Hotspots and frontiers of genetic research on pediatric cataracts from 2013 to 2022: a scientometric analysis
Yuan Tan, Wei Jiang,2, Le-Yi Hu, Yan-Yu Shen, Hui Chen, Ying-Shi Zou, Li-Xia Luo,Guang-Ming Jin, Zhen-Zhen Liu
1State Key Laboratory of Ophthalmology, Zhongshan Ophthalmic Center, Sun Yat-sen University, Guangdong Provincial Key Laboratory of Ophthalmology and Visual Science, Guangdong Provincial Clinical Research Center for Ocular Diseases,Guangzhou 510060, Guangdong Province, China
2Zhongshan Medical School, Sun Yat-sen University,Guangzhou 510060, Guangdong Province, China
Abstract
● KEYWORDS: gene; pediatric cataract; next generation sequencing; genotype phenotype association
INTRODUCTION
Pediatric cataracts account for 5%-20% of the world’s childhood blindness[1], bringing significant socioeconomic burdens, especially to developing countries[2].Genetic factors were estimated to attribute to 10%-50% of pediatric cataracts, and the percentage was expected to climb with the popularization of sequencing technologies[3].The scope and depth of research in the area of genes associated with pediatric cataracts have expanded quickly in recent years, generating complex networks of research information.It is, therefore,necessary to develop a more comprehensive understanding of the patterns of scientific publications on genes associated with pediatric cataracts, as well as to analyze the hotspots and frontiers in the field.
Over the past few years, several reviews have been published on the genetic etiology and prevalence of pediatric cataracts aiming at specific issues in basic sciences and/or clinical practices[2,4].However, a more comprehensive, objective, and quantitative overview of the research development on genes associated with pediatric cataracts has yet to be put forward.The scientometric approaches enable the identification of research hotspots and frontiers in a research field by providinga quantitative study of the predominant actors and the dynamic shifts in a particular field using statistical and mathematical methods[5-6].
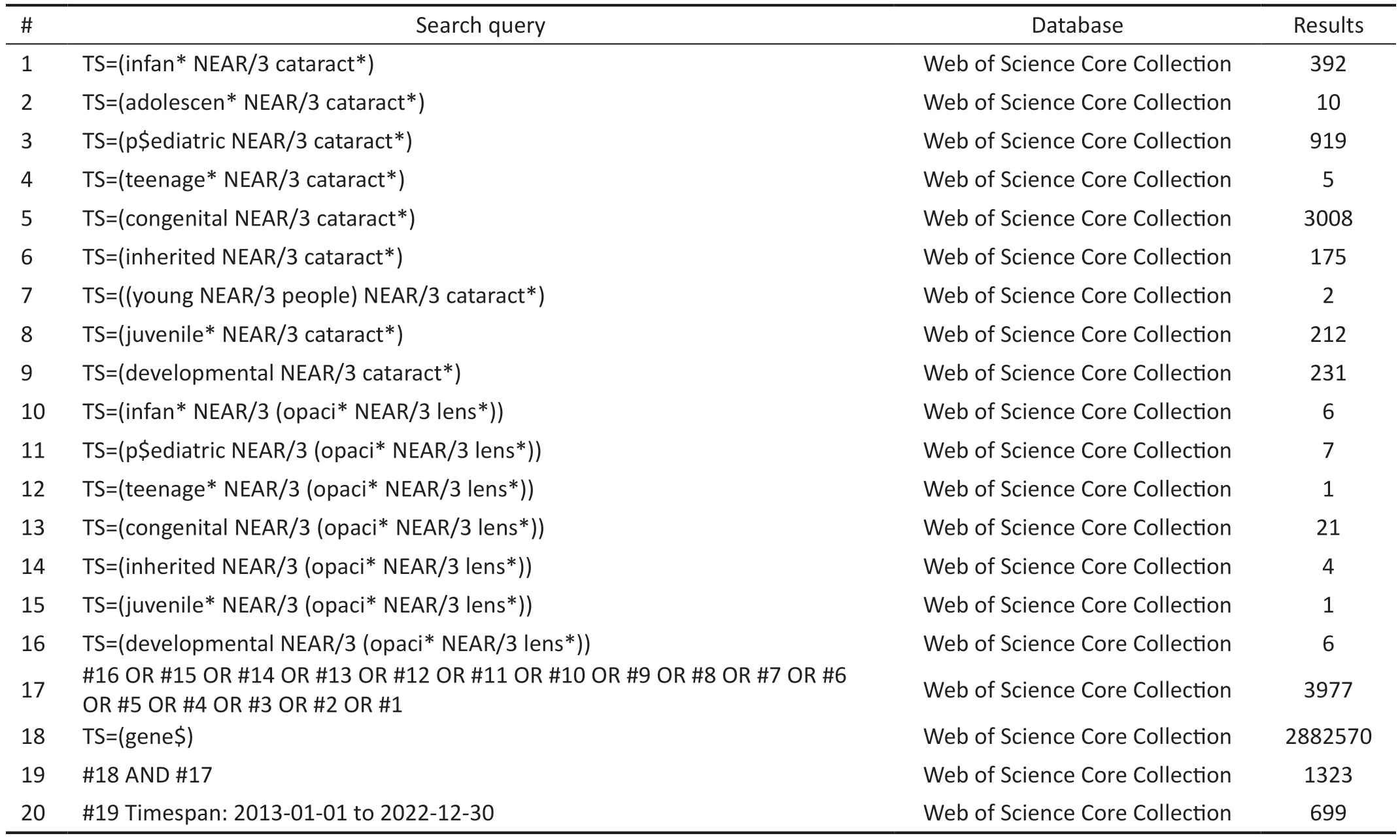
Table 1 Search strategy
This scientometric study has been conducted to explore the hotspots and frontiers of genetic research on pediatric cataracts from 2013 to 2022, aiming to enable researchers to understand the main and cutting-edge content of research in this field, as well as the collaborations between different disciplines, as to provide a reference for exploring the future research directions and strategies.
