些研究表明,与单基因调控的绿壳表型相比,褐壳有着更为复杂的遗传基础。在不同品种,甚至不同环境下,对褐壳颜色发挥调节作用的遗传机制可能并不相同。【本研究切入点】略阳乌鸡是一个产自陕西略阳县一带的地方品种。该品种因蛋肉品质优良、药食同源等优点而深受消费者的喜爱[28]。略阳乌鸡蛋壳颜色以褐色调为主,有少部分个体产颜色偏绿的蛋。蛋壳颜色在整个产蛋周期中维持稳定,但在个体之间呈现出由浅到深的连续性变异。这些表型特征肯定了遗传效应对蛋壳颜色的决定作用。但是,我们尚缺乏对遗传效应的定量认识,也不清楚之前报道的候选基因与褐壳颜色的关系在略阳乌鸡中是否也存在。【拟解决的关键问题】本研究在用L*a*b体系量化略阳乌鸡蛋壳颜色的基础上,研究了3个色度指标的分布特征和与蛋壳色素含量的相关性,估计了L, a, b值的遗传力,分析了和表达量与褐壳颜色深浅的关系,期望建立对蛋壳颜色变化有较高灵敏度和准确度的量化体系,获得品种特异性色度指标遗传力估计结果,发现参与略阳乌鸡蛋壳颜色调控的候选基因,为略阳乌鸡褐壳性状的选育提纯奠定理论基础。
1 材料与方法
1.1 略阳乌鸡的饲养
从略阳乌鸡蛋用系核心群四世代62个半同胞家系中随机选取841只母鸡。四世代母鸡出壳时间为2019年12月,淘汰时间为2020年10月,在略阳县龙昊乌鸡种源繁育中心饲养。育雏期(0—6周龄)采用笼养育雏,养殖密度为40—60只/m2,育雏温度28—33 ℃,4周龄脱温,光照时间18—22 h。在10周龄,将青年鸡转入产蛋鸡舍。在产蛋周期内,鸡舍维持16 h恒定光照,单笼养殖,自由采食、饮水。育雏期(0—6周龄)用全价配合料(杨凌海大饲料)饲喂,青年期和产蛋期用35%浓缩料(杨凌海大饲料)、60%玉米和5%麸皮混合饲喂,粗蛋白16%—20%,能量12 MJ·kg-1,粗纤维<5%,钙0.8%—3.5%,氯化钠0.7%—1.8%,蛋氨酸>0.45%,粗灰分<8%。
1.2 蛋壳颜色的测定
本研究用L*a*b色度体系量化蛋壳颜色,L为亮度值,取值范围为0—100,L值越大反映物体的颜色越白,反之越黑;a值为红绿度值,无取值范围限制,正值表示物体颜色偏红,负值表示颜色偏绿;b值为蓝黄度值,无取值范围限制,正值表示颜色偏黄,负值为颜色偏蓝。蛋壳颜色的L, a, b值用MiniScan EZ 4000便携式分光光度计(Hunter Associates Laboratory,Inc.)在D65光源和10°入射角条件下测定。在母鸡32周龄时,每只鸡测定3枚蛋,3枚蛋L, a, b测定结果的平均值作为该样本蛋壳颜色的代表值,用于后续分析。蛋壳颜色测定时间为2020年7月,在略阳县龙昊乌鸡种源繁育中心测定。
1.3 L, a, b值遗传力估计
本研究用单变量动物模型y=Xb+Za+e估计L, a, b值的遗传力,y为L, a, b测定值向量,X为设计矩阵,b为色度指标群体均值,Z为育种值效应设计矩阵,a为各色度指标的育种值,e为误差项[29]。各色度指标后验遗传力在贝叶斯框架下用马尔科夫链蒙特卡洛(Markov Chain Monte Carlo,简称MCMC)算法进行估计,运算过程由R语言MCMCglmm软件完成[30]。育种值及残差项方差的先验分布由逆γ分布指定,逆γ分布的参数分别为V=1,nu=0.002[31]。MCMC迭代参数为nitt=130 000,burnin=30 000,thin=100[31]。迭代结束后,用posterior.mode命令计算遗传力后验分布众数,用HPDinterval命令计算遗传力的95%置信区间[31]。
