DFT study on ORR catalyzed by bimetallic Pt-skin metals over substrates of Ir,Pd and Au
Xueqing Qi ,Tingting Yng ,Pingbo Li ,Zidong Wei
a College of Chemistry and Chemical Engineering,Chongqing University of Technology,Chongqing,400054,China
b The State Key Laboratory of Power Transmission Equipment & System Security and New Technology,College of Chemistry and Chemical Engineering,Chongqing University,Chongqing,400044,China
Keywords:ORR DFT Pt-skin Catalysts
ABSTRACT Bimetallic Pt-skin catalyst is a class of near-surface alloy (NSA) that owns a high degree of control over composition.Herein,density functional theory (DFT) is used to calculate the energetics of oxygen reduction reaction (ORR) on Pt-skin over Ir,Pd and Au substrates.A Brønsted-Evans-Polanyi (BEP) relationship can be determined for the oxygen molecule dissociation.The binding energy of both atomic oxygen and hydroxyl radical is found to correlate well with the d band center of Pt-skin atoms.Their catalytic activities show the volcano relationship as the positions of each substrate in the periodic table.The effect of surface strain,band structure and charge transfer on the d band center is well studied,and it can be found that the surface strain effect plays a dominant role for all Pt-skin catalysts.Ir substrate makes the d band center of Pt-skin go far away from the Fermi level,while Au substrate makes it move towards the Fermi level.Being different from both Ir and Au,Pd substrate makes the d band center of Pt-skin comparable with the monometallic Pt.
1.Introduction
Platinum is the most effective cathode catalyst in proton exchange membrane fuel cells (PEMFCs) owing to its outstanding electrocatalytic characteristics.However,the commercialization of FCs for automotive applications still requires more economical and effective catalytic materials which can be operated with a much smaller amount of expensive platinum[1].Better and less-expensive catalysts are needed to make the widespread use of low-temperature fuel cells viable.Over the last decades,it has been reported that the electrocatalytic performance of platinum-based materials can be improved by controlling the morphology of platinum nanocrystals or alloying platinum with other transition metals [2–5].In particular,the catalytic properties of bimetallic surfaces consisting of a metal monolayer on monometallic metal surfaces have attracted scientists’ attention both in experimental and theoretical fields [6–8].The formation of a surface metal-metal bond which produces significant changes for both structural and electronic properties of the metal overlayer displays much difference from the monometallic surfaces[9,10].
The ORR is a complex reaction involving the transfer of four electrons and protons.The effect of the substrate metals on the catalytic activity of ORR has been studied with some experimental methods,including scanning tunneling microscopy (STM) [11],photoelectron [12],and vibrational spectroscopy [13].However,those methods could only give indirect information about adsorption strengths unable to provide as intrinsic and direct information as they were used to reveal the altered properties of this kind of catalytic system.Theoretical calculations that may interpret the tuned activity of the alloying have also been performed.Fern′andez et al.[14]proposed a simple thermodynamic principle in which they assumed a simple mechanism only based on the dissociation of oxygen molecule and the adsorption of intermediate atomic oxygen.Zhuang et al.[15]further studied the enhanced catalytic activity of Pd-based catalysts for the ORR based on the dissociation of oxygen molecule,a volcano relationship between the activity and the degree of alloying has been determined.They came to elucidate the contrary influences from the lattice strain and surface ligand effects.Furthermore,Adzic [9]and Nørskov [16]presented another method based on the basis of both the dissociation of O–O bond and the formation of O–H bond,and constructed a volcano curve of catalytic activity as a function of oxygen and hydroxyl adsorption energies for calculating the optimum catalytic activity for ORR.For a good ORR electrocatalyst,the kinetics of both the dissociation of oxygen molecule and the hydrogenation of reactive intermediates have to be facile at the cathode.Nevertheless,Henkelman [17]thought that the trends in O and OH binding are similar on transition metal surfaces so only O binding type was used for the catalytic reactivity measurement when they studied the bimetallic core-shell nanoparticles.
