Effects of obstructive sleep apnea on retinal microvasculature
Mjdat Karabulut, Semai Bek, Sinem Karabulut, Aylin Karalezli, Glnihal Kutlu
1Ophthalmology Department, Mugla Sıtkı Kocman University Training and Research Hospital, Mugla 48300, Türkiye
2Neurology Department, Mugla Sıtkı Koçman University Faculty of Medicine, Mugla 48300, Türkiye
3Ophthalmology Department, Mugla Sıtkı Koçman University Faculty of Medicine, Mugla 48300, Türkiye
Abstract
● KEYWORDS: apnoea-hypopnoea index; deep capillary plexus; obstructive sleep apnea; superficial capillary plexus;vessel density; retinal microvasculature
INTRODUCTION
Obstructive sleep apnea syndrome (OSAS) is a chronic sleep disorder designated nocturnal cessation (apnea)or reduction (hypopnea) in ventilation resulting in a significant decline in oxygen saturation due to upper respiratory system collapse.This condition is associated with many systemic disorders, including neurocognitive, cardiovascular, metabolic,and ocular pathologies[1-2].An increased prevalence of central serous retinopathy, glaucoma, floppy eyelid syndrome,ischemic optic neuropathy, retinal and choroidal pathologies have been reported in OSAS[3-4].
Nocturnal cardiorespiratory monitoring of full night sleep in a polysomnography (PSG) unit is used to diagnose disease severity by calculating the apnoea-hypopnoea index (AHI), the number of apnoeas, and hypopnoeas per hour during sleeping.
The severity of OSAS is classified depending on AHI (number/hour) as none (AHI<5/h), mild (5/h≤AHI<15/h), moderate (15/h≤AHI<30/h), and severe (AHI≥30/h)[5].
Optical coherence tomography angiography (OCTA) has been commonly used for imaging retinal and choroidal vessels without requiring any contrast agent or dye.Recently, studies with OCTA showed alterations in the retinal vessel density(VD) in patients with OSAS[6].
We aimed to detect retinal microvascular variations and define the effects of disease severity on retinal microvasculatures in patients with OSAS.
SUBJECTS AND METHODS
Ethical ApprovalConsent was obtained from all participants and this research took no specific donations from public,commercial, or not-for-profit funding agencies.Muğla Sıtkı Koçman University Clinical Research Ethics Committee approved this study with a 03/I decision number and 13.02.2020 date.In this observational case-control study, we prospectively evaluated patients diagnosed with OSAS between February 2020 and December 2021 and compared them to healthy controls (patients having an AHI<5).We included patients suffering from daytime sleepiness, concentrating difficulty,snoring, morning headache, and episodic breathing cessation during asleep and attended Muğla Sıtkı Koçman University Training and Research Hospital, Neuropsychology Clinic for a routine examination.Exclusion criteria included history of ophthalmic pathologies and systemic diseases such as diabetes mellitus, arterial hypertension, vasculitis, rheumatologic and neurologic diseases.After diagnosing OSAS with PSG and defining disease severity, patients were informed and referred to Opthalmology Clinic for ocular examination and imaging.Patients were monitored according to the American Academy of Sleep Medicine manual for the scoring of sleep and associated events: rules, terminology and technical specifications[7].Electro-oculography electrodes for detecting eye movements, electroencephalography electrodes in 10-20 international systems, submental and bilateral anterior tibial electromyography electrodes, and electrocardiographic electrodes for cardiac monitoring were placed.Thoracal and abdominal respiratory movements were monitored, nasal air pressure sensors and oronasal thermistors were used for detecting airflow.A pulse oximeter was used to monitor oxygen saturation.Sleep efficiency of more than 70% was accepted as eligible.Embla RemLogic PSG Software was used.Sleep stages scoring as wake, N1 sleep, N2 sleep, N3 sleep, and rapid eye movement (REM) sleep and respiration scoring offline were conducted with two certified technicians and a sleep disorder specialist neurologist (Bek S and Kutlu G), respectively, according to the American Academy of Sleep Medicine manual[7].
