Constructing P-CoMoO4@NiCoP heterostructure nanoarrays on Ni foam as efficient bifunctional electrocatalysts for overall water splitting
Ning You ,Shuai Cao ,Mengqiu Huang ,Xiaoming Fan ,Kun Shi ,Haijian Huang ,Zhangxian Chen,Zeheng Yang,*,Weixin Zhang,**
a School of Chemistry and Chemical Engineering,Hefei University of Technology,230009,Hefei,Anhui,PR China
b Anhui Province Key Laboratory of Advanced Catalytic Materials and Reaction Engineering,230009,Hefei,Anhui,PR China
Keywords:Heterostructure P-CoMoO4@NiCoP Bifunctional electrocatalysts Overall water splitting
ABSTRACT Improving catalytic activity and durabilty through the structural and compositional development of bifunctional electrocatalysts with low cost,high activity and stability is a challenging issue in electrochemical water splitting.Herein,we report the fabrication of heterostructured P-CoMoO4@NiCoP on a Ni foam substrate through interface engineering,by adjusting its composition and architecture.Benefitting from the tailored electronic structure and exposed active sites,the heterostructured P-CoMoO4@NiCoP/NF arrays can be coordinated to boost the overall water splitting.In addition,the superhydrophilic and superaerophobic properties of P-CoMoO4@NiCoP/NF make it conducive to water dissociation and bubble separation in the electrocatalytic process.The heterostructured PCoMoO4@NiCoP/NF exhibits excellent bifunctional electrocatalysis activity with a low overpotential of 66 mV at 10 mA cm-2 for HER and 252 mV at 100 mA cm-2 for OER.Only 1.62 V potential is required to deliver 20 mA cm-2 in a two-electrode electrolysis system,providing a decent overall water splitting performance.The rational construction of the heterostructure makes it possible to regulate the electronic structures and active sites of the electrocatalysts to promote their catalytic activity.
1.Introduction
Energy and the environment are among the most important concerns of the current era.However,most of the energy we are using still comes from nonrenewable fossil fuels obtained from reserves that are ultimately unsustainable and that result in environmental pollution.The conversion of energy from renewable energy sources could reduce the dependence on fossil fuels significantly [1–3].Among these renewable energy methods,electrochemical catalytic water-splitting is considered to be one of the most effective.
Efficient electrolysis of water usually requires developing highperformance electrocatalysts with high stability,fast kinetics and low overpotential[4].Noble metal-based materials such as Pt and Ru-based catalysts are the most widely used catalysts in the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER),respectively.However,optimum operating conditions and reaction mechanisms are different for HER and OER electrocatalysts.Catalysts that are good for HER do not perform well in OER,and vice versa [5].Pt and Ru-based catalysts,although they are effective bifunctional electrocatalysts,perform poorly in overall water splitting.Moreover,the expense and scarcity of these precious metals severely limits their large-scale application,and it is not economical to produce single-function electrocatalysts for each HER and OER,as this would increase manufacturing costs [6–10].The development of efficient bifunctional electrocatalysts for water splitting will greatly reduce the preparation cost and simplify the electrolytic system as well.However,designing and preparing a catalyst that promotes both HER and OER in the same electrolyte remains a major challenge.
In recent years,transition metal phosphides (TMPs)[9,11],carbides[12],nitrides[13,14]and sulfides[15,16]have been widely reported as potential electrocatalysts for OER or HER[17].In particular,TMPs have attracted great interest from researchers due to their outstanding catalytic performance [18].The negatively charged P atoms in the catalyst are capable of attracting protons and acting as active sites for H2evolution.In the oxygen evolution reaction,transition metal phosphates are transformed into transition-metal oxyhydroxides on the surface of the catalysts which can act as active catalytical sites for O2evolution [19].Additionally,TMPs have been considered as advanced catalysts because of their superior electrical conductivity [20].These properties make TMPs promising electrochemical catalysts for water splitting [21].However,many non-noble metal phosphates do not exhibit satisfactory bifunctional properties.For instance,NiCoP catalysts have been reported as promising electrocatalysts of non-noble metals for overall water splitting in recent years [22,23],but they show inferior HER catalytic activity relative to the noble metal-based catalysts in the alkaline condition.
For this reason,different strategies have been implemented to improve their bifunctional performance,such as developing single atom catalysts[24],doping other transition-metals and heteroatoms[25]and modulating the structure and composition[26].It has been proved that the electrocatalytic performance of catalysts is tremendously affected by their morphology,active surface sites and electronic structure [27],which can be tailored by designing their heterostructures.Generally,heterostructured materials demonstrate better electrocatalytic performance than their individual building units because of the benefits from the strong coupling between different building components.It is an efficient approach to introduce a “collaborator” that can form heterostructures to further improve HER and OER activity.
