A novel strategy of constructing 2D supramolecular organic framework sensor for the identification of toxic metal ions
Ying Wng ,Ning Hn ,Cho-Qun M ,Hui Liu ,Shengsheng Yu ,Rongzhou Wng ,Vijy Kumr Thkur,Ling-Bo Xing,**
a School of Chemistry and Chemical Engineering,Shandong University of Technology,Zibo,255000,PR China
b Department of Materials Engineering,KU Leuven,Leuven,3001,Belgium
c Biorefining and Advanced Materials Research Center,Scotland's Rural College (SRUC),Kings Buildings,West Mains Road,Edinburgh,EH9 3JG,UK
Keywords:Two-dimensional supramolecular organic framework Encapsulation-enhanced donor-acceptor interaction Luminescent detection
ABSTRACT Two novel two-dimensional (2D) supramolecular organic frameworks were fabricated in water based on the encapsulation-enhanced donor-acceptor interaction between the methyl viologen (MV) units,methoxy naphthyl(MN) units,and CB[8].The tetraphenylethylene(TPE) derivatives 1 with four MV units were employed as rigid building blocks and the two MN units modified oligoethylene glycol derivatives 2 and 3 served as flexible edges,respectively.The obtained two SOFs have obvious sheet-like structures and exhibit fluorescence emission at 350–500 nm.In addition,these two SOFs were employed for the luminescent detection of Cr(VI) and Mn(VII)in aqueous solutions,and the detection limits of Cr,Cr2,and Mn were calculated in a very low concentration range,indicating that these two SOFs can serve as a potential sensor for Cr(VI)and Mn(VII)detection in water.This work constructs two SOFs in an aqueous solution through a facile method and further enriches the applications of SOFs.
1.Introduction
As an interdisciplinary subject of polymer science and supramolecular chemistry,supramolecular polymers have aroused extensive research interest due to their diverse self-assembled structures,stimuli responsiveness,and reversibility [1–6].Supramolecular polymers can be formed based on monomeric units held together with a variety of non-covalent interactions,including host-guest interactions [7–9],hydrogen bonding [10],hydrophobic interactions [11],coordination interactions [12,13],and electrostatic interactions [14,15].Typical linear supramolecular polymers could be formed based on the two-headed monomeric units [16,17].In addition,based on the multi-headed monomeric units with more binding sites,supramolecular polymers with precise self-assembled structures can be obtained.Those special supramolecular polymers with the ordered periodic porous structure are called the supramolecular organic framework (SOF) [18].The structure of SOFs can be regulated and controlled based on the supramolecular assembly strategy.For example,two-dimensional (2D)SOFs can be fabricated based on planar building blocks,while three-dimensional(3D)SOF structures can be obtained by building with polyhedral building blocks.In 2021,Ma et al.reported a room-temperature phosphorescence 2D SOF through planar dendritic guest[19].In 2019,Li et al.reported a 3D SOF based on the co-assembly between tetracationic monomers and CB [8,20].
CB[n]is a pumpkin-shaped macrocyclic compound that has a large hydrophobic cavity.CB[n]has good selectivity and strong bonding ability with many types of guest molecules,and is commonly used for the construction of supramolecular polymers[21–26].In recent years,many SOFs driven by host-guest interactions between CB[8]and the building blocks have been reported[27–37].In 2001,Kim et al.reported that the hydrophobic cavity of CB [8]can selectively accommodate both electron-deficient guests and electron-rich guests to form a stable 1:1:1 ternary complex [38].Based on this classical model,many SOFs have been constructed[39–41].For example,in 2015,Zhao et al.fabricated a 2D SOF based on the encapsulation-enhanced donor-acceptor interaction between the methyl viologen(MV)units and the 2,6-dihydroxynaphthalene units[39].In 2016,Li et al.fabricated a single-layer SOF driven by the host-guest interaction between the viologen units and the 2,6-deoxy or 2-hydroxy-6-amino naphthalene units[40].In addition,in 2015,Feng et al.reported a single-layer 2D SOF,in which the tris(methoxynaphthyl)units and N-methyl viologenyl moieties act as donor and acceptor monomers,respectively,and CB[8]is employed as host monomer[41].Although there have many examples of SOFs have been reported,only a few applications of SOFs have been reported in these years,such as luminescent detection [42,43],explosive detection [44],drug delivery[45],and photodynamic therapy [35,46],which has greatly stimulated people's interest in fabricating SOF.However,compared with other porous organic polymers,such as metal-organic frameworks(MOFs)and covalent organic frameworks (COFs),there are still few reports on the construction of SOFs,and the applications of SOFs still need to be further enriched.