1.4 蛋壳色素含量测定
从四世代略阳乌鸡中依据主观颜色评定,挑选产浅褐壳、褐壳和绿壳蛋的略阳乌鸡各8只。每只鸡收集连续产出的3枚蛋,分离蛋壳,测定蛋壳原卟啉和胆绿素浓度。3枚蛋的平均值作为该样本蛋壳色素浓度的代表值。敲破鸡蛋,弃蛋液,剥离蛋壳膜,用去离子水洗净蛋壳,置65 ℃烘箱过夜干燥。每枚蛋称取0.25 g蛋壳,置于4 mL蛋壳溶解液(无水甲醇﹕37.5%的盐酸=2﹕1)中,室温避光过夜,直至蛋壳完全溶解[32]。3 500 r/min离心45 min,取上清液,避光保存备测。
将4 mg原卟啉Ⅺ标准品(Sigma)溶解于4 mL蛋壳溶解液中,配成1 mg·mL-1标准品储液。取0.12 mL储液与1.88 mL蛋壳溶解液混合配成1×标样(0.06 mg·mL-1),按2倍稀释倍数依次稀释前一标样,配制2-1(0.03 mg·mL-1)— 2-9(0.000117 mg·L-1)标样。将4 mg胆绿素标准品(Sigma)溶解于4 mL蛋壳溶解液中配成胆绿素标准品储液。将0.087 mL储液与1.913 mL蛋壳溶解液混合配成1×胆绿素标样(0.043 mg·mL-1)。按2倍稀释倍数依次稀释前一标样,获得2-1(0.0215 mg·mL-1)—2-9(0.000084 mg·L-1)标样。取200 μL样品,用酶标仪(BioTek)在412 nm处测定原卟啉吸光度值,在670 nm处测定胆绿素吸光度值[32]。依据标样建立的吸光值与浓度回归方程,计算待测样本原卟啉和胆绿素的浓度。原卟啉和胆绿素浓度用μg·g-1蛋壳表示。蛋壳色素含量测定时间为2021年1月,在西北农林科技大学动物营养科研平台测定。
1.5 CPOX和ALAS1在蛋壳腺中的表达量检测
将上述参与蛋壳色素浓度测定的浅褐壳(n=8)和褐壳(n=8)鸡宰杀,采集蛋壳腺,置1 mL RNAstore(康为世纪)中,剪碎组织,在4 ℃浸泡过夜后置-80 ℃冰箱长期保存备用。取大约100 mg组织样,吸干RNAstore,用TRNzol Universal总RNA 提取试剂(天根)按操作说明提取蛋壳腺总RNA。用1%琼脂糖凝胶电泳检查RNA的完整性,用NanoDrop2000(ThermoFisher Scientific)分光光度计测定RNA浓度。取1 µg总RNA,用SumOnetube RT Mixture Ⅲ(Summer Biotech)反转录试剂盒按操作说明合成cDNA。用实时荧光定量PCR(qPCR)检测和在蛋壳腺中的表达量。qPCR反应体系由1 μL cDNA, 10 μL 2×FastHotstart SYBR QPCR Mixture (Summer Biotech), 0.4 μL正链引物(10 μmol·L-1),0.4 μL反链引物(10 μmol·L-1)和8.2 μL ddH2O组成。