CoMoO4is considered an ideal choice as a “collaborator” to provide active hydrolytic dissociation sites because Co and Mo have hydrogen adsorption energy close to Pt,and their binary metal oxides have higher constitutive activity than monomeric metal oxides,which significantly facilitates the proton supply.Nevertheless,due to the weak intrinsic conductivity of CoMoO4,its application in alkaline HER is limited.The synthesis of oxide catalysts with the incorporation of a P atom to tailor the electronic structure is an efficient strategy to address the problem of low conductivity [28].Yaqiong Gong et al.[28]utilized phosphorus-doping modulation to fabricate monoclinic P-CoMoO4with an optimized electron structure supported on nickel foam(P-CoMoO4/NF) for alkaline HER via a facile hydrothermal method,followed by low-temperature phosphidation.Ivan P.Parkin et al.[29]synthesized a series of P-doped CoMoO4nanostructures on Ni foam by facile hydrothermal annealing and phosphidation modification,which enhanced their electrochemical performance significantly.
Herein,we report a novel P-CoMoO4@NiCoP/NF heterostructured nanoarray catalyst as an efficient bifunctional electrocatalyst to promote the overall water splitting.P-CoMoO4@NiCoP/NF nanoarrays have been successfully prepared through the phosphatization of the CoMoO4@Ni-Co2O4nanoarray precursor with NaH2PO2⋅H2O as the P source under heat treatment in the N2atmosphere.Heterostructured P-CoMoO4@Ni-CoP rooted on Ni foam possesses a novel tree-like 3D structure,in which P-CoMoO4nanosheets (leaves) are assembled on the surface of NiCoP nanowires (trunk).Due to the unique heterostructure,more exposed active sites and coordinated electronic structure,the P-CoMoO4@NiCoP/NF presents excellent HER and OER catalytic activity and shows outstanding overall water splitting performance.
2.Experimental section
2.1.Materials
Analytical grade Ni(CH2COOH)2⋅6H2O (≥98%),Co(CH2-COOH)2⋅6H2O (≥99%),Na2MoO4⋅2H2O (≥99%),NH4F (≥96%),NaH2-PO2⋅H2O (98%–103%),KOH (≥85%),urea (≥99%) and ethanol were ordered from the Sinopharm Chemical Reagent Co.Commercial ruthenium dioxide(RuO2,99.9%)and platinum on activated carbon(Pt/C,20 wt%) were ordered from Aladdin Chemical Reagents Co.Ni foam(denoted as NF) was ordered from Shenzhen Meisen Electromechanical Equipment Co.,Ltd.All chemical reagents were used without further purification.The experimental water was deionized water.
2.2.Synthesis of NiCo2O4/NF nanoarrays
A piece of Ni foam (2 cm × 4 cm) was degreased in an acetone solution,ultrasonicated in 3.0 M HCl solution for 3-5 min,then thoroughly washed with deionized water and ethanol alternately to clean the surface.Urea (10 mmol),NH4F (8 mmol),Ni(CH2COOH)2⋅6H2O (0.333 mmol)and Co(CH2COOH)2⋅6H2O(0.667 mmol)was dissolved in 36 mL of deionized water and stirred continuously to form a clear solution.The pre-treated NF was then transferred to a Teflon lined stainless steel autoclave(50 mL)containing the solution and maintained at 120°C for 3 h.After natural cooling,the NiCo-based precursor of NF (denoted as NiCo-OH/NF)was taken out,rinsed with deionized water until there was no residue,washed with ethanol three times,and finally dried at 60°C for 12 h NiCo2O4nanowire arrays grown on Ni foam (denoted as NiCo2O4/NF)were obtained after annealing the NiCo-OH/NF sample at 450°C for 2 h in air.
2.3.Synthesis of CoMoO4@NiCo2O4/NF composite nanoarrays
The prepared NiCo2O4/NF nanoarrays were immersed in a solution containing 36 mL of deionized water,1 mmol Na2MoO4⋅2H2O and 1 mmol Co(CH2COOH)2⋅6H2O.The reaction was carried out at 100°C in a Teflon-lined stainless steel autoclave for 12 h.After natural cooling,the sample was taken out,washed with deionized water until there was no residue,washed with ethanol three times,and finally dried at 60°C for 12 h to obtain CoMoO4@NiCo2O4/NF.
2.4.Synthesis of heterostructured P-CoMoO4@NiCoP/NF composite nanoarrays
P-CoMoO4@NiCoP/NF composite nanoarrays were obtained through phosphatization with NaH2PO2⋅H2O as the P source.The prepared CoMoO4@NiCo2O4/NF composite nanoarrays and NaH2PO2⋅H2O were put separately in a porcelain boat with the NaH2PO2⋅H2O powder at the upstream side,and then heated in a tube furnace at 300°C(ramp rate of 5°C min-1)for 180 min under a N2atmosphere.
As comparison samples,NiCoP/NF and P-CoMoO4/NF nanoarrays based on Ni foam were prepared separately by the same method,as detailed in Supporting Information 1.