In this work,the energetics of O2dissociative adsorption,as well as the OH adsorption on various bimetallic Pt-skin surfaces compared with monometallic Pt(111),were studied with DFT method.Furthermore,the effect of surface strain,d band structure and charge transfer on the d band change was analyzed in detail.In order to eliminate the effect of crystalline orientation,all the surfaces of bimetallic Pt-skin catalysts were centralized on the most commonly studied(111)crystal facet.Then the relationship between binding energy and d band center of the Pt-skin,and that between d band and surface lateral strain for Pt-skin surface have been studied.In addition,the d band of each substrate metal was calculated so as to give a much more vivid illustration of the basic rules of designing the new nanocatalysts.Together with calculating the Ir,Pd and Au substrate metals,it can be determined how the energetics of ORR and the electronic properties of Pt-skin surface change,and which are the primary factors that relate to the reactivity of the catalysts.Our results also suggested that the improvement in overall fuel cell efficiency can be combined with substantial cost savings through using much palladium and less platinum at the cathode.
2.Methods and models
The periodic,self-consistent DFT calculation is used to investigate the energy and electronic properties of bimetallic Pt-skin catalysts.The Perdew-Wang (PW91) functional of the Generalized Gradient Approximation (GGA) is used to describe non-local exchange and correlation effects.All calculations have been finished in the Dmol3program package.And the transition state is searched with linear synchronous transit(LST) method.The metal atoms are treated by DFT Semi-core Pseudopotentials which have a non-local contribution for each channel up to l(ell)=2,as well as a non-local contribution to account for higher channels.The DNP basis set,equivalent in accuracy to the commonly used 6-31G**Gaussian orbital basis set,is selected since it can give more accuracy for hydrogen involved calculations.The convergence thresholds for energy change,maximum force and maximum displacement are set to be 2×10-5Hartree,0.004 Hartree Å-1and 0.005 Å,respectively.As shown in Fig.1,four metal layers are chosen for all the calculations,in which the three bottom layers are fixed in their bulk positions,Pt-skin atoms and all adsorbates (including O2molecule,O atom,OH radical and H atom)are allowed to relax in the DFT calculations.A vacuum region with 11.33 Å thickness,which is 5 times as long as the distance between two Pt layers,is used to eliminate the interactions along the normal surface of bimetallic metal catalysts.This truly creates a twodimensional metal surface that fully takes into account the metallic nature of platinum and the interaction among the true atoms and their periodic images.In all calculations,the 4×4×1 k-point grid is used to describe the first Brillouin zone.A Fermi surface smearing of 0.01 a.u.is utilized to speed up the convergence.
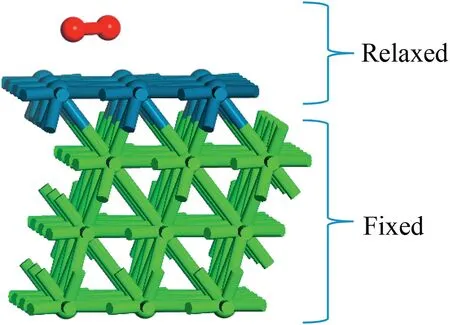
Fig.1.The MPt⋀(111)model used in the calculation(red:O,blue:Pt,green:M,the same below.).
The binding energy(Ebin) is calculated according to formula(1)
in whichEB-Xis the calculated total energy of the system with species adsorbed on the (111) faceted bimetallic catalysts,EXrepresents the energy of isolated adsorbate which can be O2molecule,oxygen atom or OH,EM-Ptis the energy of(111)faceted clean bimetallic Pt-skin catalyst.A more positiveEbinin formula (1) implies that the adsorption is thermodynamically more favorable.