扩增引物为5′-GAGAGGACGGTATGTGGAGT -3′和5′-TTTGGGATTGCGGAGAAC-3′,扩增引物为5′-GCATCTATGTCCAAGCAATC-3′和5′-CA ACATCCTTCCATGTAGCC-3′,持家基因扩增引物为5′-ATACACAGAGGACCAGGTTG-3′和5′- AAACTCATTGTCATACCAGG-3′。qPCR在LightCycler® 96 System荧光定量PCR仪(Roche)中进行。qPCR反应条件为95℃变性2 min,(95℃变性10 s,60℃退火10 s,60℃延伸30 s)×40个循环,扩增结束后执行qPCR仪默认融解曲线程序,验证qPCR扩增的特异性。每个样品执行3个技术重复,以为内参基因,以浅褐壳组为对照,用2-ΔΔCt法以相对于浅褐壳组表达倍数的形式呈现表达量检测结果。和表达量检测时间为2020年12月,在西北农林科技大学动物营养科研平台检测。
1.6 统计分析
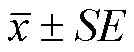
2 结果
2.1 略阳乌鸡蛋壳颜色的量化和变异研究
略阳乌鸡蛋壳颜色以褐色调为主,从褐壳、浅褐壳、白壳到浅绿壳呈现出连续性变异的特征(图1-A)。在用L*a*b体系量化蛋壳颜色后,研究发现a值不仅能灵敏地捕捉到褐壳颜色逐渐变浅的变化规律,而且能准确区分褐色和绿色调,其逐渐递减的变化趋势与褐壳→浅褐壳→白壳→浅绿壳的视觉观感最为接近。L值随褐壳颜色变浅逐渐增大,b值逐渐减小(1#—6#样本),但L值和b值并不能准确区分褐色和绿色调。例如,3#和12#、5#和10#的L值和b值较为接近,但在视觉观感上二者呈现为完全不同的色调;8#和9#颜色较为接近,但b值相差1倍之多。
除了分析色度指标与主观视觉观感的吻合度外,研究也在大样本(n=841)内分析了色度指标的群体分布特征(图1-B)。L值变异范围为59.7—89.8,均值为76.0±0.15,50%的样本的L值集中于73.3—79.1之间。a值变异范围为-6.7—15.5,均值为4.3±0.13,50%的样本的a值集中于1.4—7.1之间。b值变异范围为4.5—26.9,均值为15.6±0.13,50%的样本的b值集中于13.1—18.2之间。在3个色度指标中,a值的变异系数为88.2%,显著高于L值(5.7%)和b值(24.0%)的变异系数。
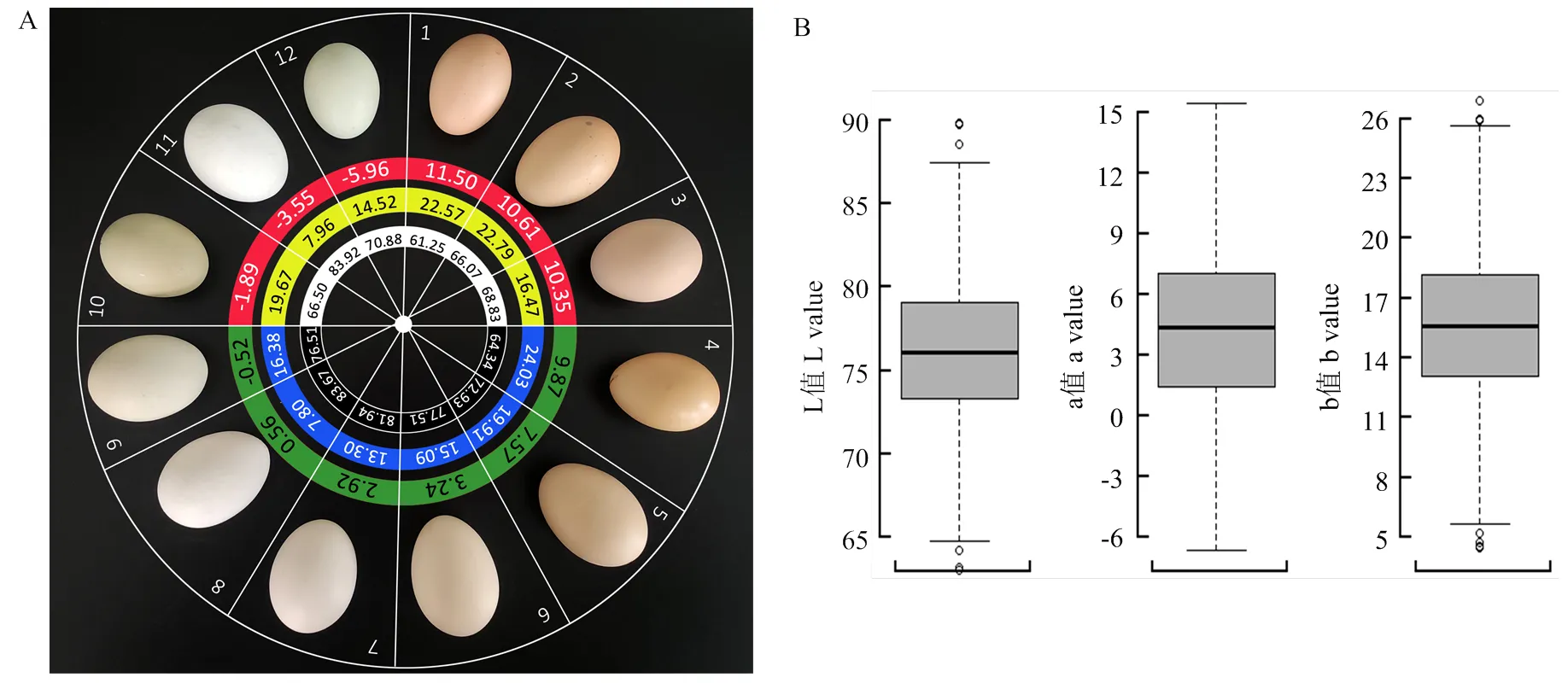
A:略阳乌鸡蛋代表性蛋壳颜色。红绿色圈内标注的数字代表各枚蛋的a值,黄蓝色圈内的数字代表b值,白黑色圈内的数字代表L值;B:略阳乌鸡蛋壳颜色(n=841)L, a, b值分布。LBC=Lüeyang Black-boned Chicken
2.2 蛋壳颜色L、a和b值遗传力估计
略阳乌鸡蛋壳颜色表现出连续性变异特征,符合数量性状的遗传特点。为了定量评估遗传效应对略阳乌鸡蛋壳颜色变异的决定性,本研究在量化蛋壳颜色的基础上,进一步估计了L, a, b值的遗传力。在3个色度指标中,L值和b值维持中等偏低遗传力,遗传力估计结果为0.46和0.37。a值维持了较高遗传力,估计结果为0.77。3个色度指标遗传力估计精度较为接近,95%置信区间宽度均为0.4左右(图2)。
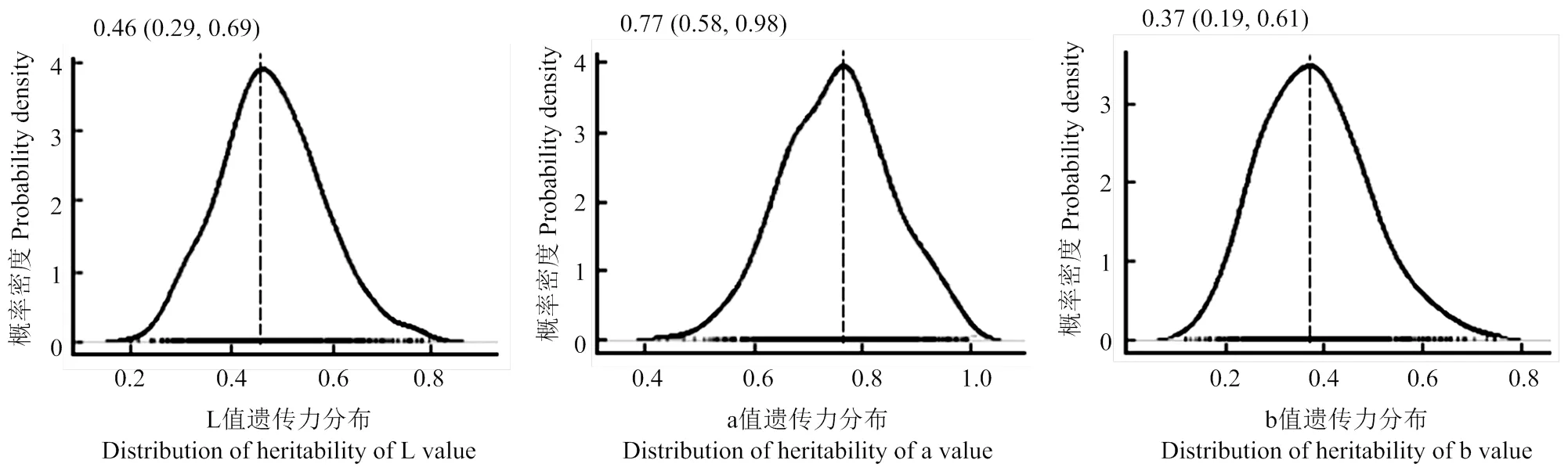
曲线描绘1000个后验遗传力概率分布,图上方数字指各颜色指标后验遗传力众数,括号中的数字为各指标后验遗传力的95%置信区间