3.Results and discussion
Fig.1 illustrates the process of synthesizing P-CoMoO4@NiCoP/NF nanoarrays.Briefly,NiCo-OH/NF(Fig.S1)was synthesized through hydrothermal synthesis,followed by annealing to form NiCo2O4/NF nanowire arrays (Fig.S2).The CoMoO4nanosheets were then synthesized on the NiCo2O4nanowires to form CoMoO4@NiCo2O4/NF(Fig.S3).Finally,heterostructured P-CoMoO4@NiCoP/NF nanoarrays were synthesized through phosphatizing the prepared CoMoO4@Ni-Co2O4/NF sample.

Fig.1.Schematic illustration of preparing heterostructured P-CoMoO4@NiCoP arrays on Ni foam.
Fig.2a shows the X-ray diffraction (XRD) pattern of the PCoMoO4@NiCoP powder sample which was peeled off the Ni foam substrate.The reflections in the XRD pattern could be indexed to NiCoP(PDF No.71-2336) and CoMoO4(PDF No.21-0868),indicating the partial phosphatization of the CoMoO4@NiCo2O4/NF sample.Under the experimental conditions,NiCo2O4was completely phosphatized into NiCoP,while CoMoO4did not show an obvious conversion to a phosphatized product.The 2θ values of 41.14°and 45.06°corresponded to the(111)and(201)crystal planes of NiCoP,and it was found that the 2θ values were shifted positively about 0.15°compared with the standard card of NiCoP (PDF No.71-2336).This was ascribed to the higher amounts of Co atoms and the mixed valence states of the Co ions[6,30].Meanwhile,the 2θ values corresponding to the (002) and (021) crystal planes of CoMoO4were 26.66°and 23.54°,exhibiting 0.15°and 0.21°deviations from the standard values of CoMoO4(PDF No.21-0868),probably due to the incorporation of P in it.Further element mapping characterization(line scanning of P-CoMoO4@NiCoP in Fig.S4)revealed that the P elements were highly concentrated in the core nanowires,but also with a uniform distribution of relative low content in the CoMoO4nanosheets,indicating that the P element was partially incorporated into the CoMoO4matrix [11].Combining it with the XRD analysis,the phosphatized sample was denoted as P-CoMoO4@NiCoP/NF.
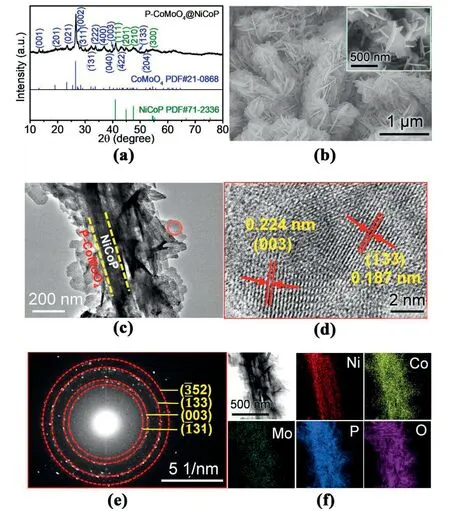
Fig.2.(a)X-ray diffraction(XRD)pattern of P-CoMoO4@NiCoP;(b)FESEM images of P-CoMoO4@NiCoP/NF;(c)TEM image;(d)HRTEM image and(e)SAED pattern taken from the nanosheet of P-CoMoO4@NiCoP;(f) TEM image and elemental mapping of Ni,Co,Mo,P and O for whole P-CoMoO4@NiCoP.(P-CoMoO4@NiCoP sample was peeled off from the Ni foam substrate by ultrasonication).
The morphological and structural characteristics of the synthesized samples were investigated through field emission scanning electron microscopy (FESEM).First,the FESEM images of NiCoP/NF(Fig.S5) with different magnifications showed that the NiCoP nanowire arrays were uniformly grown on the Ni foam.The length of the NiCoP nanowires was about 1.3 μm,and the diameter of the nanowires was~100 nm.Each nanowire was directly in contact with the Ni foam,which can ensure efficient electron transport between the electrocatalyst and the Ni foam substrate,thereby contributing to the water splitting[31].After a second hydrothermal reaction and phosphatization,the P-CoMoO4nanosheets were closely and homogeneously covered on the Ni foam (Fig.S6).Fig.2b shows the FESEM image of the P-CoMoO4@NiCoP/NF sample.It can be found that the P-CoMoO4nanosheets with a thickness of about 40 nm (Fig.S7) were uniformly assembled on the NiCoP nanowire arrays,constructing heterostructured P-CoMoO4@NiCoP/NF nanoarrays.The heterostructured P-CoMoO4@NiCoP anchored on the Ni foam exhibited a novel tree-like 3D structure in which the P-CoMoO4nanosheets were assembled like leaves on the NiCoP nanowire trunk.The transmission electron microscopy(TEM)image of the P-CoMoO4@NiCoP(Fig.2c)also attests to the heterostructure,with the P-CoMoO4nanosheets tightly aggregating around the NiCoP nanowires.