MATERIALS AND METHODS
Literature Search and SelectionScientometric research typically requires the use of a single database to avoid duplicate acquisition of literature and maintain consistency in the definition of evaluation indicators[7-8].The keywords covering genetic research on pediatric cataracts were searched in the Web of Science Core Collection (WoSCC; Clarivate Analytics, Philadelphia, PA, USA; Table 1).The WoSCC is a widely recognized and reliable resource that provides citation data and facilitates the examination of research frontiers.In this study, we utilized several sub-databases within the WoSCC to acquire comprehensive and diverse information in the field.These sub-databases included the Science Citation Index Expanded from 1999 to present, the Social Sciences Citation Index from 2002 to present, the Arts & Humanities Citation Index from 2002 to present, the Conference Proceedings Citation Index-Science from 2000 to present, the Conference Proceedings Citation Index-Social Sciences & Humanities from 1990 to present, the Emerging Sources Citation Index from 2018 to present, the Current Chemical Reactions from 1985 to present, and the Index Chemicus from 1993 to present.The search terms included variations of “infant”, “adolescent”,“pediatric”, “teenage”, “congenital”, “inherited”, “young people”, “juvenile”, and “developmental” in close proximity to “cataract” or “lens” and “opacity.” Additionally, the search included the term “gene”.All of the search terms were applied to the Title, Abstract, Author Keywords, and Keywords Plus fields.The time range was set from 2013 to 2022.The search was carried out on November 27, 2022.Therefore,the publication volume for 2022 is limited to a cut-offdate of November 27, 2022 and does not include publications from the entire year.Figure 1 details the study selection and analysis procedures.Pediatric cataracts here refer to cataracts that occur in children with hereditary backgrounds.
Data Extraction and CollectionRelevant data were downloaded to analyze annual publications, annual citations,countries, journals, authors and publication time.The full records of the selected publications including the cited references were collected for keyword analysis, cited reference analysis, and subject category analysis.

Figure 1 Flow chart of the literature selection and analysis procedures WoSCC: Web of Science Core Collection.
