Dry eye disease among patients infected with the SARSCoV-2 Omicron variant: a cross-sectional study
Qi-Fang Chen, Juan Yu, Han Chu, Pan Hu, Li Chen, Ting Liu, Chong-Yi Li
1Department of Ophthalmology, Daping Hospital, Third Military Medical University, Chongqing 400042, China
2Center of Growth, Metabolism and Aging, Key Laboratory of Bio-Resources and Eco-Environment, College of Life Sciences, Sichuan University, Chengdu 610064, Sichuan Province, China
Abstract
● KEYWORDS: COVID-19; dry eye disease; risk factors
INTRODUCTION
C orona virus disease 2019 (COVID-19) pandemic has considerably affected the general health of individuals globally[1-2].Owing to the frequent development of new viral variants, a wave of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Omicron variant infection was reported in Shanghai, China, in late February 2022.A case fatality rate has been reported in unvaccinated individuals regardless of the lower virulence of the Omicron strain.
Since the ocular surface is an important route for SARSCoV-2 transmission and infection, ocular symptoms, including blurred vision, eye soreness, and eye itch, have garnered the attention of researchers[3-4].As a common disease of the ocular surface, dry eye disease (DED) affects the vision and quality of life of people[5-6].While the symptoms of DED in patients with COVID-19 have been reported, limited information is available about the current status of patients with DED symptoms in clinical settings owing to the limitations of the COVID-19 pandemic.In addition, considering the different infection characteristics, a survey on the development of DED symptoms in patients infected with the SARS-CoV-2 Omicron variant infection should be developed urgently.
The aim of this cross-sectional study was to investigate the incidence of DED and the associated risk factors in patients with COVID-19 infected with the SARS-CoV-2 Omicron variant to provide a theoretical basis and appropriate clinical guidelines for thediagnosis and treatment of the disease in the population.
SUBJECTS AND METHODS
Ethical ApprovalThe study was approved by the Ethics Committee of Chongqing Daping Hospital (No.2022-121-02),all patients received a thorough explanation of the study design and aims followed by a signed informed consent.The study was conducted in compliance with the tenets of the Declaration of Helsinki.
Sampling, Survey, and Data CollectionThe cross-sectional,observational study was conducted on 993 patients with COVID-19 at the National Exhibition and Convention Center(Shanghai) Fangcang Shelter Hospital, from April 10 to May 26, 2022.Concurrently, 944 uninfected control participants were recruited from the community dwelling population.
Information on demographic characteristics, video display terminal (VDT) use, wearing contact lenses (CL), history of corneal refractive surgery (CRS), sleep quality and length of hospital stay (LOS) was collected using questionnaires.
Definition and Symptomatic Evaluation of DED SymptomsBased on the total ocular surface disease index (OSDI) scores,participants were divided into four categories as follows:1) OSDI ≤12 (normal); 2) 13≤OSDI≤22 (mild DED); 3)23≤OSDI≤32 (moderate DED); and 4) OSDI ≥33 (severe DED)[7-8].
Briefly, two ophthalmologists observed the patients with COVID-19 after entering the isolation zone.After the purpose,procedures, and requirements of the study were explained, the patients with COVID-19 voluntarily filled out the questionnaire administered, such as the OSDI questionnaire[7,9-10].OSDI questionnaires are regarded as a gold-standard instrument for assessing DED symptoms according to the Dry Eye Work Shop (DEWS) consensus[11-12].
Definition of Risk FactorsAccording to the questionnaire,six questions were potential risk factors, namely “SARS-CoV-2 infection”, “long-term VDT use”, “wearing CL”, “poor sleep quality”, “CRS history”, and “LOS”.“VDT use” indicated“spending excess time on VDT devices, such as iPad, smart telephone, or computers, per day during the week before the test”.“Wearing CL” indicated “wearing CL at least once a week for the last three months”.“Poor sleep quality” indicated“difficulty in falling asleep”.
Nucleic Acid-Based Detection of SARS-CoV-2 Omicron Variant in Nasopharyngeal SwabsNasopharyngeal swabs samples collected from patients with COVID-19 werepreserved in a viral-transport medium at 2°C to 8°C and then assessed by reverse transcription-polymerase chain reaction(RT-PCR) using a 2019-nCoV nucleic acid detection kit(Wuhan EasyDiagnosis Biomedicine Co., Ltd., China).