The high-resolution TEM(HRTEM)image(Fig.2d)on the nanosheet(marked in red circle)reveals two clear lattice distances of 0.224 nm and 0.187 nm,corresponding to the(003)and(33)crystal plane of CoMoO4,respectively.The corresponding selected area electron diffraction(SAED)image (Fig.2e) shows several bright rings with discrete spots,which match well with the(31),(003),(33)and(52)planes of the CoMoO4.The corresponding elemental mapping image of P-CoMoO4@NiCoP(Fig.2f) illustrates that the Ni element is only distributed on the nanowires and Mo only on the nanosheets,while the P and Co elements are uniformly distributed on the NiCoP nanowires and P-CoMoO4nanosheets.This confirms that the P-CoMoO4nanosheets are uniformly grown on the NiCoP nanowires.These results demonstrate the successful preparation of the heterostructured P-CoMoO4@NiCoP/NF composite.
The chemical composition and valence states of the elements were studied by XPS for the heterostructured P-CoMoO4@NiCoP/NF,along with those of bare NiCoP/NF and P-CoMoO4/NF.XPS survey spectra of PCoMoO4@NiCoP/NF (Fig.S8a) shows that P-CoMoO4@NiCoP/NF is mainly composed of Ni,Co,Mo,P and O elements.The peaks of Ni in PCoMoO4@NiCoP/NF are weaker than those in NiCoP/NF because there is a dense vegetation of P-CoMoO4nanosheets wrapped on the surface of the NiCoP nanowires,thus weakening the Ni peak intensity of the PCoMoO4@NiCoP sample.The XPS spectrum of the Ni 2p in P-CoMoO4/NiCoP displays two peaks at 875.08 and 856.58 eV,attributed to Ni2+2p1/2and Ni2+2p3/2(Fig.3a)[32].The peaks at 870.98 eV and 852.98 eV are assigned to the peaks of P-CoMoO4@NiCoP/NF,related to Ni02p1/2and Ni02p3/2,respectively.Compared to those of NiCoP/NF,the Ni2+peaks of P-CoMoO4@NiCoP/NF are negatively shifted about 0.1 eV.Likewise,Fig.3b demonstrates the Mo 3d spectrum.The appearance of Mo4+and Mo6+can be attributed to the CoMoO4and Mo-P species.In Fig.3b,the Mo 3d signal in the P-CoMoO4@NiCoP/NF exhibits a positive shift of about 0.3 eV compared with that in the P-CoMoO4/NF.Generally,the increase of the valence electron charge will lead to the decrease of binding energy,and vice versa [33].Therefore,the negative shift of binding energy for Ni 2p and the positive shift of binding energy for Mo 3d strongly demonstrate that there are strong electronic interactions between the NiCoP nanowire and P-CoMoO4nanosheet,which can significantly accelerate the charge transfer.The Co 2p core-level spectrum(Fig.3c)exhibits Co2+species at 797.98 eV and 781.78 eV.As can be seen,there are no peaks of Coδ+species in CoMoO4@NiCo2O4/NF,indicating that Coδ+(793.88 eV and 778.88 eV)is due to the formation of Co-P bonds (Fig.S8b) [4].Moreover,the peak intensity of the Coδ+species in NiCoP/NF and P-CoMoO4/NF is weaker than that in P-CoMoO4@NiCoP/NF,indicating that the formation of the heterogeneous interface facilitates the formation of Coδ+species,thereby improving the OER or HER performance [19].In Fig.3d,the high-resolution P 2p spectra of P-CoMoO4@NiCoP/NF,with the peaks of P-M 2p3/2and P-M 2p1/2,locate at a binding energy of 129.58 and 130.43 eV,respectively.Compared to those of bare NiCoP/NF and P-CoMoO4/NF,the peak intensities of P-Metal(P-M 2p3/2and P-M 2p1/2)demonstrate that the redistribution of the charge is caused by the strong interaction at the NiCoP and P-CoMoO4interface in P-CoMoO4@Ni-CoP/NF,which could significantly enhance the electrocatalytic activity[34].The peak at 133.9 eV represents P-O species oxidized(P,etc.)due to air exposure[22].

Fig.3.XPS spectra of P-CoMoO4@NiCoP/NF,NiCoP/NF and P-CoMoO4/NF: (a) Ni 2p,(b) Mo 3d,(c) Co 2p and (d) P 2p.
Generally,a large number of air bubbles will be generated on the surface of the electrode during the electrocatalytic reaction under high current density.The gas bubbles produced in-situ,if not released immediately,will seriously hinder the electrolyte diffusion and produce dead areas that cannot participate in the catalytic reaction,thus hindering the mass transfer process and causing the catalytic performance to deteriorate[35].