Analysis of Publication AttributesThe publication volume and predominant actors, including countries, journals, and authors, were analyzed with Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, USA) and GraphPad Prism version 8.3.0 (GraphPad Software, La Jolla, CA, USA).In counting the country publication volume, the publication volume attributed to the United Kingdom represents the combined publication volume of Scotland, England, Wales,and Northern Ireland.The annual growth rate was calculated by dividing the value of the difference between publication counts in a given year and publication counts in the prior year by the publication counts in the prior year.The absence of growth was considered to be represented by a value of 0.A year with more than 100 publications and an annual growth rate of over 10% was defined as a year with remarkable publication activity[9].
The co-authorship analysis and the high-frequency keyword co-occurrence analysis were performed using VOSviewer 1.6.5 (Leiden University’s Centre for Science and Studies,Leiden, the Netherlands).Through the co-authorship analysis,the collaboration networks were obtained.The association strength among authors was determined by the total link strength, which indicates the number of publications where two authors were present together[10].The clusters in the coauthor collaboration network analysis were named by the most collaborative authors in corresponding clusters.
Analysis of Research HotspotsIn the keyword co-occurrence analysis, the keywords are weighted by their occurrence counts to form different keyword clusters according to the relevance between keywords by using VOSviewer.Both the frequency and the characteristics of the keywords in each cluster were taken into consideration for the identification of research hotspots that each cluster represented[11].The clusters were named by the identified research hotspots.
Analysis of Research TrendsWe evaluated the total citation counts of articles to show the overview focus characteristics of the area because articles that were cited more frequently suggested more academic attention.
The co-citation analysis of cited references and co-occurrence analysis of networks of subject categories were performed using CiteSpace V version 6.1.4 (Drexel University, Philadelphia,PA, USA).To identify research frontiers, the cited references were clustered according to relevance and displayed in a timeline view, in which the clustered cited references were arranged on a horizontal timeline according to the publication time.The visualization of the timeline can provide an intuitive overview of the development of a certain cluster[6].Clusters were labelled according to the subject content of the citing articles in corresponding clusters.Research frontiers were defined as clusters with the most recent mean publication year or the most recently recruited members, implying that they represented newly formed topics or topics with continual development.A significant betweenness centrality (BC), that is BC>0.1, of the cited references was used to identify the cited references that served as important turning points.
To determine the most involved subject categories in the field with ophthalmology, pediatrics, and genetics, all subject categories were analyzed.The significant BC of the subject categories were used to identify their bridging roles among disciplines.
RESULTS
Annual Global OutputThe annual global output and citations in the field of genes associated with pediatric cataracts from 2013 to 2022 are displayed in Figure 2A.There were 699 publications in the WoSCC, with the highest in 2021 (n=96;13.73%) and the lowest in 2015 (n=58; 8.30%).The annual global output in 2021 was more than 1.5 times that of 2015.There were 7321 citations from 2013 to 2022, with an average of 10.47 citations per publication.The annual citations peaked in 2021 (n=1531).The years 2017, 2020, and 2021 were identified as years with remarkable publication activities.
Predominant Actors
CountriesThere were67 countries that contributed to the publications in the field of genes associated with pediatric cataracts from 2013 to 2022 (Figure 2B).Among them, China had the most publications (n=240, 34.33%), followed by the USA (n=177, 25.32%), the UK (n=79, 11.30%), Germany(n=46, 6.58%), Japan (n=45, 6.44%), Italy (n=34, 4.86%),India (n=34, 4.86%), Canada (n=32, 4.58%), Turkey (n=29,4.15%), and Spain (n=29, 4.15%).

Figure 2 Overview of publication information on genes associated with pediatric cataracts from 2013-2022 A: The overall trends of publications and citations, and the annual publication growth rates; B: Top 10 countries by the number of publications; C: Top 13 journals by the number of publications.