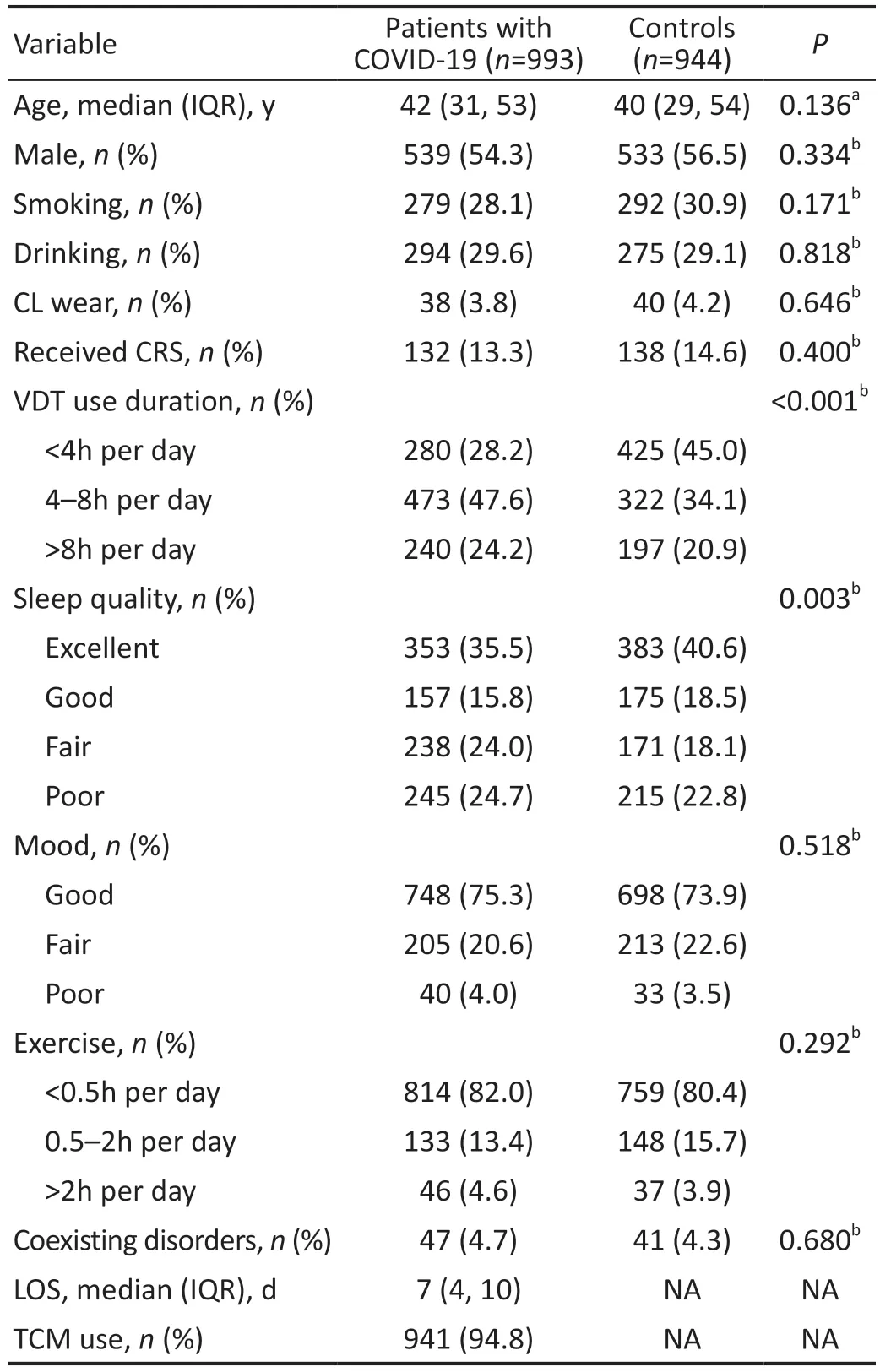
Table 1 Demographic characteristics of participants
Statistical AnalysisPvalues were calculated using the Mann-WhitneyUtest and Pearson’sχ2test.Risk factors were analyzed by univariate and multivariate logistic regression models.Relative risks were calculated as odds ratios (ORs)with a 95% confidence interval (CI).P<0.05 indicated a significant difference.Data were analyzed using SPSS version 21.0 (SPSS, Chicago, IL, USA).