2.3 蛋壳原卟啉和胆绿素含量与L, a, b值的相关性
为了阐明蛋壳色素浓度与略阳乌鸡蛋壳颜色变异的关系,本研究分析了蛋壳原卟啉和胆绿素浓度与L, a, b值的相关性。原卟啉的浓度与L值存在强负相关性,与a值和b值均呈现出强正相关性(图3)。蛋壳胆绿素浓度与a值呈现出显著负相关性,但与L值和b值的相关关系均不显著(图3)。
2.4 CPOX和ALAS1表达量与褐壳颜色深浅的关系
已知原卟啉是影响略阳乌鸡蛋壳颜色的关键色素。为了筛选出调控略阳乌鸡褐壳颜色的候选基因,本研究比较了原卟啉前体物合成酶基因和在褐壳和浅褐壳间的表达差异。褐壳的L值显著低于浅褐壳,a值、b值和原卟啉含量均显著高于浅褐壳(表1),支持所选样本蛋壳颜色量化和原卟啉含量测定结果与视觉观感一致,样本具有代表性。在褐壳鸡蛋壳腺的表达水平是浅褐壳鸡的(1.5±0.4)倍,但表达水平在褐壳和浅褐壳组间无显著差异(图4)。
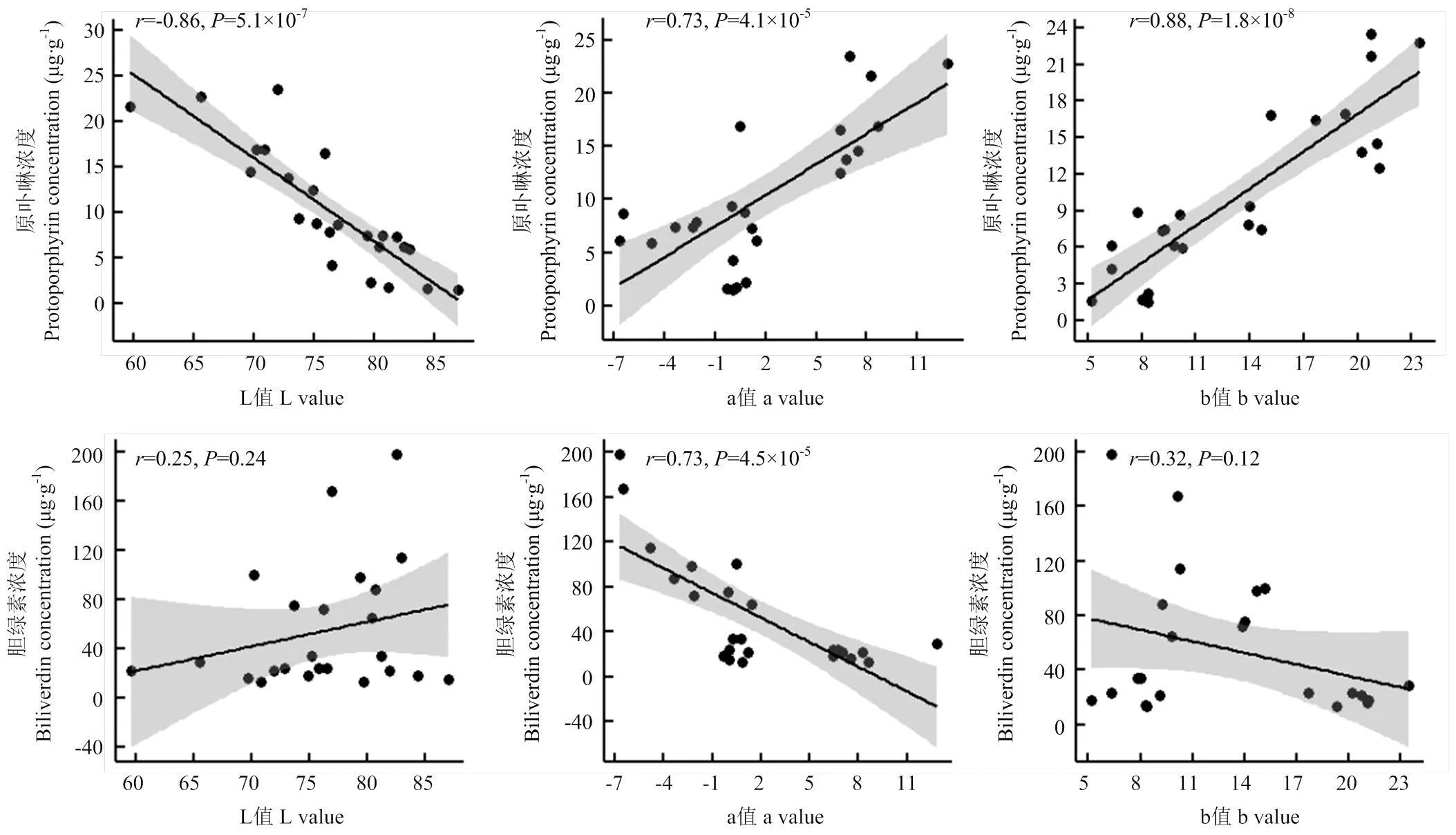
图中每个散点代表一个样本,直线为回归直线,灰色带为回归直线95%置信区间。图上方数字为相关系数(r)和相关系数显著性检验的P值
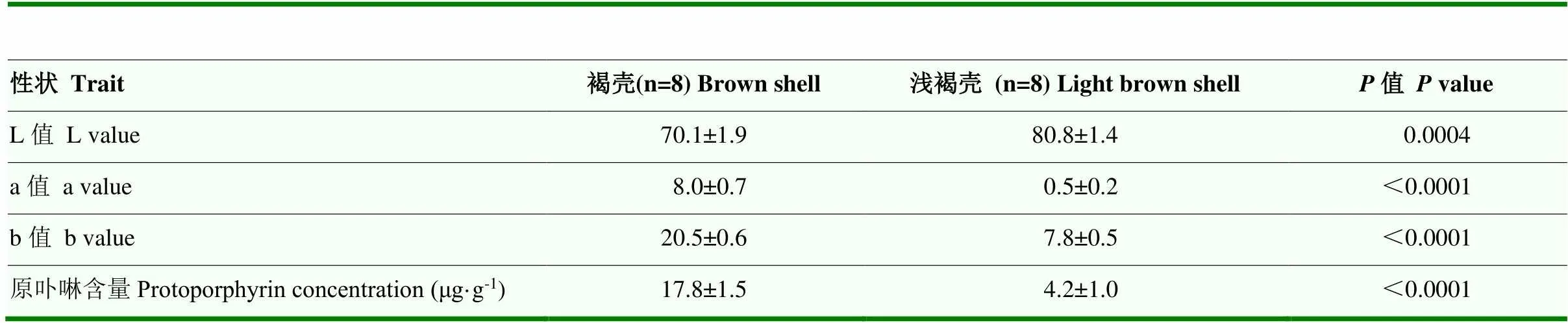
表1 褐壳和浅褐壳蛋壳颜色指标和原卟啉含量的比较
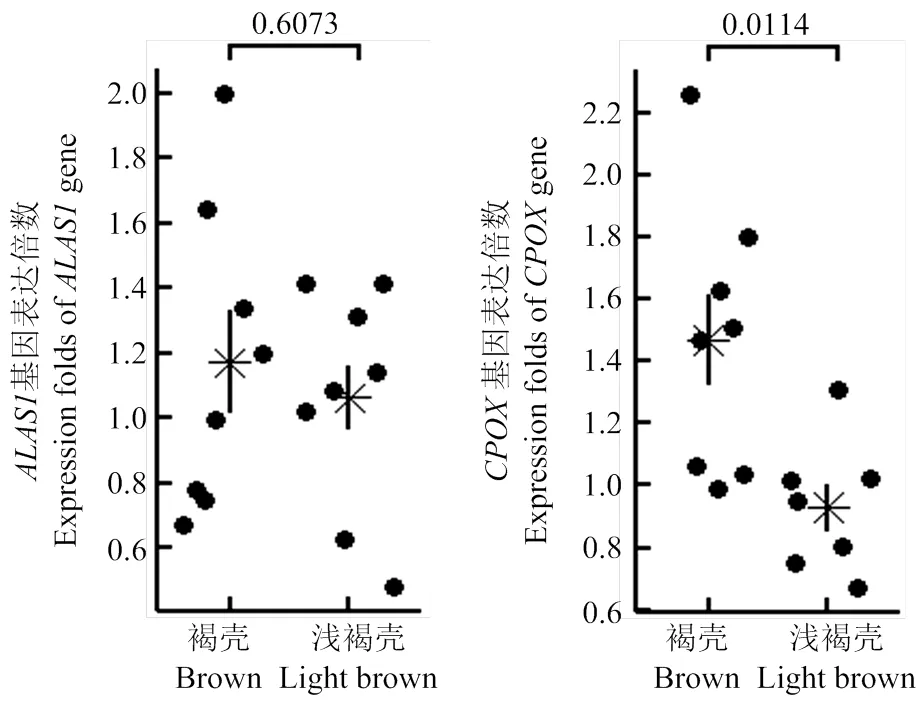
每个散点代表一个样本,星号代表组内均值(n=8),星号两侧直线代表标准误,图上数字代表组间差异显著检验的P值
3 讨论
3.1 a值是捕捉蛋壳颜色变异性灵敏度最高的指标
鉴于略阳乌鸡蛋壳颜色连续性变异的特点,建立一种能够准确度量蛋壳颜色变异,且数值变化与视觉观感保持一致的量化方法,是研究其遗传基础的前提。在用L*a*b色度体系对略阳乌鸡蛋壳颜色的变异情况进行定量分析后,本研究发现L值和b值虽然受个别极值影响呈现出较宽的变异范围,但50%的样本的L值和b值集中于一个较窄取值区间内,而a值在变异范围内分布更为均匀,其变异系数(88.2%)也明显高于L值(5.7%)和b值(24.3%)。Kamanli[27]在横斑洛克鸡、Sasaki等[16]在白来航与洛岛红的F2杂交群体、Goger等[25]在洛岛红中均发现,a值的变异系数(74.97%,40.2%,34.9%)要明显高于L值(9.22%,6.3%,5.76%)和b值(14.67%,27%,7.55%)。在罗曼褐蛋壳颜色随产蛋时间变化的研究中, a值变化幅度也要明显高于L值和b值[26]。这些研究表明a值在响应褐壳颜色变化的灵敏度方面要高于L值和b值,是量化蛋壳颜色变异、用于蛋壳颜色选育的理想指标[34]。
3.2 原卟啉是影响略阳乌鸡蛋壳颜色的关键色素
Wang等[32]在分析绿壳蛋和褐壳蛋蛋壳色素含量时发现两种色素同时存在于绿壳和褐壳蛋中,但褐壳蛋的原卟啉含量显著高于绿壳蛋,而胆绿素含量显著低于绿壳蛋。这些结果支持对绿壳和褐壳蛋的着色而言,两种色素都在发挥作用,但可能有主次之分,即一种色素决定主色调,另一种色素起修饰作用。在绿壳蛋中,随着原卟啉含量的升高,绿壳蛋的蛋壳颜色将由浅蓝向蓝绿、橄榄绿变化[35]。略阳乌鸡褐壳颜色表现出连续性变异的特征,且颜色偏浅的蛋略带有绿色光泽。