Herein,we designed and synthesized a tetraphenyl ethylene derivative1with four methyl viologen (MV) units and oligoethylene glycol derivatives2and3with two methoxy naphthyl(MN)units.The MV units could serve as electron-deficient acceptors,and the MN units of compound2or3serve as electron-rich donors.Based on the encapsulationenhanced donor-acceptor interaction between the MV units,MN units,and CB [8],the MV units of compound1and the MN units of building compound2or3can be encapsulated by CB[8]to form two SOFs(SOF-1 and SOF-2) with sheet-like structure (see Scheme 1).The SOFs can be obtained by mixing three compounds in a certain proportion in an aqueous solution,and no tedious synthesis steps and harsh reaction conditions are required.The obtained SOFs have fluorescence emission at 350–500 nm,which could be used to realize the specific detection of Mn,Cr2,and Crin an aqueous solution.After the addition of these three anions into the solution of SOF-1 or SOF-2,the fluorescence emission of SOFs was reduced by more than 60%.The detection limits are determined to be 1.88×10-4M and 1.79×10-4M for Cr,1.59× 10-4and 2.22 × 10-4for Cr2,1.98 × 10-4and 1.98 × 10-4for Mnrespectively,which indicate that these two SOFs can be used as sensitive detectors to realize specific luminescent detection in water.
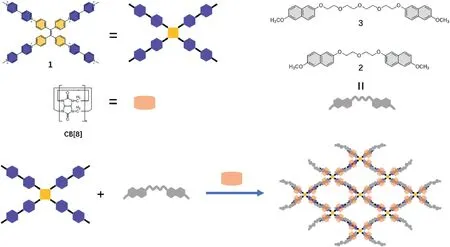
Scheme 1.Illustration of the 2D SOF based on the compound 1,2,3 and CB[8].
2.Experimental
All the details of materials preparation and characterizations are given in the supporting information.
3.Results and discussion
The detailed procedures for the synthesis of compounds1,compound2,and3are given in Fig.1.Compound5was obtained by the McMurry reaction.Four viologen units were attached to the periphery of the tetraphenyl ethylene core to form compound6as an intermediate,and compound1was obtained by further methylation.The structures of compound1,compound2and3were determined by1H NMR in supporting information.
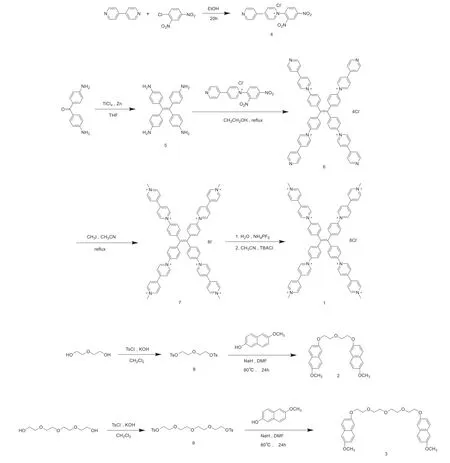
Fig.1.Synthetic route of compound 1,2 and 3.
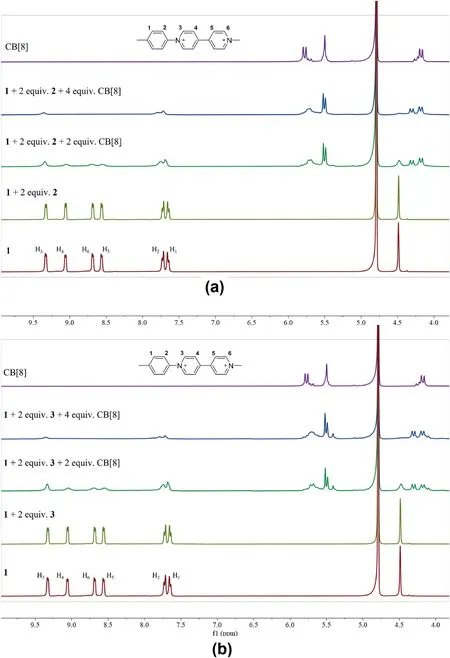
Fig.2.1H NMR spectra (400 MHz) of (a) the mixed solution of 1,2, CB [8].(b) the mixed solution of 1,3, CB [8]in D2O.
We first investigate the formation of the SOFs through1H NMR titration experiments.As shown in Fig.2a,after addition 2 equiv.of2into a solution of1in D2O,there are almost no changes in the spectrum of1observed.This is probably due to the extremely low solubility of compound2in D2O.After the addition of CB[8]into the mixture of1and2in D2O,the signals of MV units of1show a significant decline,and the peaks of MV are significantly broadened.These results show that the MV units were encapsulated by CB[8].When adding 4 equiv.of CB[8],the H4,H5and H6signals of MV units disappeared completely,which indicates the binding ratio of1,2and CB [8]was 1: 2: 4.Similar experimental phenomenon can be observed in the1H NMR titration experiments of compounds1,3and CB[8],indicating the same binding ratio(1:2:4)between1,3and CB[8](Fig.2b).In addition,the1H NMR spectrums of these two three-component mixtures were carried out in D2O after the solutions were left standing for three days.As shown in Fig.S4,the1H NMR spectrum of these two SOFs did not show significant changes,indicating the high stability of the SOFs in an aqueous solution.