All patients underwent a complete ocular examination in the ophthalmology clinic, including ocular history inquiry, visual acuity, refraction, intraocular pressure (IOP) measurements,anterior and posterior segment biomicroscopic checks.Axial length (AL) was automatically measured with an optical biometer (IOLMaster 700, Carl Zeiss Meditec AG, Jena,Germany).The device estimates the average of scans and offers a standard deviation (SD).The SD under 27 µm was accepted as reliable.
Macula-centered, 6.0×6.0 mm2sized OCTA images were automatically taken by an expert at the same period of time(9:00-12:00a.m.) to lower the effect of diurnal variations using RTVue-XR Avanti (Optovue, CA, USA) device.The VD of both superficial and deep capillary plexus (SCP, DCP) in four segments (foveal, perifoveal, parafoveal, whole), the flow area of the outer retina and choriocapillaris, foveal avascular zone(FAZ) area, and foveal thickness were automatically explored and quantified by software available in the device with the segmentation of retinal layers (Figures 1-4).

Figure 1 The whole image, foveal (A), parafoveal (B), and perifoveal (C)areas divided into 1 (1), 3 (2), and 6 (3) mm diameter rings in the superficial capillary plexus, which is located between the internal limiting membrane and the inner plexiform layer (D).

Figure 2 The whole image, foveal (A), parafoveal (B), and perifoveal(C) areas divided into 1 (1), 3 (2), and 6 (3) mm diameter rings in the deep capillary plexus, which is located between the inner plexiform layer and the outer plexiform layer (D).
The image quality was determined with the signal strength index.

Table 1 Demographic characteristics, ocular and polysomnographic findings of the groups mean±SD (range)
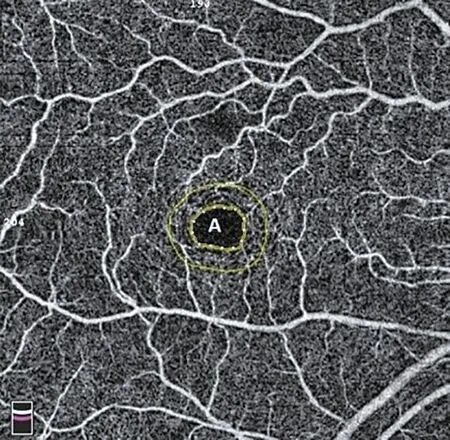
Figure 3 The foveal avascular zone automatically encircled by the device.
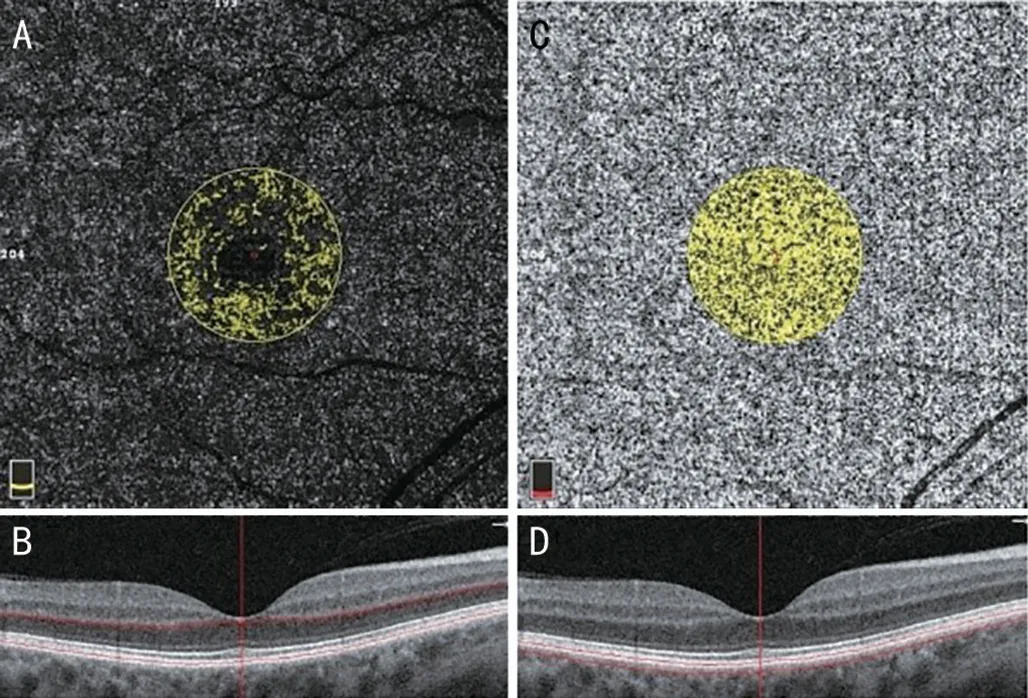
Figure 4 The 3.142 mm2 flow area in the outer retina (A, B) and choriocapillaris (C) segmented 30 µm deep from Bruch’s membrane(D).