JournalsA total of 258 journals contributed to the publications on genes associated with pediatric cataracts during the study period.PLoS Onehad the most publications(n=33, 4.72%), followed byMolecular Vision(n=26, 3.72%),Investigative Ophthalmology Visual Science(n=25, 3.58%),Ophthalmic Genetics(n=23, 3.29%) andAmerican Journal of Medical Genetics Part A(n=18, 2.58%).The number of publications of the top 13 journals is displayed in Figure 2C.
AuthorsIn general, 4629 authors participated in the publication of genes associated with pediatric cataracts, with an average of 6.62 authors per article.Ke Yao (18 publications;2.58%) and J.Fielding Hejtmancik (16 publications; 2.29%)were the most prolific authors, followed by Kathryn P.Burdon(14 publications; 2.00%).Figure 3A shows the top 11 coauthors by the number of publications during the study period.The author collaboration network included 78 authors and 349 collaborations (Figure 3B).Prolific authors showed a tendency to form independent clusters and make collaborations with each other.Eight clusters were formed in the author collaboration analysis.The S.Amer Riazuddin and Sheikh Riazuddin cluster (17 authors), the Qiwei Wang cluster (15 authors), and the Ke Yao cluster (14 authors) were the three largest clusters.
Research Hotspots and Frontiers
Hot topics based on high-frequency keywordsThe hotspots of genes associated with pediatric cataracts were determined according to 93 high-frequency keywords (Figure 4).The research hotspots were: the crystallin gene mutations (26 items), pathogenicity evaluation (24 items), phenotypes of ocular and neurodevelopmental abnormalities (20 items),genes encoding membrane proteins (13 items), and diagnosis of multisystemic disorders (10 items).

Figure 3 The most active authors in genetic research on pediatric cataracts A: Top 11 co-authors by the number of publications; B:Author collaboration network map.
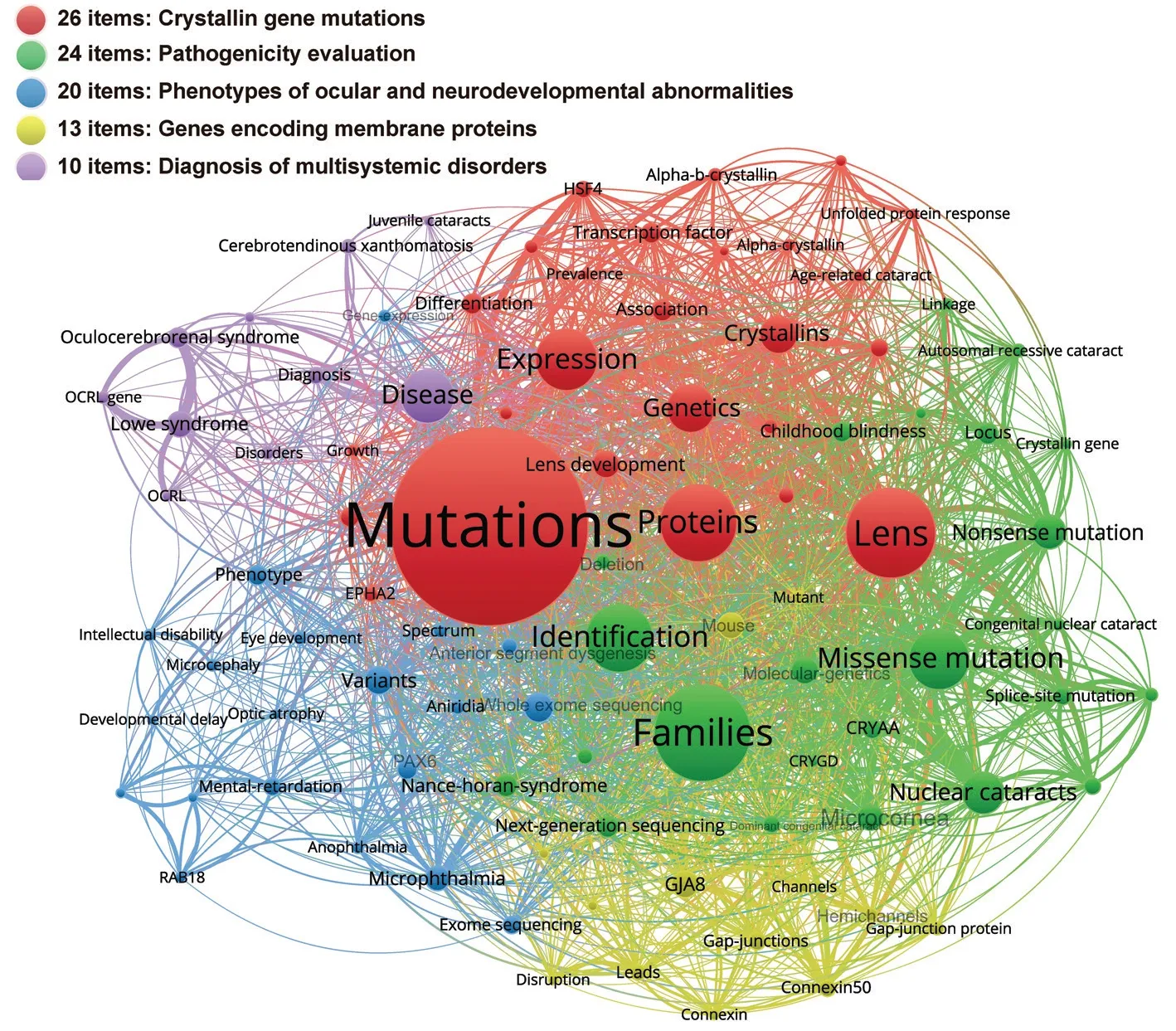
Figure 4 Keyword-based research hotspots of genetic research on pediatric cataracts A node represents a keyword.Larger nodes indicate more frequent keywords.Clusters are formed based on keyword co-occurrence analysis.Nodes that are closely related belong to the same cluster and are represented by the same color.
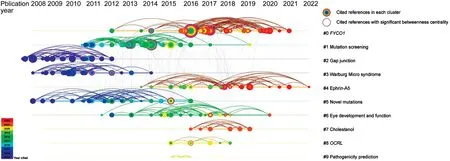