RESULTS
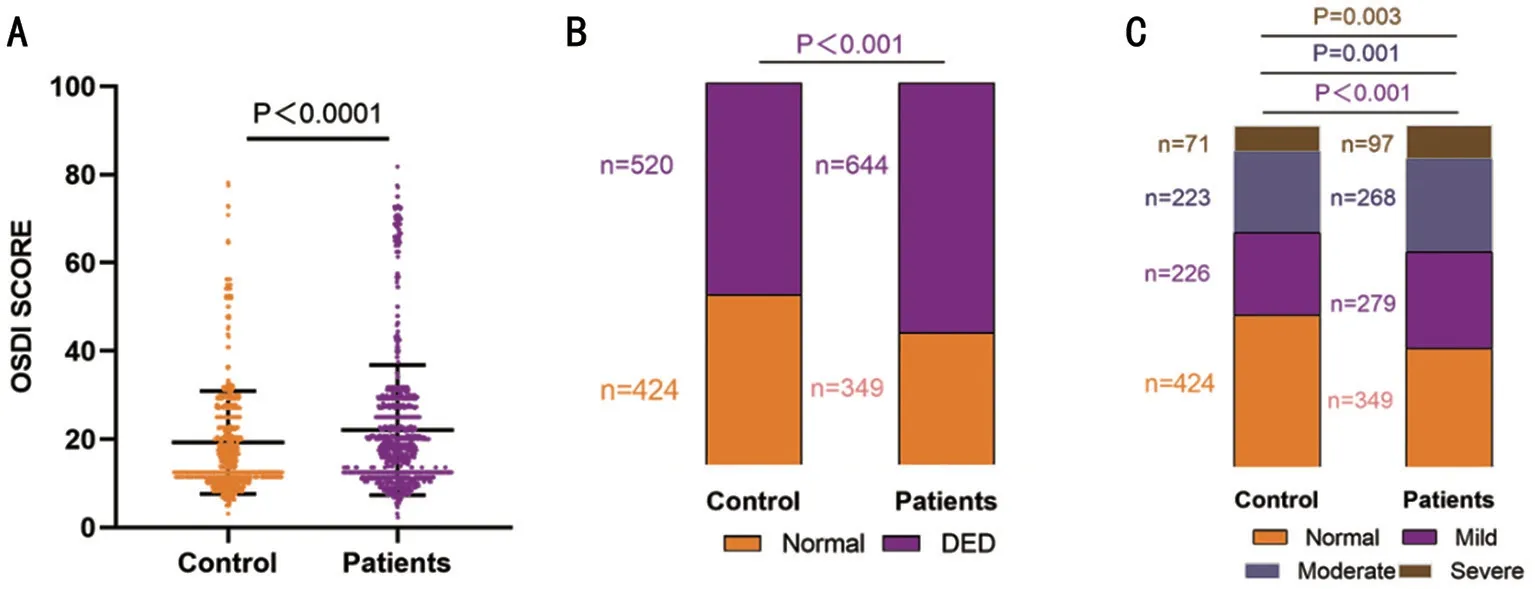
Figure 1 Incidence of DED in patients with COVID-19 and controls A: Comparison of OSDI scores between patients with COVID-19 and controls.Wilcoxon-Mann-Whitney test.B: Individuals with DED among control participants and patients with COVID-19.χ2 test results.C:Individuals with different DED severity grades among control participants and patients with COVID-19.χ2 test results.DED: Dry eye disease;OSDI: Ocular surface disease index.P (mild)<0.001; P (moderate)=0.001; P (severe)=0.003.