3.Results and discussion
3.1.Mechanism of molecular oxygen dissociation
The adsorption of oxygen molecule on monometallic Pt and bimetallic Pt-skin over Ir,Pd and Au substrates (named as IrPt⋀,PdPt⋀and AuPt⋀respectively was studied first since it is the initial step in the whole ORR.It can be found that the oxygen molecule prefers to adsorb at the bimetallic Pt-skin(111)surface with one oxygen atom at the top site and the other at the bridge site (namely t-b type).As listed in Table 1,the binding energy got the highest value of 61.18 kJ mol-1when the oxygen molecule adsorbed on monometallic Pt(111),while it decreases to the lowest value of 52.07 kJ mol-1on the bimetallic Pt-skin (111) over Ir substrate.There are no significant differences for the adsorption of molecular oxygen on various Pt-skin bimetallic surfaces.The mechanism of O–O bond dissociation on various (111) faceted bimetallic Pt-skin catalysts were further studied.The oxygen molecule with an aligned t-b type geometry was used as the initial state (IS),and two oxygen atoms adsorbed at two adjacent fcc sites were used as the final state (FS).The calculated results showed that all the dissociation routes of oxygen molecule on these surfaces share a similar schematic diagram except for the activation energy barrier.Fig.2 shows the diagrams of O–O bond rupture proceeding on Pt-skin over various substrates.The oxygen atom which was at top site in the IS will transfer to fcc site,the other one at bridge site in the IS will cross another nearby bridge site and finally adsorbs at another adjacent fcc site when the oxygen molecule dissociates on these Pt-skin surfaces.For the transition state (TS),the oxygen molecule adsorbs at two adjacent fcc and bridge sites on Pt-skin (111)surfaces with an elongated O–O bond.The correlation between the energy barrier for O2dissociation and the binding energy of reaction product(oxygen atoms)are further studied on various Pt-skin surfaces.A BEP relationship can be determined for the oxygen molecule dissociation proceeding on the IrPt⋀(111),PdPt⋀(111),Pt(111) and AuPt⋀(111) as shown in Fig.3.The binding energy of the product oxygen atom is inversely correlated to the energy barrier of the oxygen molecule dissociation.For the case of Pt-skin over Au substrate,the product,atomic oxygen,forms the strongest adsorption with the binding energy of 369.50 kJ mol-1.And the reactant,molecular oxygen,dissociates with the least energy barrier of 68.82 kJ mol-1.Whereas Pt-skin over Ir substrate shows the weakest adsorption with the binding energy of 306.41 kJ mol-1in FS but the largest energy barrier of 88.89 kJ mol-1for molecular oxygen dissociative reaction in TS.However,monometallic Pt has shown intermediate values for both the energy barrier in TS and the binding energy in FS,and Pt-skin over Pd substrate shares almost equivalent interaction with those on monometallic Pt(111).When the trends in the energy barrier and binding energy are put into a kinetic model of the surface chemistry,a volcano peak in catalytic activity can be found at an optimal compromise between a low reaction barrier and a weak product binding energy.This result is consistent with the research performed by R.R.Adzic [9]who confirmed that Pt-skin over Pd substrate showed the best catalytic activity for ORR.Thus it can be inferred that the catalysts which possess similar geometry structure and energetics with monometallic Pt(111)will show a better catalytic activity for the ORR,and Pt-skin over Pd substrate is the best candidate among all the three bimetallic Pt-skin metals.
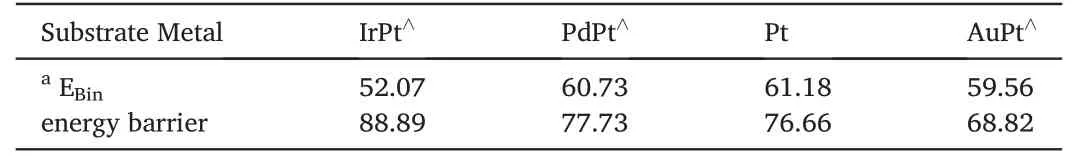
Table 1Binding energy(EBin,kJ mol-1)of adsorbed O2 and energy barrier(Ea,kJ mol-1)of O2 dissociation on MPt⋀(111) surface.
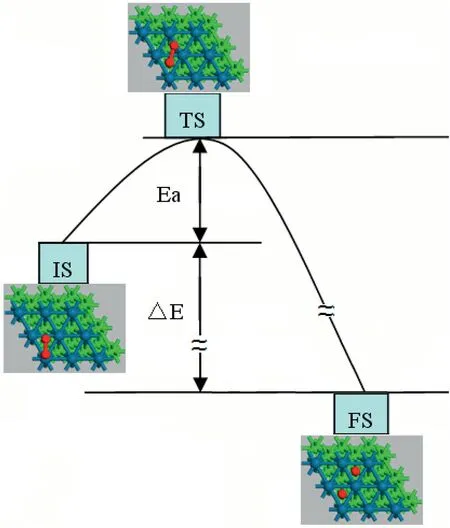
Fig.2.Diagram of O–O bond rupture on MPt⋀over substrate M.