The absorption spectra of these two three-component mixtures were then used to investigate the formation of the polymeric structures.As shown in Fig.3a,the absorption curve of compound1has two absorption peaks at 225 nm–300 nm and 300 nm–500 nm,which can be attributed to the absorption of the MV unit and the TPE unit respectively.After the addition of 2 equiv.of2into a solution of1in water,the absorption of compound1shows no significant change.In contrast,after the addition of 2 equiv.of2and 4 equiv.of CB [8],the absorption peaks at 225 nm–300 nm decrease significantly.At the same time,the absorption peak at 365 nm showed an obvious red shift.The results above indicate that the MV units of1and the MN units of2were encapsulated by CB[8],and the red shift of the absorption peak at 365 nm may be attributed to the electron transfer between the electron-deficient MV unit and the electron-rich MN unit.The three-components UV absorption of compounds1,3and CB [8]showed a similar phenomenon,the absorption peak at 365 nm shows a larger redshift (Fig.3d).In addition,the absorption spectra of these two three-component mixtures at different concentrations were also made (Fig.3b and e).All absorptions of the mixtures at the tested concentrations follow Lambert Beer's law,which shows that the supramolecular structures are stable in the studied concentration range.
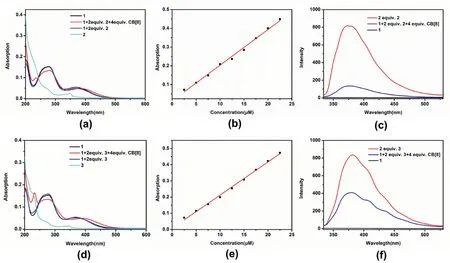
Fig.3.UV–Vis spectra(a)1,1+2 equiv.2,1+2 equiv.2+4 equiv.CB[8]and 2(d)1,1+2 equiv.3,1+2 equiv.3+4 equiv.CB[8]and 3 in water.(b)The plot of the absorption of the mixture of 1,2 and CB[8](1:2:4)at 376 nm vs[1].(e)The plot of the absorption of the mixture of 1,3 and CB[8](1:2:4)at 378 nm vs[1].Fluorescence emission spectra of(c)1,2,1+2 equiv.2+4 equiv.CB[8].(f)1,3,1+2 equiv.3+4 equiv.CB[8].([1]=2.5×10-6 M,[2]or[3]=5×10-6 M[CB[8]],=1 × 10-5 M) in water.
To further investigate the formation of polymeric structures,we also studied the fluorescence emission of these two three-component systems.As shown in Fig.3c,compound1has almost no fluorescence emission in water,and the solution of compound2has a fluorescence peak at 372 nm when excited at 276 nm.In contrast,under the same concentration,the fluorescence emission of compound2decreases significantly after mixing compound2with1and CB [8].The obvious decrease in fluorescence emission of compound2may be attributed to the charge transfer since the electron-deficient MV unit and the electron-rich MN unit are co-encapsulated in the cavity of CB[8].The fluorescence of the mixture of compounds1,3and CB [8]in water also is much lower than that of compound3when excited at 276 nm,indicating that the MV unit of1and the MN unit of3are co-encapsulated by CB[8](Fig.3f).And1+2 equiv.3+4 equiv.CB [8]mixtures exhibit stronger fluorescence emission than1+2 equiv.2+4 equiv.CB[8]mixtures in aqueous solution by comparison.In addition,the fluorescence emission of SOF-1 and SOF-2 when excited at 365 nm was also investigated.As shown in Fig.S5,When excited at 365 nm,compounds2and3are not fully excited.In contrast,compound1has weak emission at 450 nm–600 nm.The weak emission of compound1may be due to the electron transfer between the MV unit and the TPE unit.After the formation of SOF,the MV units of1and the MN units of2or3were co-encapsulated by CB[8],there will be electron transfer between the electron-deficient MV unit and the electron-rich MN unit.Therefore,the emission of TPE unit at 450 nm–600 nm is enhanced slightly.However,the fluorescence emission intensity of TPE is still very low compared to that of SOF-1 and SOF-2 under the excitation of 276 nm.