Demographic Characteristics of ParticipantsA total of 993 patients hospitalized with COVID-19 and 944 control participants were recruited for the study.The demographic data, CRS history, details of treatment with traditional Chinese medicine (TCM), LOS, and other factors are shown in Table 1.The median age of COVID-19 patients and control participants is respectively 42y and 40y.A similar proportion of males and females (539/454 and 533/411, respectively) were present in the two groups.Two hundred and seventy-nine (28.1%)patients with COVID-19 reported a smoking habit, and 294(29.6%) patients with COVID-19 reported a drinking habit.Only 3.8% of the patients with COVID-19 wore CL, and 13.3% of the patients with COVID-19 received CRS.For the majority (47.6%) of patients with COVID-19, the duration of VDT use was 4-8h per day, and 45.0% of the control participants reported a VDT time less than 4h per day.In the control group, 40.6% of the participants reported excellent sleep quality, and in the patient group, 245 (24.7%) patients reported poor sleep quality.Similarly, in the control group,73.9% of the participants reported a good mood, and 205(20.6%) patients with COVID-19 reported a fair mood.A small percentage of patients (4.6% and 3.9%, respectively) exercised for more than 2h per day.Among patients with COVID-19 and control participants, most did not have coexisting disorders(95.3% and 95.7%, respectively).The LOS of patients was 7d.TCM was administered to almost all patients with COVID-19.
Incidence of DED in Patients with COVID-19 and ControlsTo date, the precise incidence of DED in patients with COVID-19 is unclear[13-14].The OSDI scores of patients with COVID-19 were higher than those of controls in Figure 1A.Additionally, patients with COVID-19 had 644 (64.9%) cases with DED symptoms and controls had 520 (55.1%) cases with DED symptoms, indicating a higher DED incidence in patients with COVID-19 than in controls in Figure 1B.Moreover,among patients with COVID-19, 279 (28.1%) showed mild DED, 268 (26.9%) showed moderate DED, and 97 (9.7%)showed severe DED.The ratios were significantly higher than those of controls [mild DED: 226 (23.9%); moderate DED:223 (23.6%); severe DED: 71 (7.5%)], indicating the highest incidence of mild DED among patients with COVID-19(Figure 1C).
Risk Factors for DED Symptoms in Patients with COVID-19 and ControlsIn view of the above results,it is essential to investigate the important risk factors for DED symptoms.In the cohort including both patients with COVID-19 and controls, SARS-CoV-2 infection [ORs(95%CI): 1.271 (1.038, 1.556)], wearing CL [ORs (95%CI):9.350 (3.676, 23.783)], CRS history [ORs (95%CI): 2.047(1.494, 2.804)], poor sleep quality [ORs (95%CI): 2.657 (2.029,3.480)], and VDT use for more than 8h per day [ORs (95%CI):6.348 (4.720, 8.538)] were risk factors for DED symptoms(Table 2).SARS-CoV-2 infection was an independent risk factor (Figure 2A).In addition, SARS-CoV-2 infection, wearing CL, poor sleep quality, and VDT use for more than 8h per day were also important risk factors for different DED severities(Figure 2C).However, SARS-CoV-2 infection [ORs (95%CI):1.346 (1.064, 1.704)] was a key risk factor for only mild DED in patients with COVID-19 and controls, as indicated by the results of multivariate logistic regression analysis.
For patients with COVID-19, LOS [ORs (95%CI): 1.196(1.134, 1.262)], wearing CL [ORs (95%CI): 20.423 (2.680,155.632)], CRS history [ORs (95%CI): 2.166 (1.321, 3.553)],poor sleep quality [ORs (95%CI): 3.650 (2.381, 5.597)], and VDT use for more than 8h per day [ORs (95%CI): 7.740(4.918, 12.180)] were significant risk factors associated with DED symptoms (Table 3), and LOS was also an independent risk factor (Figure 2B).Additionally, LOS, wearing CL, CRS history, poor sleep quality, and VDT use for more than 8h per day were significant risk factors for different DED severities by both univariate and multivariate logistic regression analysis(Figure 2D).
Prediction Model of DED Symptoms in Patients with COVID-19A clinical prediction model has auxiliary value for the diagnosis and treatment of DED symptoms.First,a nomogram can graphically present a logistic regression equation.As shown in Figure 3A, the nomogram illustrated thatSARS-CoV-2 infection was an independent risk factor for DED symptoms.Second, a decision tree is easier to use for estimating the model through static testing and determining the reliability of the model.As shown in Figure 3B, the decision tree model of DED symptoms in patients with COVID-19 indicated that the LOS was also a risk factor for DED symptoms.Lastly, it is necessary to perform a quantitative evaluation to assess the performance of the prediction model.The basis for modeling should consider 70% of the training model results and 30%of the test model results.Surprisingly, the receiver-operating characteristic (ROC) curve of the prediction model of DED symptoms in patients with COVID-19 indicated a better specificity and sensitivity because of the training area under the ROC curve (AUC) of 79.1% and test AUC of 77.33% in Figure 3C.