通过相关分析,本研究发现原卟啉浓度与3个色度指标均存在强相关关系,但胆绿素仅与a值存在显著负相关关系。原卟啉相关分析结果与Zeng等[36]报道的结果一致,但胆绿素的结果与Zeng等[36]报道的结果有所不同,在东乡绿壳蛋鸡中胆绿素与L值(=-0.67)和b值(=0.48)也存在显著的相关关系。Li等[34]在分析长顺鸡不同颜色蛋壳色素含量时发现,原卟啉在褐壳中的含量显著高于绿壳,但胆绿素在褐壳和绿壳组间并无差异。这些结果说明,在略阳乌鸡中,原卟啉对蛋壳颜色的调节作用要大于胆绿素,而胆绿素所引起的细微变化只有灵敏度更高的a值能捕获到。东乡鸡绿壳颜色的深度要远远高于略阳乌鸡,在胆绿素的含量和蛋壳颜色的变异上可能产生更大幅度的变化。因此,这种变化在L和b值上也能反映出来。
在罗曼褐蛋鸡中,褐壳理想的蛋色标准为L=60,a=20,b=30[26]。略阳乌鸡蛋颜色(L=76.0±4.4,a=4.3±3.8,b=15.6±3.8)与此标准仍有较大差距。鉴于原卟啉含量与L、a、b值间的强相关关系,通过育种和营养手段提高原卟啉含量是促使略阳乌鸡褐壳蛋色朝向理想蛋色标准靠近的有效手段。
3.3 CPOX是影响略阳乌鸡蛋壳颜色的一个候选基因
在原卟啉合成通路中,ALAS1是催化甘氨酸和琥珀酰辅酶A合成δ-氨基乙酰丙酸的限速酶,而CPOX是促化粪卟啉原Ⅲ转化为原卟啉原Ⅲ的酶[22]。尽管(位于12号染色体上)和(1号染色体)基因均未落在已知的蛋壳颜色QTL内[16-18],但鉴于这两个候选基因在原卟啉前体物合成中的重要作用,它们仍有可能作为受主效应基因调控的下游基因参与褐壳蛋色的调控。Samiullah等[22]发现抗球虫药尼卡巴嗪主要通过下调致使褐壳颜色变浅。Li等[19]和Lu等[20]发现和在深褐壳样本中的表达水平显著高于浅褐壳。本研究发现在深褐壳中的表达量显著高于浅褐壳组,而并未呈现这种表达差异。在蛋壳腺中的表达水平随蛋的形成而变化,而的表达量在蛋形成的整个过程中维持恒定[22]。为此,本研究推测表达的时间依赖性增大了表达量比较的难度,即在无法保证样本采集时间一致性的情况下将很难消除采样时间不同造成的试验误差,发现组间表达差异。尽管如此,本研究所取得的结果证实是与略阳乌鸡蛋壳中原卟啉含量和褐壳颜色深浅密切相关的一个候选基因。
3.4 3个色度指标遗传力的差异
本研究发现略阳乌鸡蛋壳颜色L值(h2=0.46)和b值(h2=0.37)的遗传力估计结果均位于前人报道的遗传力估计范围(L值:h2=0.2—0.65,b值:h2=0.22—0.64)内,但a值遗传力(h2=0.77)高于目前已报道的结果(a值:h2=0.21—0.74)[24-27]。此外,前人报道的3个色度指标的遗传力较为接近[26,27],但本研究所得a值遗传力要明显高于L值和b值,与Goger等[25]在洛岛红中估计的结果(a值:h2=0.74 vs. L值:h2=0.37和b值:h2=0.64)较为接近。本研究推测a值遗传力高可能与以下两方面原因有关:第一、a值能响应于胆绿素含量变化。在3个色度指标中,a值是唯一一个能对胆绿素含量变化做出灵敏反应的色度指标。胆绿素在蛋壳中的沉积及其引起的绿壳色泽主要受遗传效应调控[14-15,35]。因此,在引起a值变异的因素中,遗传效应将起着非常重要的作用。第二、a值度量蛋壳颜色变化的高灵敏度。本研究基于半同胞数据用单变量动物性模型估计遗传力。高遗传力性状在表型方面应该表现出家系内具有较高的一致性,但家系间存在较大的差异。a值较高的灵敏性和较大的变异系数表明,它更有可能捕捉到家系间蛋色的遗传差异,获得较高的遗传方差和遗传力估计结果。