In order to study the hydrophilic and aerophobic properties of the samples,the contact angle of the water droplet and the underwater contact angle of the air bubble were tested.Dynamic testing showed that the water droplet spread immediately on the surface of the PCoMoO4@NiCoP/NF,as well as the NiCoP/NF and P-CoMoO4/NF,once in contact with them,while the water droplet remained on the surface of the blank Ni foam(see the testing video in Supporting Information 2).As shown in Fig.S9,the P-CoMoO4@NiCoP/NF,as well as NiCoP/NF and PCoMoO4/NF,displayed a liquid contact angle of 0°,showing its superhydrophilic property,while the blank Ni foam showed its hydrophobic property,with a large static liquid contact angle of 120°.Fig.S10 displays the measurement images of the underwater air bubble contact angles for those samples.The P-CoMoO4@NiCoP/NF demonstrates superaerophobic properties,and the air bubble shows complete estrangement from the surface of the contact film,which is beneficial to the release of the gas bubbles produced during the electrocatalytic water splitting.Similarly,the components comprising NiCoP/NF and P-CoMoO4/NF also exhibit superaerophobic properties,and the air bubbles become estranged from the surface of the film upon cessation of contact,while the air bubble can retain a contact angle of 134°on the surface of the Ni foam,implying the underwater aerophilic property of the bare Ni foam surface.The videos about the underwater air bubble contact angle testing are provided in Supporting Information 3.The superhydrophilic and superaerophobic properties of P-CoMoO4@NiCoP/NF make it conducive to water dissociation and bubble separation in the electrocatalytic process,thus improving the catalytic performance.
Supplementary video related to this article can be found at https://doi.org/10.1016/j.nanoms.2021.05.004
To determine the HER performance,a standard three-electrode cell was used to perform linear sweep voltammetry in a 1.0 M KOH electrolyte.The iR-compensated polarization curves of P-CoMoO4@NiCoP/NF,NiCoP/NF,P-CoMoO4/NF,Pt/C/NF and Ni foam at 5 mV s-1are displayed in Fig.4a,and their corresponding overpotentials at current densities of 10,50,and 100 mA cm-2are presented in Fig.4b.The PCoMoO4@NiCoP/NF offers outstanding HER performance with a low overpotential of 66 mV at 10 mA cm-2,which is very close to that of the commercial Pt/C/NF catalyst(45 mV).In contrast,the NiCoP/NF and PCoMoO4/NF exhibit inferior HER performance with high overpotentials of 139 mV and 125 mV at 10 mA cm-2,respectively,suggesting significantly enhanced effects of the heterostructure catalyst on the HER performance.Furthermore,the P-CoMoO4@NiCoP/NF delivers a high current density (j=209 mA cm-2) at an overpotential of 200 mV(Fig.S11),which is about 4 times higher than those of NiCoP/NF(j=55 mA cm-2) and P-CoMoO4/NF (j=56 mA cm-2),indicating strong synergic effects derived from the P-CoMoO4@NiCoP/NF heterostructured interface[36].In addition,Tafel plots were investigated to determine the HER rates of these catalysts (Fig.4c).The P-CoMoO4@NiCoP/NF presents the smallest Tafel slope of 75 mV dec-1among the three catalysts,including NiCoP/NF(135 mV dec-1)and P-CoMoO4/NF(103 mV dec-1),demonstrating the fastest HER kinetics of the P-CoMoO4@NiCoP/NF catalyst[37].Compared with some reported catalysts in recent literature,the P-CoMoO4@NiCoP/NF catalyst shows superiority in HER performance(Table S1).
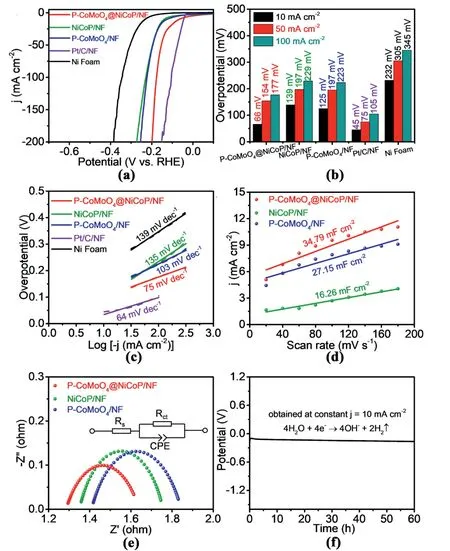
Fig.4.Electrocatalytic performance of different catalysts for HER in1.0 M KOH.(a)Polarization curves at 5 mV s-1.(b)Overpotentials required at 10,50 and 100 mA cm-2.(c)Tafel plots derived from figure(a).(d)The fitting plots for Cdl and(e)Nyquist plots at the overpotential of 399.6 mV.(f)Chronopotentiometry curve of the PCoMoO4@NiCoP/NF for HER at 10 mA cm-2 in 1.0 M KOH solution.