Moreover,we further investigate the morphology of the formed SOFs through transmission electron microscopy (TEM).As shown in Fig.4a,the TEM image of1+2 equiv.2+4 equiv.CB [8]mixture shows an obvious sheet-like structure with large sizes,which proved the formation of a 2D supramolecular structure.And the TEM image of1+2 equiv.3+4 equiv.CB[8]mixture also shows a large sheet-like structure(Fig.4b).In addition,the dynamic light scattering(DLS)experiments of compound1and the two three-component mixtures were performed to determine their hydrodynamic diameter (DH) values.As shown in Fig.4c,the DHvalue of compound1in water was determined to be 190 nm.And the DHvalues of the solution of the two three-component mixtures were 400 nm and 525 nm,respectively (Fig.4d and e).The apparent increase in DHvalues may be attributed to the formation of larger-sized SOFs.These results clearly show that the SOFs have been successfully prepared through the self-assembly strategy.In addition,we also studied the ordered periodicity of 2D SOF through small angle X-ray scattering(SAXS)in an aqueous solution.As shown in Fig.S6,for the solution of SOF-1,a clear scattering peaks at 3.82 nm-1was observed.And thedspacing of SOF-1 was determined as 1.64 nm through formula(D=2π/q).

Fig.4.TEM images of samples(a)1+2 equiv.2+4 equiv.CB[8],(b)1+2 equiv.3+4 equiv.CB[8]in water.DLS results of(c)1,(d)the mixtures of 1,2,and CB[8],(e) the mixtures of 1, 3,and CB [8]in water.([1]=5 × 10-6 M,[2]or [3]=1 × 10-5 M [CB [8]],=2 × 10-5 M).

Fig.5.Fluorescence emission spectra of(a)SOF-1 with the different anions.(d)SOF-2 with the different anions in water.The quenching efficiency of(b)SOF-1 with the addition of the anions.(e)SOF-2 with the addition of the anions([1]=7.5×10-6 M,[2]or[3]=1.5×10-5 M[CB[8]],=3×10-5 M).UV–vis absorption spectra of Cr2,Cr,and Mn in aqueous solutions and the emission spectra of (c) SOF-1,(f) SOF-2.

Fig.6.Fluorescence emission spectra of SOF-1 with the gradual addition of(a)Cr,(b)Cr2,(c)Mnin water(λex=270 nm).The Stern-Volmer plots of I0/I versus the concentration of (d) Cr,(e) Cr2,(f) Mn.
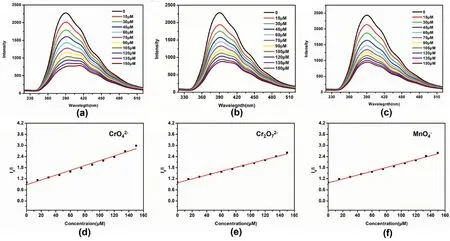
Fig.7.Fluorescence emission spectra of SOF-2 with the gradual addition of(a)Cr,(b)Cr2,(c)Mnin water(λex=270 nm).The Stern-Volmer plots of I0/I versus the concentration of (d) Cr,(e) Cr2,(f) Mn.
4.Conclusion
In summary,we fabricated two novel three-components 2D SOFs in water based on the encapsulation-enhanced donor-acceptor interaction between MV units,MN units,and CB[8].Unlike most reported SOFs,the SOFs herein are fabricated through the three-components supramolecular self-assembly strategy.The planar compound1with four MV units served as rigid units and the oligoethylene glycol derivatives2and3served as flexible edges to realize the 2D structure.And the SOFs can be obtained by mixing three compounds in a certain proportion in an aqueous solution,no tedious synthesis steps,and harsh reaction conditions are required.In addition,these SOFs can realize the specific detection of Cr(VI)and Mn(VII)anions in an aqueous solution,showing their application potential in luminescent detection.At present,compared with other porous organic polymers,such as MOFs and COFs,there are still few reports on the construction and application of SOFs.In this context,it is hoped that more new strategies for building SOFs will be reported,and the application of SOFs in other fields will be discovered.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
We are grateful for the financial support from the National Natural Science Foundation of China(52205210 and 22005179)and the Natural Science Foundation of Shandong Province (ZR2020MB018,ZR2022QE033,ZR2021QB049,and ZR2020QB113).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2023.01.002.
- Namo Materials Science的其它文章
- PtZn nanoparticles supported on porous nitrogen-doped carbon nanofibers as highly stable electrocatalysts for oxygen reduction reaction
- Flexible and electrically robust graphene-based nanocomposite paper with hierarchical microstructures for multifunctional wearable devices
- Piezoresistive behavior of elastomer composites with segregated network of carbon nanostructures and alumina
- Surface reconstruction,modification and functionalization of natural diatomites for miniaturization of shaped heterogeneous catalysts
- DFT study on ORR catalyzed by bimetallic Pt-skin metals over substrates of Ir,Pd and Au
- Constructing P-CoMoO4@NiCoP heterostructure nanoarrays on Ni foam as efficient bifunctional electrocatalysts for overall water splitting