相对于蛋壳绿色调主要受基因调控的特点,褐壳蛋壳颜色有着更为复杂的遗传基础,且易受年龄、药物、疾病、应激、产蛋时间等多种非遗因素的影响[1,13]。Li等[19]发现L值与原卟啉含量存在强负相关关系,并建议L值作为度量褐壳蛋颜色深浅和原卟啉含量的一个指标。本研究发现除了L值外,b值也与原卟啉含量存在强正相关关系。基于上述结果,本研究推测L值和b值遗传力偏低可能与褐壳复杂的遗传基础和易受环境影响的特点有关。
4 结论
研究发现a值是量化略阳乌鸡蛋壳颜色准确度和灵敏度最高的色度指标,且主要受遗传效应调控。对a值向上选择,将有望在褐壳深度和均匀度上取得较大遗传进展。鉴于a值与蛋壳色素间的强相关关系,将a值作为蛋壳色素含量的间接评价指标,在基因定位之类的大样本研究中将为蛋壳色素含量不易测定、成本高提供一种解决方案。原卟啉是决定略阳乌鸡蛋壳颜色变异的关键色素,是与原卟啉浓度和褐壳颜色深浅显著关联的一个候选基因。
[1] SAMIULLAH S, ROBERTS J R, CHOUSALKAR K. Eggshell color in brown-egg laying hens - a review. Poultry Science, 2015, 94(10): 2566-2575.
[2] ŞEKEROĞLU A, DUMAN M. Effect of egg shell colour of broiler parent stocks on hatching results, chickens performance, carcass characteristics, internal organ weights and some stress indicators. Kafkas Universitesi Veteriner Fakultesi Dergisi, 2011, 17(5):837-842.
[3] JAVU016FRKOVÁ V G, POKORNÁ M, MIKŠÍK I, TU016FMOVÁ E. Concentration of egg white antimicrobial and immunomodulatory proteins is related to eggshell pigmentation across traditional chicken breeds. Poultry Science, 2019, 98(12): 6931-6941.
[4] DRABIK K, KARWOWSKA M, WENGERSKA K, PRÓCHNIAK T, ADAMCZUK A, BATKOWSKA J. The variability of quality traits of table eggs and eggshell mineral composition depending on hens' breed and eggshell color. Animals, 2021, 11(5): 1204.