Double-layer capacitance (Cdl) reflects the electrochemical surface area of the catalyst.The capacitive current density difference between the anode and the cathode is proportional to the scan rate [15].Cyclic voltammetry (CV) was used to study the electrochemical surface area(ECSA)at different scan rates(Fig.S12).The Cdlof the three catalysts was calculated based on their current density under different scan rates,as shown in Fig.4d,with 34.79 mF cm-2,27.15 mF cm-2and 16.26 mF cm-2for P-CoMoO4@NiCoP/NF,P-CoMoO4/NF and NiCoP/NF,respectively.The heterostructured P-CoMoO4@NiCoP/NF possesses the largest electrochemically active surface area,which endows it with the best electrocatalysis activity.The excellent performance of the hydrogen evolution is also related to the superhydrophilic and superaerophobic properties of the P-CoMoO4@NiCoP/NF.The superhydrophilicity of the P-CoMoO4@NiCoP/NF can facilitate electrolyte diffusion and make full contact with the electrolyte in the reaction process.In addition,the superaerophobic property is conducive to the release of gas bubbles for exposing more active sites and clearing the pathways for electrolyte diffusion,thus accelerating the hydrogen evolution reaction.
Electrochemical impedance spectroscopy(EIS)was investigated in an alkaline medium to further reveal the relevant properties.The fitting circuit consisted of Rct(charge transfer resistance between electrolyte and catalyst interface)in parallel with CPE and then in series with Rs(the intrinsic resistance of the electrode and electrolyte).According to the equivalent circuit diagram shown in Fig.4e,the Rsof P-CoMoO4@Ni-CoP/NF (1.286 Ω) is smaller than that of NiCoP/NF (1.350 Ω) and PCoMoO4/NF (1.409 Ω).Similarly,the Rctof P-CoMoO4@NiCoP/NF(0.353 Ω) is also smaller than that of NiCoP/NF (0.406 Ω) and PCoMoO4/NF(0.435 Ω).The smaller Rsand Rctof the P-CoMoO4@NiCoP/NF electrode resulted from the tree-like 3D architecture with a heterostructured interface,which reduced the internal resistance and promoted electron transfer,thus improving the electrocatalytic performance [38].As shown in Fig.S13,the Rs(1.723 Ω) and Rct(0.918 Ω) of the CoMoO4@NiCo2O4/NF are significantly larger than those of P-CoMoO4@NiCoP/NF,indicating that phosphide and the incorporation of P can effectively increase the charge transfer property of the electrocatalyst.
In addition,the P-CoMoO4@NiCoP/NF shows high durability during the 60 h chronopotentiometry test at 10 mA cm-2,and the overpotential displays a negligible increase(Fig.4f).It basically maintains the original tree-like 3D heterostructure (Fig.S14).This is attributed to the superaerophobic property of the P-CoMoO4@NiCoP/NF.The superaerophobic property of the P-CoMoO4@NiCoP/NF accelerates the release of bubbles,and will not produce a dead zone in the catalytic reaction,maintaining good performance in the stability test [35].Fig.S15 shows the XPS spectra of the P-CoMoO4@NiCoP/NF before and after the 60 h chronopotentiometry test for HER at 10 mA cm-2in 1.0 M KOH solution.Of note,the XPS spectra of the Ni 2p region does not change,but the Coδ+peaks disappear.The XRD of the P-CoMoO4@NiCoP/NF after the chronopotentiometry test for HER in 1.0 M KOH solution (Fig.S16a) shows the presence of Co(OH)2,confirming the partial change from P-CoMoO4@NiCoP/NF to Co(OH)2[9].The high-resolution TEM(HRTEM) image (Fig.S16c) on the nanosheet (marked in red circle in Fig.S16b)reveals a clear lattice distance of 0.237 nm,corresponding to the (101) crystal plane of Co(OH)2.The corresponding selected area electron diffraction(SAED)image(Fig.S16d)shows several bright rings with discrete spots,which match well with the (101),(102) and (111)planes of Co(OH)2.Phase transformation may cause the disappearance of Coδ+.And the overall decrease in the peak intensity of P 2p and Mo 3d may be caused by the partial leaching out of P and Mo from the P-CoMoO4@NiCoP sample during the reaction process [39].The existence of Coδ+related to the formation of the Co-P bond has been proved in Fig.S8b and it has been reported in some literature [4,40].The decrease of P-M bonds may be one of the reasons for the disappearance of Coδ+.