Figure 5 Reference-based research frontiers in genetic research on pediatric cataracts Each node represents a cited reference of included studies.Larger nodes indicate that higher citation frequency.All nodes are arranged in a timeline according to their publication time.The node color corresponds to the citation years.Nodes with a purple outer ring indicate they have a significant betweenness centrality (betweenness centrality>0.1), showing their role as a turning point in the shiftof research direction.The main clusters are ordered by cluster size as clusters#0-9, with #0 containing the largest number of members.The labels of the clusters were derived from the citing articles in each cluster.FYCO1:FYVE and coiled-coil domain autophagy adaptor 1; OCRL: OCRL inositol polyphosphate-5-phosphatase.
Research frontiers based on co-cited articlesWe listed the top 20 cited articles on genetic research on pediatric cataracts(Table 2).Ten research topic clusters were formed in the cited reference analysis and were displayed in a timeline view as shown in Figure 5.TheFYCO1(FYVE and coiled-coil domain autophagy adaptor 1) cluster (56 items, mean publication year=2017) was the largest cluster, as well as one of the most recently formed clusters.The mutation screening cluster (43 items, mean publication year=2013) had no member that was cited after 2020.The gap junctioncluster (29 items,mean publication year=2010), the Warburg Micro syndrome cluster (29 items, mean publication year=2010), and the novel mutation cluster (24 items, mean publication year=2012)formed before 2017.The ephrin-A5cluster (28 items, mean publication year=2017) continuously recruited members as of 2022 and was one of the most recently formed clusters.There were 22 members in the eye development and function cluster (mean publication year=2015).The cholestanol cluster(7 items, mean publication year=2017) was one of the most recently formed clusters in the past decade.There were 6 members in theOCRL(OCRL inositol polyphosphate-5-phosphatase)cluster (mean publication year=2016).There were 3 members in the pathogenicity prediction cluster (mean publication year=2015).FYCO1, ephrin-A5, and cholestanolwere listed as research frontiers in the research trends because they were relatively active in terms of corresponding members’recruitment time (Ephrin-A5 cluster) and cluster mean publication year (FYCO1, Ephrin-A5, and Cholestanol cluster;mean publication year=2017), respectively.Table 3 lists the top cited reference and citing article for each cluster in order of the mean cluster publication year to show the evolution of the clusters.