Table 2 Logistic regression models to evaluate the risk factors for DED symptoms in patients with COVID-19 and controls
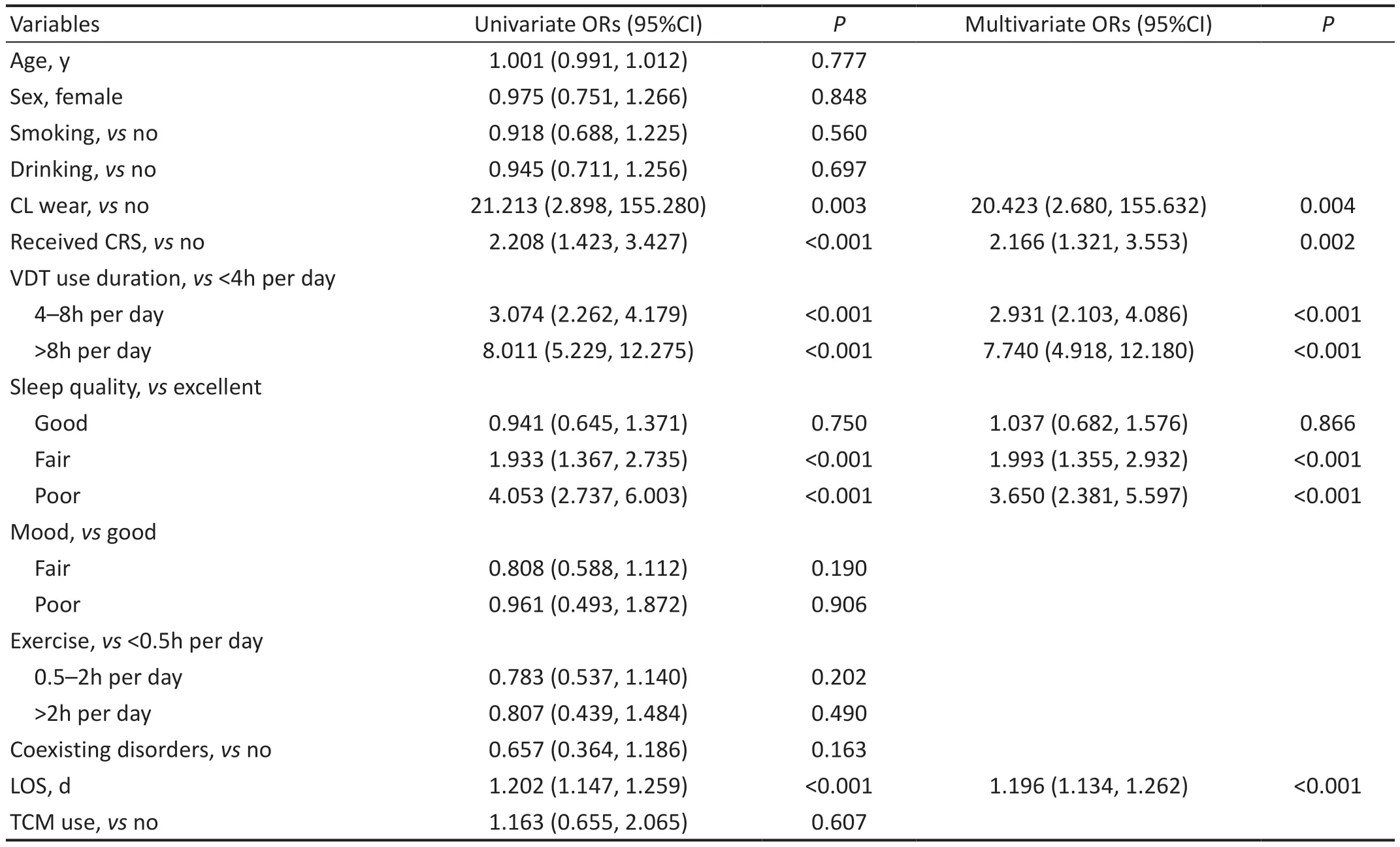
Table 3 Logistic regression models to evaluate the risk factors for DED in patients with COVID-19

Figure 2 Risk factors associated with DED symptoms in the total cohort, including both patients with COVID-19 and control participants A:Risk factors for DED symptoms in patients with COVID-19 and controls; B: Risk factors for DED symptoms in patients with COVID-19; C: Risk factors for different DED severities in patients with COVID-19 and controls; D: Risk factors for different DED severities in patients with COVID-19 with multivariate logistic regression models.DED: Dry eye disease; CL: Contact lenses; CRS: Corneal refractive surgery; VDT: Visual display terminal; LOS: Length of hospital stay.