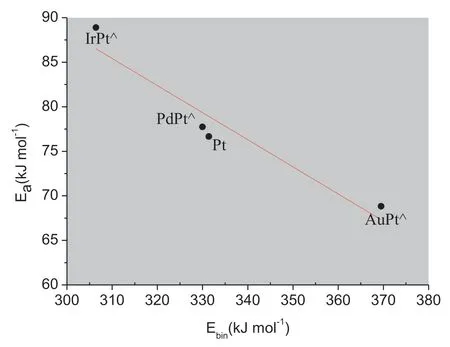
Fig.3.Correlation between energy barrier of molecular oxygen dissociation and binding energy of oxygen atom on various MPt⋀bimetallic catalysts.The most effective catalyst for ORR should possess the optimum compromise between the energy barrier and the binding energy.
3.2.Correlation of binding energies with the d band center of Pt-skin
The d band center of pseudomorphic overlayer has direct effect on its own property and the adsorption of adsorbate[18,19].Thus we studied the correlation between the d band center of Pt-skin over different substrates and the binding energy of both oxygen atom and hydroxide radical.As shown in Fig.4,it can be found that the binding energy of oxygen atom adsorbed on Pt-skin over Ir substrate surface which owns the lowest d band center shows the minimum value of 306.41 kJ mol-1,while Pt-skin over Au substrate which owns the highest d band center has the maximum value of 369.50 kJ mol-1.Pt-skin over Pd substrate shares almost equivalent d band center with monometallic Pt and has a similar binding energy with an energy gap of only 5 kJ mol-1.The binding energy of the intermediate oxygen atom decreases as the d band center going far away from the Fermi level,which is consistent with Mavrikakis's experimental and theoretical studies[20,21].The optimum catalyst should possess a kind of d band center which is neither too high nor too low,a higher one leads to a stronger adsorption for the intermediate oxygen atom product,thus blocking the subsequent reaction,a lower one cannot make the reactant form effective adsorption,thus the reaction cannot be triggered.From the aspect of the relationship between binding energy and d band center,it can be deemed that the catalytic activities will show the volcano relationship as the positions of each substrate Ir,Pd and Au in the periodic table,and that the Pt-skin over Pd substrate will be the most potential candidate as the ORR catalyst.Compared with the adsorption of oxygen atom,the much weaker interaction and the similar adsorption trend for hydroxide radical on various Pt-skin(111)surfaces can be determined.The linear trend between the d band centers of various Pt-skins and the binding energy of the species is expected,but it does not provide an explanation for why different substrate metals shift the d band center of Pt-skin.Thus we further studied the structural and electronic properties.
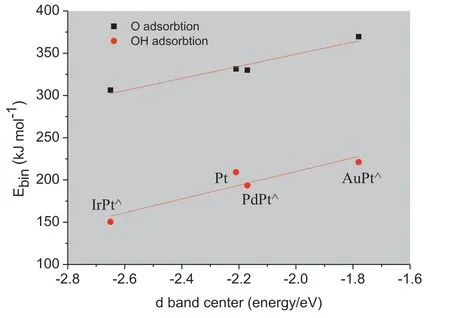
Fig.4.Correlation between binding energy of intermediates (O and OH) on MPt⋀(111)over various substrates and d band center(red:binding energy of O,black: binding energy of OH,the Fermi level is 0eV.).
3.3.Correlation of d band center with strain effect,d band structure and charge transfer
Two types of effect are usually used to describe the properties of nearsurface alloys,one is the strain effect involving the change of morphology,and the other is the ligand effect involving the modifications of electronic structure.Fig.5 is a plot of the strain effect that shows the d band center as a function of the average bond length of Pt-skin atoms.The compressive strain corresponds to the decrease of the average Pt–Pt bond,while the tensile strain corresponds to the increase of the average Pt–Pt bond.It can be found that the compressive strain leads the d band center to shift far away from the Fermi level while the tensile strain towards the Fermi level.The reason lies in that a compression of the metal leads to an increasing overlap between electrons and a widening d band,whereas expansion reduces the d orbital overlap and makes the d band narrow.Considering the d band of Pt-skin over Ir,Pd,Pt and Au as shown in Fig.6 share the similar bandwidth,in order to maintain a constant filling,a widening band shifts its mean value,d band center,far away from the Fermi level,while a narrowing one brings the d band center close to the Fermi level.However,special attention should be paid to Pt-skin over Pd substrate.The Pt–Pt bond length of Pt-skin decreases 0.02 Å,which should lead the d band center of Pt-skin to shift far away from the Fermi level.On the contrary,the d band center moves towards the Fermi level with a value of 0.04 eV.Thus the strain effect is not the exclusive factor that determines the change of the d band center.