The OER catalytic activities of these samples were also investigated in 1.0 M KOH solution.The LSV curves are shown in Fig.5a.To reach a current density of 100 mA cm-2,the heterostructured P-CoMoO4@Ni-CoP/NF requires an overpotential of only 252 mV,which is much lower than that of NiCoP/NF(287 mV)and P-CoMoO4/NF(262 mV).To reach even higher current densities of 200 and 300 mA cm-2,only 292 and 313 mV overpotentials are required for the P-CoMoO4@NiCoP/NF catalyst.The capacitance behavior of the non-Faradaic capacitance current range for P-CoMoO4@NiCoP/NF,NiCoP/NF,P-CoMoO4/NF and Ni foam was measured (Fig.S17).The results show that the P-CoMoO4@NiCoP/NF,NiCoP/NF and P-CoMoO4/NF samples exhibit very strong capacitive behavior in comparison with the Ni foam sample.This may be one of the reasons that the polarization curve is raised in the non-Faradaic capacitance current range.In addition,the formation of Ni and Co oxidation peaks also raises the current,to some extent,which causes an obvious peak in the non-Faradaic region of the polarization curve [41,42].Moreover,as shown in Fig.5b,it has the smallest Tafel slope of the three samples,P-CoMoO4@NiCoP/NF (126 mV dec-1),NiCoP/NF (150 mV dec-1) and P-CoMoO4/NF (148 mV dec-1),demonstrating the fastest OER kinetics of the P-CoMoO4@NiCoP/NF catalyst.Similarly,the P-CoMoO4@NiCoP/NF achieves a higher current density (j=231 mA cm-2) at an overpotential of 300 mV than those of NiCoP/NF (j=124 mA cm-2) and the P-CoMoO4/NF (j=183 mA cm-2) (Fig.S18),indicating the synergistic effect of NiCoP and P-CoMoO4for improving the OER performance [36].P-CoMoO4@NiCoP/NF's superhydrophilicity enables it to adsorb water molecules well and promotes the wettability of the electrolyte,thus promoting the surface activity of the catalyst,showing excellent oxygen evolution performance.In the EIS spectra(Fig.5c),the heterostructured P-CoMoO4@NiCoP/NF exhibits the lowest semicircle.The Rsof P-CoMoO4@NiCoP/NF(1.041 Ω)is lower than that of NiCoP/NF(1.181 Ω)and P-CoMoO4/NF(1.200 Ω),indicating that the P-CoMoO4@NiCoP/NF presents the lowest intrinsic resistance of the electrode and electrolyte among the three catalysts.Similarly,the Rctof P-CoMoO4@NiCoP/NF (0.646 Ω) is significantly smaller than that of NiCoP/NF (0.993 Ω) and P-CoMoO4/NF (0.858 Ω),indicating a faster charge transfer between the P-CoMoO4@NiCoP/NF electrode and the electrolyte [19].Compared with the Rs(1.615 Ω) and Rct(1.717 Ω) of CoMoO4@NiCo2O4/NF (Fig.S19),the charge transfer can be increased through phosphating,and then improve the electrical conductivity of the electrocatalyst [20].In addition,a minimal increase in overpotential is observed even after continuous chronopotentiometry testing over 50 h at 100 mA cm-2(Fig.5d),confirming the high stability and durability of the P-CoMoO4@NiCoP/NF.Its superaerophobic property enables P-CoMoO4@NiCoP/NF to release bubbles rapidly to maintain stability.The morphology observation in Fig.S20 shows that the original heterostructure is basically retained.

Fig.5.Comparison of electrocatalytic OER in alkaline media.(a) Polarization curves and (b) Tafel plots of P-CoMoO4@NiCoP/NF,NiCoP/NF,P-CoMoO4/NF and RuO2/NF.(c)Nyquist plots of P-CoMoO4@NiCoP/NF,NiCoP/NF and P-CoMoO4/NF in 1.0 M KOH at the overpotential of 320.4 mV.(d)Chronopotentiometry curve of the P-CoMoO4@NiCoP/NF for HER at 100 mA cm-2 in 1.0 M KOH solution.
Generally,the proposed mechanism of OER under alkaline conditions are considered as follows(M,surface metal sites)[43].
This indicates that oxides and hydroxides are all intermediates in the oxygen evolution reaction.It was also reported that the OER electrocatalytic activity of NiCo phosphides are attributable to the Ni-Co oxo/hydroxo species,which are key OER intermediates during oxygen evolution and are partially derived from the oxidization of Ni and Co atoms on the surface of the catalyst [4,9,44].Fig.S21 shows the XPS spectra of P-CoMoO4@NiCoP/NF before and after the 50 h chronopotentiometry test for OER at 100 mA cm-2in 1.0 M KOH solution.Of note,the peaks of Ni0and Coδ+disappeared after 50 h OER testing,indicating Ni and Co have been oxidized.Meanwhile,the sole presence of the P-O bond and the disappearance of the P-M bonds are in close correlation with the oxidation of Ni and Co during the catalytic processes[45].In order to further determine the surface oxidation,the samples were characterized by XRD after the OER stability test (Fig.S22a),and the presence of NiO was found,indicating that surface oxidation did exist on the P-CoMoO4@NiCoP/NF sample.The high-resolution TEM(HRTEM) image (Fig.S22c) on the nanosheet (marked in red circle in Fig.S22b)reveals a clear lattice distance of 0.205 nm,may correspond to the crystal plane of Co oxo/hydroxo species.The selected area electron diffraction (SAED) image (Fig.S22d) on the nanowire shows several bright rings with discrete spots,which match well with the(111),(200)and(220)planes of NiO.These results elucidate that an additional oxide catalyst layer is gradually formed on the surface of the P-CoMoO4@NiCoP.