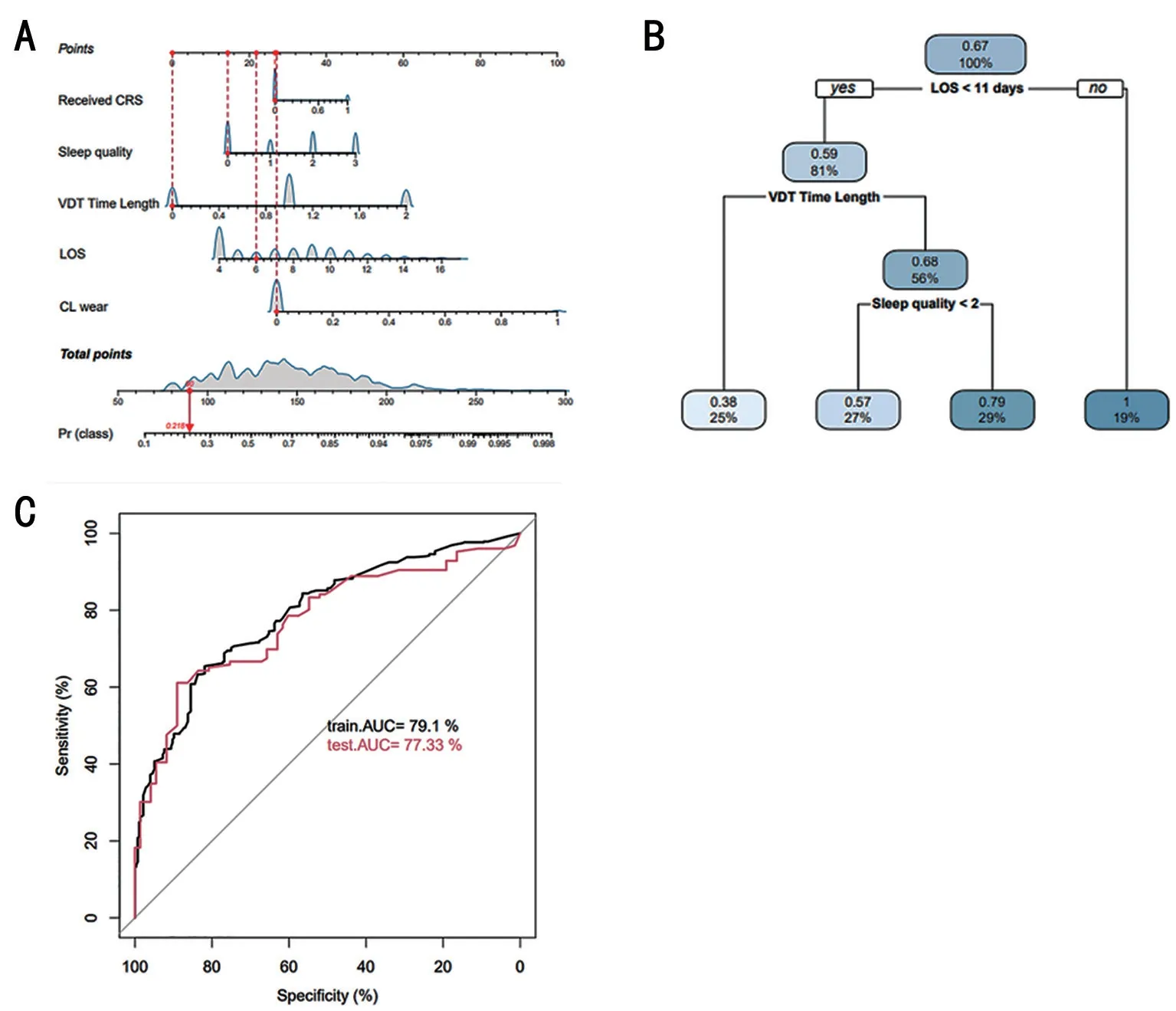
Figure 3 Prediction model of DED symptoms in patients with COVID-19 A: Nomogram for DED symptoms in patients with COVID-19; B:Decision tree model of DED symptoms in patients with COVID-19; C: ROC curve of the prediction model for DED symptoms in patients with COVID-19.DED: Dry eye disease; CL: Contact lenses; CRS: Corneal refractive surgery; VDT: Visual display terminal; LOS: Length of hospital stay;ROC: Receiver-operating characteristic; AUC: Area under the ROC curve.