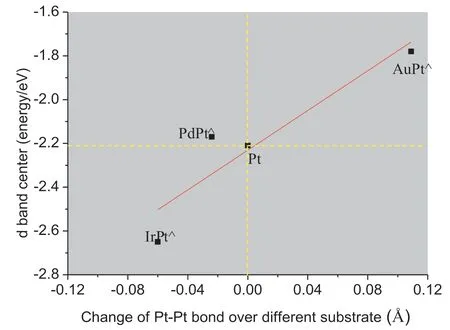
Fig.5.Correlation between the d band center and the change of Pt–Pt bond length in MPt⋀(Fermi level is set to 0 eV).
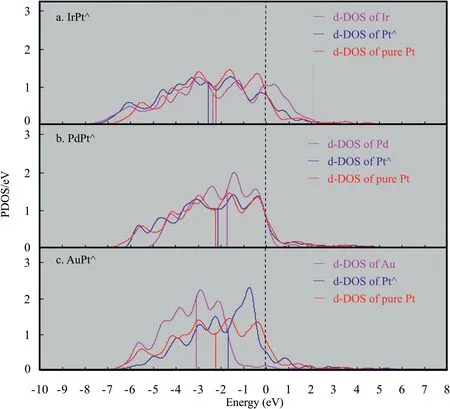
Fig.6.PDOS of the outmost layer of various monometallic substrate metals (pink)and the bimetallic MPt⋀covered on substrates (blue) compared to pure platinum(red).The Fermi level is set to 0 eV.The vertical lines above the x-axis indicate the d-band centers.
In order to study the ligand effect on the change of d band center,the partial density of state (PDOS) of Pt-skin over various substrates compared with the monometallic substrate,and the charge transfer proceeding in various Pt-skin bimetallic catalysts are further studied.As shown in Fig.6a,the d band center of monometallic iridium is-2.25 eV and it is -2.21 eV for monometallic Pt with a gap of only 0.04 eV.However,their catalytic activity is very different from each other as described before,the reason may lie in that the d-DOS around Fermi level is different from each other.Pt-skin over Ir substrate has the lowest d band center compared with both monometallic Ir and Pt,and the order of the d band centers is Pt-skin Charge transfer proceeding in various Pt-skin bimetallic catalysts is then studied compared with the strain effect.Fig.7 shows the effect of charge transfer on the d band center.According to the Hirshfeld charge analysis,when Ir is used as the substrate in the bimetallic Pt-skin catalyst,every skin Pt atom transfers about 0.04 electrons to Ir substrate,which leads to a charge decrease in the d band of Pt-skin,and this effect will make the d band center of Pt-skin go far away from the Fermi level.The reason may lie in that the changes of both d band shape (Fig.6) and d band filling co-determine the d band center.And the rearrangement of d electrons also happen when the d electrons increase or decrease.Strain effect and charge transfer effect now show a consistent trend for the change of the d band center,and their synergistic effect makes the d band center of the Pt-skin 0.44 eV father away from Fermi level.For Pt-skin over Au substrate,every Pt-skin atom takes 0.02 electrons from Au substrate and the electrons accumulation in the d band of Pt-skin will facilitate the d band center to move towards the Fermi level.Furthermore,the significant tensile strain with the average Pt–Pt bond of Pt-skin 0.11longer than that of monometallic Pt–Pt bond will make the d band center move towards the Fermi level.In accordance with the rules aforementioned,the strain effect and the charge transfer effect on the d band center also present a consistent trend and make the d band center of Pt-skin 0.43 eV closer to the Fermi level.However,the surface strain and the charge transfer show a contrary effect on the d band center of Ptskin over Pd substrate.Every Pt-skin atom will accumulate 0.06 electrons from the Pd substrate,which will make the d band center move towards the Fermi level.But the shortened Pt–Pt bond which corresponds to a constrain strain effect will drive the d band center to go far away the Fermi level.Their compromise effect makes the d band center Pt-skin over Pd substrate 0.04 eV closer to the Fermi level compared with that of monometallic Pt.The change of d band center is only about 1/11 of that in both IrPt⋀(111) (0.44 eV) and AuPt⋀(111) (0.43 eV).While the charge transfer in PdPt⋀(111) is 1.5 times of that in IrPt⋀(111) and 3.0 times in AuPt⋀(111),and the changes of the Pt–Pt bond length in PdPt⋀(111) is about 1/3 and 1/5 of that in IrPt⋀(111) and AuPt⋀(111),respectively.The charge transfer effect and the surface strain effect codetermine the change of d band center.And the surface strain effect