[5] CASSEY P, PORTUGAL S J, MAURER G, EWEN J G, BOULTON R L, HAUBER M E, BLACKBURN T M. Variability in avian eggshell colour: A comparative study of museum eggshells. PLoS ONE, 2010, 5(8): e12054.
[6] SKRADE P D B, DINSMORE S J. Egg crypsis in a ground-nesting shorebird influences nest survival. Ecosphere, 2013, 4(12): 1-9.
[7] LAHTI D C. Population differentiation and rapid evolution of egg color in accordance with solar radiation. The Auk, 2008, 125(4): 796-802.
[8] BAKKEN G S, VANDERBILT V C, BUTTEMER W A, DAWSON W R. Avian eggs: Thermoregulatory value of very high near-infrared reflectance. Science, 1978, 200(4339): 321-323.
[9] GOSLER A G, HIGHAM J P, JAMES REYNOLDS S. Why are birds’ eggs speckled? Ecology Letters, 2005, 8(10): 1105-1113.
[10] MORENO J, OSORNO J L. Avian egg colour and sexual selection: Does eggshell pigmentation reflect female condition and genetic quality? Ecology Letters, 2003, 6(9): 803-806.
[11] LANG M R, WELLS J W. A review of eggshell pigmentation. World’s Poultry Science Journal, 1987, 43(3): 238-246.
[12] KENNEDY G Y, VEVERS H G. A survey of avian eggshell pigments. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry, 1976, 55(1): 117-123.
[13] LU M Y, XU L, QI G H, ZHANG H J, QIU K, WANG J, WU S G. Mechanisms associated with the depigmentation of brown eggshells: A review. Poultry Science, 2021, 100(8): 101273.
[14] WANG Z P, QU L J, YAO J F, YANG X L, LI G Q, ZHANG Y Y, LI J Y, WANG X T, BAI J R, XU G Y, DENG X M, YANG N, WU C X. An EAV-HP insertion in 5' Flanking region of SLCO1B3 causes blue eggshell in the chicken. PLoS Genetics, 2013, 9(1): e1003183.
[15] CHEN L, GU X R, HUANG X T, LIU R, LI J X, HU Y Q, LI G Q, ZENG T, TIAN Y, HU X X, LU L Z, LI N. Two cis-regulatory snps upstream of abcg2 synergistically cause the blue eggshell phenotype in the duck. Plos Genetics, 2020, 16(11): e1009119.
[16] SASAKI O, ODAWARA S, TAKAHASHI H, NIRASAWA K, OYAMADA Y, YAMAMOTO R, ISHII K, NAGAMINE Y, TAKEDA H, KOBAYASHI E, FURUKAWA T. Genetic mapping of quantitative trait loci affecting body weight, egg character and egg production in F2intercross chickens. Animal Genetics, 2004, 35(3): 188-194.
[17] SCHREIWEIS M A, HESTER P Y, SETTAR P, MOODY D E. Identification of quantitative trait loci associated with egg quality, egg production, and body weight in an F2resource population of chickens1. Animal Genetics, 2006, 37(2): 106-112.
[18] GOTO T, ISHIKAWA A, YOSHIDA M, GOTO N, UMINO T, NISHIBORI M, TSUDZUKI M. Quantitative trait loci mapping for external egg traits in F2chickens. The Journal of Poultry Science, 2014, 51(2): 118-129.
[19] LI G Q, CHEN S R, DUAN Z Y, QU L J, XU G Y, YANG N. Comparison of protoporphyrin IX content and related gene expression in the tissues of chickens laying brown-shelled eggs. Poultry Science, 2013, 92(12): 3120-3124.
[20] LU M Y, WANG W W, QI G H, XU L, WANG J. Mitochondrial transcription factor A induces the declined mitochondrial biogenesis correlative with depigmentation of brown eggshell in aged laying hens. Poultry Science, 2021, 100(3): 100811.
[21] ZHENG C W, LI Z S, YANG N, NING Z H. Quantitative expression of candidate genes affecting eggshell color. Animal Science Journal, 2014, 85(5): 506-510.
[22] SAMIULLAH S, ROBERTS J, WU S B. Downregulation of ALAS1 by nicarbazin treatment underlies the reduced synthesis of protoporphyrin IX in shell gland of laying hens. Scientific Reports, 2017, 7: 6253.
[23] HAN G P, KIM J M, KANG H K, KIL D Y. Transcriptomic analysis of the liver in aged laying hens with different intensity of brown eggshell color. Animal Bioscience, 2021, 34(5): 811-823.
[24] GUO J, WANG K H, QU L, DOU T C, MA M, SHEN M M, HU Y P. Genetic evaluation of eggshell color based on additive and dominance models in laying hens. Asian-Australasian Journal of Animal Sciences, 2020, 33(8): 1217-1223.
[25] GOGER H, DEMIRTAS S E, YURTOGULLARI S. A selection study for improving eggshell colour in two parent lines of laying hens and their hybrids. Italian Journal of Animal Science, 2016, 15(3): 390-395.
[26] CAVERO D, SCHMUTZ M, ICKEN W, PREISINGER R. Attractive eggshell color as a breeding goal. Lohmann Information, 2012, 47(2): 16-21.
[27] KAMANLI S. Estimation of genetic parameters for some performance traits in a selected Barred Rock line. Ankara Üniversitesi Veteriner Fakültesi Dergisi, 2019, 66:391-396.
[28] 党李苹, 周雯馨, 刘瑞芳, 白云, 王哲鹏. 略阳乌鸡体重和产蛋数性状遗传参数估计. 中国农业科学, 2020, 53(17): 3620-3628.
DANG L P, ZHOU W X, LIU R F, BAI Y, WANG Z P. Estimation of genetic parameters of body weight and egg number traits of Lueyang black-boned chicken. Scientia Agricultura Sinica, 2020, 53(17): 3620-3628. (in Chinese)
[29] WILSON A J, RÉALE D, CLEMENTS M N, MORRISSEY M M, POSTMA E, WALLING C A, KRUUK L E B, NUSSEY D H. An ecologist’s guide to the animal model. The Journal of Animal Ecology, 2010, 79(1): 13-26.