We excluded patients with any corneal, lenticular, vascular,macular, and optic nerve pathology; amblyopia, nystagmus,glaucoma; history of previous ocular surgery; AL greater than 26 mm and less than 18 mm; spherical equivalent (SE) more than two diopters , and images with signal strength index less than 8/10.
The patients were divided into four groups for their AHI results as the control group (AHI<5), mild (5≤AHI<15), moderate(15≤AHI<30), and severe (AHI≥30) OSAS groups.
Statistical AnalysisData was recorded into the Statistical Package for the Social Sciences program, version 21.0.0.0(IBM Corporation and other[s] 1989, 2012).Following the evaluation of normality with the Shapiro-Wilk test, Kruskal-Wallis and Chi-squared tests were applied to compare continuous and categorical variables among groups.Mann-WhitneyUpost-hoc test was performed to assess the differences between the groups.Spearman’s rank correlation coefficient was used for assessing relations.Statistical significance was indicated asP<0.05.
RESULTS
One hundred and eighteen eyes of 59 patients were enrolled.Mild, moderate, severe OSAS and control groups had 30,26, 36, and 26 eyes, respectively.The groups were similar for gender, age, AL, SE, IOP, and body mass index (BMI;P=0.354, 0.156, 0.462, 0.738, 0.591, 0.229, respectively).The mean best-corrected visual acuity was 0.0 logMAR in all groups.Pulse oximetry measurements (SpO2) and AHI were significantly different among the groups (P<0.001; Table 1).
In the SCP and DCP, the control group had a significantly higher whole image, parafoveal, and perifoveal VD than theVD of OSAS groups (P=0.034, 0.036, 0.033, 0.041, 0.032,0.028, respectively).Foveal VD was not different among the groups (P=0.341, 0.141, respectively).Foveal thickness,FAZ area, outer retinal, and choriocapillaris flow area were also similar for the groups (P=0.858, 0.485, 0.098, 0.532,respectively; Table 2).

Table 2 Comparison of the mean vessel density in SCP and DCP among the groups mean±SD

Table 3 Relation between AHI, SpO2, and the mean vessel density in SCP and DCP
REM sleep AHI significantly and reversely correlated with whole (Rho=-0.195,P=0.034), parafoveal (Rho=-0.242,P=0.008), perifoveal (Rho=-0.187,P=0.045) VD in SCP,and whole (Rho=-0.186,P=0.046), parafoveal (Rho=-0.260,P=0.004), perifoveal (Rho=-0.189,P=0.043) VD in DCP,though the mean and non-REM AHI related with only parafoveal VD in SCP (Rho=-0.213,P=0.020; Rho=-0.191,P=0.038) and DCP (Rho=-0.254,P=0.005; Rho=-0.194,P=0.035).Moreover, whole, parafovea, and perifovea VD in SCP and DCP were not related to SpO2(Table 3).
DISCUSSION
Vascular diseases in patients with OSAS are considered to result from systemic inflammation due to intermittent hypoxia.Endothelial dysfunction and excessive platelet activation accelerate coagulation and atherosclerotic processes, leading to vascular complications[8].Additionally, chronic hypoxemia and hypercapnia induce some neuroendocrine, autonomic, and hemodynamic responses that result in endothelial dysfunction,endothelin production, peripheric vasoconstriction, and eventually peripheral vascular diseases[9].