Table 2 The 20 top-cited studies in genetic research on pediatric cataracts
Frontiers in research subject categoriesTo show the overall distribution of subject categories, we identified the top 12 subject categories in terms of their BC (Table 4).Cell biology(n=41, BC=0.44), biochemistry & molecular biology (n=108,BC=0.41), and medicine, research & experimental (n=54,BC=0.30) had the highest BC values, serving as bridging subject categories (Figure 6).
DISCUSSION
The research attributes and trends were identified according to 699 WoSCC articles on genes associated with pediatric cataracts in the past 10y, and the results showed that the crystallin gene mutations, pathogenicity evaluation, phenotypes of ocular and neurodevelopmental abnormalities, genes encoding membrane proteins, and diagnosis of multisystemic disorders were the research hotspots.FYCO1, ephrin-A5,and cholestanol were identified as research frontiers, with cell biology being a crucial bridging subject category among different disciplines.

Figure 6 Subject categories of genetic research on pediatric cataracts The distribution map of subject categories.Seven bridging subject categories (betweenness centrality >0.1) indicated in a purple outer ring, were shown.
The overall research profile of genes associated with pediatric cataracts suggests that this field is relatively condensed yet with a variety of sources.There were fluctuated annual publication counts and several identified years with remarkablepublication activities during a decade, indicating instability in a relatively small research field, but the engagement of numerous geographical countries, journals focused on different topics, and various closely collaborating authors suggested the diversity of research topics in this field.The potential impact of socioeconomic factors on the accessibility of healthcare services for pediatric cataract patients was undefined and could contribute to variations in publication activities[1-2,12].In this study, we did not analyse research institutions.Future research could build upon our work by incorporating an analysis of research institutions.

Table 3 The top cited reference and citing article for each cluster in order of the mean cluster publication year

Table 4 Betweenness centrality based top 12 subject categories
Five research hotspots in the study of genes associated with pediatric cataracts during the last decade were identified.Hot topics in molecular genetics included the study of membrane protein-coding genes and crystallin genes, suggesting that as genes encode key structural proteins in the crystalline lens, membrane protein-coding genes and crystallin genes are receiving continuous attention.Second, the evaluation of the pathogenicity of sequence variants remained a research hotspot due to the heterogeneity of pediatric cataracts in molecular genetics and clinical manifestations[13-15].Third,the developmental abnormalities associated with pediatric cataracts were intensely studied, especially in ocular structure as well as the nervous system.It was reported that about 14.5% of congenital cataract cases involve additional ocular aberrant phenotypes[16].Furthermore, pediatric cataracts and neurodevelopmental abnormalities often coexist, typical examples include Lowe syndrome caused byOCRLmutations,and in recent years, several other related genes have been identified, such asRIC1(RIC1 homolog, RAB6A GEF complex partner 1) andFOSL2(FOS like 2, AP-1 transcription factor subunit)[15,17-18].Last but not least, great efforts had been made to identify novel cataract-related syndromes for accurate diagnosis and targeted treatment[19].To date, more than 150 genetic loci related to syndromic pediatric cataracts have been identified[20].According to our findings, the field of pediatric cataract genetics had focused attention on the clinical diagnosis and relevant evaluation of the pathogenicity of gene variants.Our knowledge of pathogenic genes and the patterns of gene mutations that cause disease was limited[3].Furthermore, little was known about multisystem triggers because systemic conditions were rarely evaluated in the majority of the evidence collected from pediatric cataract genetic research[4].Exploration of possible mechanisms of multi-system manifestations was still ongoing.