[30] HADFIELD J D. MCMC methods for multi-response generalized linear mixed models: The MCMCglmm RPackage. Journal of Statistical Software, 2010, 33(2): 1-22.
[31] Hadfield J D. MCMCglmm course notes. MCMCglmm R package. 2010. http://cran.r-project.org/web/packages/MCMCglmm/index.html.
[32] WANG X T, ZHAO C J, LI J Y, XU G Y, LIAN L S, WU C X, DENG X M. Comparison of the total amount of eggshell pigments in Dongxiang brown-shelled eggs and Dongxiang blue-shelled eggs. Poultry Science, 2009, 88(8): 1735-1739.
[33] HARRELL F E. Hmisc: Harrell Miscellaneous. R package version 4.5-0. 2021. https://CRAN.R-project.org/package=Hmisc.
[34] LI G, XU J, CHEN S, TAN S, LI H. Pigment concentrations in eggshell and their related gene expressions in uterus of Changshun blue eggshell chickens. British Poultry Science, 2022, 63(3): 421-425.
[35] PUNNETT C. Genetic studies in poultry. Journal of Genetics, 1933, 27(3): 465-470.
[36] ZENG L S, XU G Y, JIANG C Y, LI J Y, ZHENG J X. Research Note: L*a*b* color space for prediction of eggshell pigment content in differently colored eggs. Poultry Science, 2022, 101(8): 101942.
Establishment of Quantization Method and Genetic Basis Analysis of Brown Eggshell Color in the Lüeyang Black-Boned Chicken
CHEN Qiu, HUANG JingJing, WANG ZhePeng
College of Animal Science and Technology, Northwest A&F University, Yangling 712100, Shaanxi
【Background】Brown eggshell color is closely relevant to eggshell strength, concentrations of egg white antimicrobial protein and yolk carotenoid, blood and meat spots, and hatchability, and is an important index affecting quality and sale of eggs. However, due to absence of selection for eggshell color, color of brown eggs that some indigenous breeds lay is light and highly variable, which has an adverse effect on sale of eggs and creation of egg brands. 【Objective】The aims of this study are to establish a quantization method that could accurately and sensitively capture the variation of eggshell color, to estimate breed-specific heritability of eggshell color and to identify candidate genes associated with eggshell color in the Lüeyang black-boned chicken (LBC), so as to provide a theoretic basis for genetic improvement of LBC eggshell color. 【Method】841 hens from 62 half-sibling families of LBC breeding population were selected. Three eggs were collected from each hen, and then the eggshell color was quantified using the L*a*b color space, and posterior heritabilities of L, a, and b values were estimated in a Bayesian framework using the Markov Chain Monte Carlo (MCMC) algorithm. The prior distributions of breeding value and residual variances were set using an inverse-Gamma distribution. MCMC performed 130 000 iterations, dropped 30 000 iterations at the beginning and stored one every 100 iterations to obtain posterior distributions of variance components and posterior heritability estimates of color indexes. Hens that laid light brown (n=8), brown (n=8) and light blue (n=8) eggs were selected from LBC population. Three eggs were collected from each hen for the measurement of eggshell pigment, and the absorbance values were measured at 670 nm for biliverdin and at 412 nm for protoporphyrin. The concentrations of protoporphyrin and biliverdin were calculated by regression equations, which were fitted using absorbances and concentrations of standard samples. Shell glands of brown- (n=8) and light brown-shelled (n=8) hens were collected. Expression levels ofandin shell glands were detected using qPCR. 【Result】Among three color indexes, a* value kept a high consistency with the change of eggshell color based on subjective perception as a* value generally decreased with transition of egg color from brown to green hues. L and b values could accurately reflect the change of egg color from light to dark brown, but L and b values were unable to discriminate between brown and green hues reliably. L and b values showed low variation as L and b values of 50% of samples were distributed between 73.3-79.1 and 13.1-18.2. In contrast, a* values were evenly distributed among the samples as a* values of 50% of samples ranged from 1.4 to 7.1. In line with the distribution characteristics, the coefficient of variation (88.2%) of a value was higher than ones of L (5.7%) and b (24.0%) values. Estimation results of heritability showed that a* value (h2=0.77) was predominately affected by genetic factors. In contrast, L (h2=0.46) and b (h2=0.37) values were controlled by environmental effect to larger extents. For the relationship of eggshell pigment and color, protoporphyrin concentration had a strong correlation with all of three color indexes (L:=-0.86, a:=0.73, b:=0.88). But biliverdin concentration showed a strong (=-0.73) negative correlation with a* value alone.andwere two key enzymes that catalyze the biosynthensis of protoporphyrin precursors. Expression results showed that expression levels ofin shell glands of brown-shelled chickens were 1.5-fold higher (<0.05) than that in shell glands of light brown-shelled chickens.had no significant (>0.05) difference between brown- and light brown-shelled chickens.【Conclusion】These results indicated that protoporphyrin was the key pigment affecting LBC eggshell color, andwas a candidate associated with protoporphyrin concentration and color of brown eggs. The a* value was an optimal index quantifying eggshell color of LBC with high accuracy and sensitivity. In view of high heritability estimate of a* value, it was possible to increase brown hue and uniformity of LBC eggshell color via positive selection of a* value.
Lüeyang black-boned chicken; eggshell color; L*a*b color space; heritability;;
10.3864/j.issn.0578-1752.2023.17.017
2022-08-02;
2022-11-29
陕西省重点研发计划(2021NY-028)、陕西省农业厅专项计划畜禽新品种培育—略阳鸡(K3031222058)
陈球,E-mail:chenqiu_960625@163.com。通信作者王哲鹏,Tel:029-87091960;E-mail:wangzhepeng-001@163.com
(责任编辑 林鉴非)