The prevalence of retinal vascular diseases, such as retinal vein occlusion, diabetic retinopathy, hypertensive retinopathy,vascular sclerosis, narrowing, and nicking, also increased in patients with OSAS[10].Some studies showed that diabetic retinopathy and maculopathy progression positively correlated with REM-AHI value[11].Various methods can easily detect disease-related gross vascular findings after the onset of vasculopathy.However, it may be too late to treat these complications or slow down the progression after that moment.Therefore, detecting early changes in which obvious vasculopathy has not yet started may benefit for prevention of vascular complications.
This study aimed to determine the retinal microvascular changes in patients with OSAS whose eyes did not have detectable vasculopathy on fundus examination.Additionally,we planned to define the relationship between disease severity and retinal microvascular changes.
Since it was known that age, gender, best-corrected visual acuity, IOP, AL, SE, BMI, some ocular and systemic diseases could affect retinal microvascular structures, we equated the groups with seeing the pure effect of OSAS on retinal vasculature[12-13].We found that the mean whole, parafoveal,and perifoveal VD in SCP and DCP were significantly decreased in patients with OSAS.Moreover, REM-AHI value was reversely correlated with VD in these sectors.
The mean and nonREM-AHI values were only related to parafoveal VD.Interestingly, there was no significant relation between SpO2and VD in these sectors.Foveal thickness, FAZ area, choriocapillaris, and outer retinal flow area did not differ among the groups.
Studies with OCTA showed decreased retinal vessel density in the eyes of patients with OSAS.Ucak and Unver[14]reported decreased VD in SCP and DCP.They also showed a significant reverse relation between the AHI value and VDs.Likewise,Yuet al[15]found a significant decrease in parafoveal and perifoveal VD in the eyes of patients with OSAS and reported that disease severity negatively affected these VDs.
The effects of OSAS on the FAZ area are still controversial.Some studies showed an enlarged FAZ area, while others reported reduced or unchanged FAZ area in the eyes of patients with OSAS[14,16].In our study, the mean FAZ area was increased in OSAS groups, but the differences were insignificant compared among the groups.
Choroidal structural and vascular changes in patients with OSAS have been seen as an increased area of interest.Most case-control studies with optical coherence tomography have shown decreased choroidal thickness in patients with OSAS[17].However, data on choroidal microvascular changes are limited.In a case-control study, Özcanet al[18]reported no significant change in the choroidal vascular index in patients with moderate OSAS compared to controls.Some studies suggested that patients with OSAS have an adaptive and protective mechanism, such as hypercapnia-induced vasodilatation in choroidal microcirculation[19].
Additionally, some animal studies showed an autoregulatory capacity in choroidal blood flow even though it had been known that the choroid was assumed to have no autoregulation[20].We also found no significant differences in choriocapillaris flow area among the groups.It could be related to choroidal blood flow autoregulation.
This observational case-control study was unique in that the groups were equalized for gender, age, AL, SE, IOP, BMI,and systemic-ocular diseases were excluded to show the pure impact of OSAS on retinal and choroidal microvasculatures.
However, the sample size was relatively small, and the effects of smoking, caffeine use, and OSAS treatment on these microvasculatures were not investigated.
Consequently, this novel and non-invasive technique could be used for determining OSAS-related microvascular changes and disease monitorization by imaging retinal and choroid vessels.Extensive sample-sized prospective cohort studies are required to highlight the impact of OSAS treatment on these microvasculatures.
ACKNOWLEDGEMENTS
Conflicts of Interest:Karabulut M,None;Bek S,None;Karabulut S,None;Karalezli A,None;Kutlu G,None.
 International Journal of Ophthalmology2023年10期
International Journal of Ophthalmology2023年10期
- International Journal of Ophthalmology的其它文章
- A novel approach for 25-gauge transconjunctival sutureless vitrectomy to evaluate vitreous substitutes in rabbits
- Visual resolution under photopic and mesopic conditions in patients with Sjögren's syndrome
- Bibliometric analysis of research relating to refractive cataract surgery over a 20-year period: from 2003 to 2022
- Three-dimensional bioprinting in ophthalmic care
- Agreement of intraocular pressure measurement with Corvis ST, non-contact tonometer, and Goldmann applanation tonometer in children with ocular hypertension and related factors
- A combined treatment for patients with dry eye and associated laryngopharyngeal reflux: a real-life approach