may play a dominant role,since the largest charge transfer effect does not correspond to the largest change of d band center in these systems.This is consistent with that the charge transfer can be negligible because the dband filling was found to be nearly constant[22].The strain effect plays a dominant role in the shift of the d band center than the electronic property. Fig.7.Effect of electrons on the d band center of surface Pt⋀(positive value means the surplus of electrons while negative values represent deletion of electrons for Pt⋀). The dissociative adsorption of oxygen molecule on the IrPt⋀(111),PdPt⋀(111),Pt(111) and AuPt⋀(111) is studied comparatively with the DFT method.A BEP relationship can be determined for the oxygen molecule dissociation proceeding on the Pt-skin over various substrates,the binding energy of the product oxygen atom is inversely proportional to the energy barrier of oxygen molecule dissociation.The binding energy for intermediates of both O and OH in ORR adsorbed on bimetallic Pt-skin over Ir,Pd,Pt and Au substrates increase as the d band center of the Pt-skin moves towards the Fermi level.A volcano peak in catalytic activity can be found for IrPt⋀(111),PdPt⋀(111),and AuPt⋀(111)as the positions of each substrate in the periodic table.Pt-skin over Pd substrate performs comparable activity with monometallic Pt(111),since both the reaction energy barrier of O–O dissociation and the binding energy of intermediate oxygen atom are similar in the two systems. The compressive strain leads to the decrease of the d band center while the tensile strain leads to the increase of the d band center with respect to the Fermi level.The d bandwidth of Pt-skin over various substrates share similar values,though the d band structures are different from each other.Hirshfeld charge analysis shows that every Pt-skin atom will transfer 0.04 and 0.06 electrons to Ir and Pd substrate respectively,while it will take 0.02 electrons from the Au substrate.The reduction of electrons in Pt-skin will make the d band center of Pt-skin go far away from the Fermi level,while the accumulation of electrons in the Pt-skin will facilitate the d band center to move towards the Fermi level.Compared with the charge transfer effect,the strain effect plays a dominant role.Pt-skin over Pd substrate shares a similar d band center with that of monometallic Pt,and it shows the best catalytic activity for ORR.PdPt⋀can be deemed as the promising candidate catalyst for ORR. Declaration of competing interest None. Acknowledgements This work was supported by the Projects of International Cooperation and Exchanges NSFC (No.21761162015),the Foundation and Frontier Research Project of Chongqing of China (cstc2018jcyjAX0513) and the Science and Technology Research Program of Chongqing Municipal Education Commission(KJQN201801125).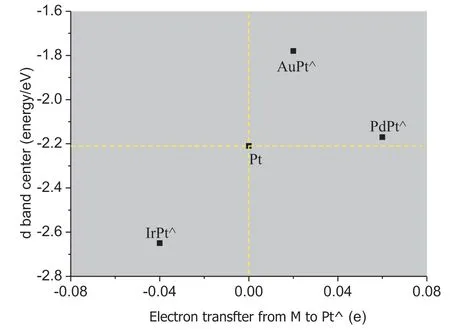
4.Conclusions
- Namo Materials Science的其它文章
- A novel strategy of constructing 2D supramolecular organic framework sensor for the identification of toxic metal ions
- PtZn nanoparticles supported on porous nitrogen-doped carbon nanofibers as highly stable electrocatalysts for oxygen reduction reaction
- Flexible and electrically robust graphene-based nanocomposite paper with hierarchical microstructures for multifunctional wearable devices
- Piezoresistive behavior of elastomer composites with segregated network of carbon nanostructures and alumina
- Surface reconstruction,modification and functionalization of natural diatomites for miniaturization of shaped heterogeneous catalysts
- Constructing P-CoMoO4@NiCoP heterostructure nanoarrays on Ni foam as efficient bifunctional electrocatalysts for overall water splitting