The above experimental results showed that the heterostructured PCoMoO4@NiCoP/NF electrocatalyst presented a superior bifunctional electrocatalytic performance on HER and OER.Therefore,a twoelectrode overall water splitting electrolyzer was constructed using PCoMoO4@NiCoP/NF as both the anode and the cathode.As shown in Fig.6a,P-CoMoO4@NiCoP/NF electrocatalysts are used as anode for OER and cathode for HER.In view of the superior bifunctional characteristics of P-CoMoO4@NiCoP/NF,its possible mechanism for overall water splitting can be illustrated by Fig.6b.During OER,electrons are transferred from the P-CoMoO4nanosheets to the NiCoP nanowires via interface action.Then they are transferred from NiCoP to the Ni foam substrate,while the electron transmission path of HER is the opposite[15].The superhydrophilic and superaerophobic properties of P-CoMoO4@NiCoP/NF make it conducive to water dissociation and bubble separation in the electrocatalytic process,thus improving the catalytic performance and stability.In addition,the heterostructured nanostructures ensure strong electronic interactions of the P-CoMoO4@NiCoP/NF [27].Because of these advantages,this electrolyzer only needs a low potential of 1.62 V to achieve the current density of 20 mA cm-2(Fig.6c),which could be maintained well with almost no degradation after the 50 h chronopotentiometry test(Fig.6d),suggesting an impressive durability of overall water splitting.Such outstanding activity and durability enable the heterostructured P-CoMoO4@NiCoP/NF to be a potential alternative to noble metal electrocatalysts for energy-efficient and cost-effective water splitting [46].Table S1 compares the overall water splitting performance of P-CoMoO4@NiCoP/NF with some representative catalysts reported recently.It can be seen that it is comparable to or even outperforms its counterparts.
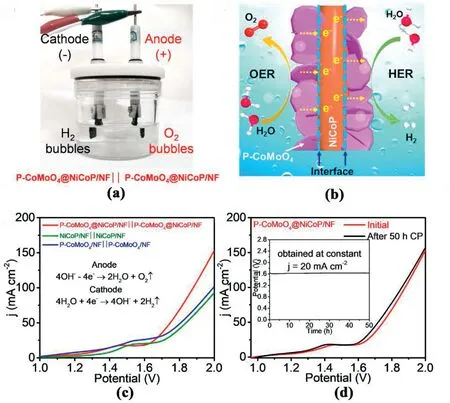
Fig.6.(a)Durability tests of the electrolyzer at 20 mA cm-2 in 1.0 M KOH.(b) Schematic illustration of the P-CoMoO4@NiCoP/NF electrodes for HER and OER.(c)Overall water splitting performance of P-CoMoO4@NiCoP/NF,NiCoP/NF and P-CoMoO4/NF.(d)Overall water splitting performance of P-CoMoO4@NiCoP/NF before and after 50 h chronopotentiometry test (Inset: Chronopotentiometry curve of the P-CoMoO4@NiCoP/NF).
4.Conclusions
In summary,we have developed an efficient strategy for improving the electrocatalytic activity of water splitting by engineering a 3D treelike heterostructure of P-CoMoO4@NiCoP/NF,which could promote electron and charge transfer and provide abundant active sites.In addition,the superhydrophilic and superaerophobic properties of the PCoMoO4@NiCoP/NF can facilitate good contact between the catalysts and electrolyte,which is very conducive to water electrolysis.In the halfcell evaluation,P-CoMoO4@NiCoP/NF exhibits excellent HER and OER performance with low overpotentials of 66 mV at 10 mA cm-2and 252 mV at 100 mA cm-2.Furthermore,it also displays small Tafel slopes of 75 and 126 mV dec-1in alkaline media,as well as high stability,even in the chronopotentiometric testing of 50–60 h.Moreover,as both the cathode and the anode,P-CoMoO4@NiCoP/NF exhibits good overall water splitting performance.To reach a current density of 20 mA cm-2,PCoMoO4@NiCoP/NF only needs a low potential of 1.62 V with 50 h durability.This excellent performance indicates that P-CoMoO4@NiCoP/NF is a promising bifunctional electrocatalyst for overall water splitting,which may make it possible to realize large scale,high efficiency catalytic electrolysis of water under high current density.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China(NSFC 91834301,21808046 and 21908037)and Anhui Provincial Science and Technology Department Foundation (201903a05020021 and 202003a05020046)for funding support.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2021.05.004.
- Namo Materials Science的其它文章
- Recent progress in graphene-based wearable piezoresistive sensors: From 1D to 3D device geometries
- Recent progress in flexible capacitive sensors: Structures and properties
- DFT study on ORR catalyzed by bimetallic Pt-skin metals over substrates of Ir,Pd and Au
- Surface reconstruction,modification and functionalization of natural diatomites for miniaturization of shaped heterogeneous catalysts
- Piezoresistive behavior of elastomer composites with segregated network of carbon nanostructures and alumina
- Flexible and electrically robust graphene-based nanocomposite paper with hierarchical microstructures for multifunctional wearable devices