DISCUSSION
To date, many studies have investigated and reported the relationship between COVID-19 and DED symptoms.For example, Wanget al[1]showed that the symptoms of DED were prevalent in patients with COVID-19.A cross-sectional study confirmed a considerable prevalence rate of DED symptoms among Chinese high school students during the COVID-19 outbreak[15].Another cross-sectional study confirmed that serious conjunctive congestion among patients with COVID-19 was highly correlated with a high hand-eye contact rate (22.2%vs7.9%)[16].The study showed that SARS-CoV-2 infection in Wuhan was related to DED symptoms which leading to excessive tearing and redness[16].However, the clinical ocular surface symptoms of SARS-CoV-2 Omicron variant infection in Shanghai are mild, whose relationship with DED symptoms is not clear.Thus, this cross-sectional study primarily focused on DED symptoms among patients with COVID-19 infected by SARS-CoV-2 Omicron variant.
Currently, many epidemiologic data focused on the prevalence of DED symptoms in COVID-19 patients infected by SARS-CoV-2, but the precise incidence of DED symptoms associated with SARS-CoV-2 Omicron variant infection is unclear.Among the DED questionnaires, the OSDI questionnaire was the key instrument.Our study demonstrated a high incidence of DED symptoms in 993 patients with COVID-19 and 944 controlsvialogistic regression model analysis (64.9%vs55.1%,P<0.001).The results suggested that patients with COVID-19 were more likely to suffer from symptomatic DED.
During the COVID-19 global pandemic, SARS-CoV-2 Omicron variant infection considerably affected the health and quality of life of patients with DED symptoms.Thus, changes that occurred in the lifestyle of patients cannot be ignored.In addition, DED is a multifactorial chronic disease, and its risk factors also warrant investigation.The risk factors included the use of VDTs, wearing CL, CRS history, mood and poor sleep quality in our study are common risk factors for DED symptoms and have been identified in previous studies.Of note, this study added two risk factors such as SARS-CoV-2 Omicron variant infection and LOS.In our present study, we found that SARS-CoV-2 Omicron variant infection, wearing CL, history of CRS, poor sleep quality and VDT use for more than 8h per day were risk factors for DED symptoms in patients with COVID-19 and control participants.Among them, SARSCoV-2 Omicron variant infection was an important risk factor for mild DED, rather than moderate or severe DED, among patients with COVID-19, as observed in multivariate logistical regression analyses.However, we currently only used the OSDI questionnaire to evaluate and diagnose DED symptoms due to the limited medical conditions and environment factors.We will be to explore more dry eye-related parameters such as tear film break up time (TBUT), Schirmer’s test, meibomian gland evaluationetc.for patients with COVID-19 in our future research.In addition, these risk factors were determined by OSDI questionnaires rather than objective measures, which would increase the detection bias of the findings, both the risk factors and the DED symptoms might be confounded by both COVID-19 quarantine and the disease itself.Thus,a causative association could not be addressed from such a study design.In recent years, the mechanism of DED symptoms has been a focus of attention.The ocular surface can inherently protect against multiple pathogens by producing active immune substances, such as immunoglobulins and lysozymes, when SARS-CoV-2 infects the eye[17].These environmental factors also play a key role in DED symptoms by perturbing mechanisms of tear homeostasis.Thus, DED symptoms may develop as a result of SARS-CoV-2 Omicron variant infection in the ocular surface epithelium and glands,which could lead to the formation of an unstable tear film or reduce tear production[18].In addition, genetic factors account for the majority of various symptoms and signs, such as eyelid inflammation, increased tear osmolality, and reduced blinking rate[19].Ocular surface inflammation, which is an important component of DED symptoms, can work together with infection and immune-mediated conditions to promote the development of chronic inflammation.Cellular molecular components, such as inflammatory cytokines, chemokines,and their receptors, reportedly contribute to the pathogenesis of DED symptoms, thus leading to immune-cell activation and associated inflammation[20-21].At the molecular mechanism level, DED symptoms results in tear-film hyperosmolarity associated with an inflammatory cascade most studies have validated.Notably, the mitogen-activated protein (MAP)kinase and nuclear factor κB signaling pathways (MAPK/NF-κB) highly regulates the tear homeostasis resulted by DED symptoms.Among these pro-inflammatory cytokines,interleukin-1α (IL-1α), interleukin-1β (IL-1β), tumor necrosis factor α (TNF-α) and matrix metalloproteinase 9 (MMP9) are susceptible[22].Meanwhile, recent research results had also found that a large amount of extracellular DNA and neutrophil extracellular traps appeared in the tears of DED patients and would eventually lead to ocular surface inflammation,which may be caused by the lack of nuclease[23].Moreover,infection by the SARS-CoV-2 Omicron variant may alter or increase cytokine activity and eventually aggravate the DED symptoms.Another novel finding was that LOS was also an important risk factor for DED symptoms in patients with COVID-19, as observed by both univariate and multivariate logistic regression analyses, but showed no relationship with DED severity.In view of this, we had a possible explanation:COVID-19 patients cannot contact the outside fresh world, and staying in a closed environment with low humidity for a long time is easy to increase screen use time, so the increased LOS may also increase the risk exposure of DED symptoms.
In conclusion, the findings of this cross-sectional study demonstrated that patients with COVID-19 are more likely to suffer from symptomatic DED.SARS-CoV-2 Omicron variant infection, long-term VDT use, use of CL, poor sleep quality, and CRS history were important risk factors for the development of DED symptoms.In addition, the LOS of patients with COVID-19 is also a significant risk factor for DED symptoms.Notably, the prediction model of DED symptoms in patients with COVID-19 showed good specificity and sensitivity.Our findings may provide clinical evidence for the early diagnosis of DED symptoms in patients with COVID-19, but the clinical value was still limited by the cross-sectional design and the descriptive nature of the study.However, there is also hard to find the inner relationship between SARS-CoV-2 Omicron variant infection, LOS and DED symptoms of COVID-19 patients based on our limited data.Thus, our further research is required to understand the potential molecular mechanism.
ACKNOWLEDGEMENTS
Authors’ contributions:Li CY conceptualized the idea.Li CY and Liu T designed the study.Yu J recruited the cases for the study and performed follow up for the patients.Chen QF and Chu H performed OSDI scores statistics and risk factors examining & its analysis.Hu P and Chen L conducted the statistical analysis.Chen QF drafted the manuscript.Li CY and Chen QF revised manuscript.All authors approved the final manuscript.
Foundation:Supported by the Natural Science Foundation of Chongqing (No.cstc2020jcyj-msxmX0241).
Conflicts of Interest: Chen QF,None;Yu J,None;Chu H,None;Hu P,None;Chen L,None;Liu T,None;Li CY,None.
 International Journal of Ophthalmology2023年10期
International Journal of Ophthalmology2023年10期
- International Journal of Ophthalmology的其它文章
- A novel approach for 25-gauge transconjunctival sutureless vitrectomy to evaluate vitreous substitutes in rabbits
- Visual resolution under photopic and mesopic conditions in patients with Sjögren's syndrome
- Effects of obstructive sleep apnea on retinal microvasculature
- Bibliometric analysis of research relating to refractive cataract surgery over a 20-year period: from 2003 to 2022
- Three-dimensional bioprinting in ophthalmic care
- Agreement of intraocular pressure measurement with Corvis ST, non-contact tonometer, and Goldmann applanation tonometer in children with ocular hypertension and related factors