In terms of research frontiers, the roles ofFYCO1, ephrin-A5,and cholestanol in lens development and transparency maintenance were found to attract great attention in recent years.Chenet al[21]discovered that mutations inFYCO1can cause autosomal recessive congenital cataracts.And the protein encoded byFYCO1plays a role in the autophagic process.In 2022, Khanet al[22]demonstrated that loss ofFYCO1function had caused decreased autophagic flux, impaired organelle clearance, and cataractogenesis.However, whether impaired organelle removal dependent on the loss of function ofFYCO1directly leads to cataracts warrants further study.Ephrin-A5 is a ligand for the Eph receptor.In different species, the disruption of Eph-ephrin signaling was found to be cataractogenesis[23-25].Vu and Cheng[26]extracted and sequenced Eph receptor and ephrin ligand transcripts in adult mice and discovered that practically all Ephs and ephrins were expressed in the adult mouse lens.The spatiotemporal specificity of the expression of other receptor-ligand pairs and their role in the lens, and whether they are affected in the ephrin-A5 mutant lens remain to be elucidated.Cholestanol was recognized as a research frontier in the field, highlighting the potential role of lens opacity as a screening marker for multisystemic disorders for pediatric patients.Mutations inCYP27A1(cytochrome P450 family 27 subfamily A member 1), the gene encoding sterol 27-hydroxylase, cause cerebrotendinous xanthomatosis, a multisystemic disorder characterized by elevated levels of cholestanol[27].A previous study showed that when genetic testing was unavailable or the results were inconclusive, ocular examinations were especially useful in diagnosing specific syndromes[28].Specifically, Freedmanet al[27]demonstrated that pediatric cataracts may be utilized as a screening marker for cerebrotendinous xanthomatosis, which is 500 times more likely to be detected in the pediatric cataract group than in the general population.
Our result showed that cell biology played the most prominent bridging role among multiple disciplines.In the field of genes associated with pediatric cataracts, cell biology may serve as the glue that integrates biological disciplines such as genetics for systematic research, and other related scientific fields such as neuroscience to address cutting-edge scientific questions.For example, congenital cataracts, a form of cataract in children, are generally characterized by defects in the structure or function of lens proteins and/or their coding genes, which can lead to lens opacity[12].Utilizing cell biology research methods, we can investigate the pathogenic genes, the abnormalities in the structure of the encoded proteins, and their consequences[15,26].For instance, changes in isoelectric point or local hydrophobicity/hydrophilicity can cause abnormal aggregation of crystallin, leading to a loss of solubility of high concentrations of intracellular proteins and resulting in lens opacity[12].Additionally, abnormalities in ion pumps on the lens cell membrane, such as aquaporin and calcium channels,can disrupt metabolic homeostasis and cause lens opacity[25].Furthermore, cell biology research methods can be used to explore the role of developmental biology in pediatric cataract genes, including the involvement of heat shock factor 4 as an eye development-related gene in cataract autophagy-related pathogenesis research[29].It is also important to note that children with cataracts can exhibit abnormalities in the nervous system[15,18].The integrative nature of cell biology enables researchers to explore interactions between ocular structures and the nervous system at the cellular level, promoting interdisciplinary research between pediatric cataracts and neuroscience and providing a deeper understanding of disease development mechanisms.
The study nature of a scientometric analysis brought some limitations to this study.Although PubMed is a commonly used database, it does not provide citation data.For this reason, we limited our literature selection for our research to the WoSCC, which does provide citation data for citation analysis.As a result, our research results cannot be generalized to literature not included in WoSCC.A literature search was conducted to ensure the research was specific to pediatric cataracts, and genetic studies in pediatric cataracts were selected.This allowed us to primarily investigate congenital,developmental, and hereditary types of pediatric cataracts.However, it was possible that some literature had included a mix of congenital, developmental, hereditary, and traumatic cataracts in children.As a result, there may be some bias in the types of cataracts represented in our findings, and they should be interpreted with caution.The analysis conducted with VOSviewer and CiteSpace, which generated keyword co-occurrence and co-citation networks, had its limitations.It only took into account single factors such as keywords and citations, while overlooking the potential influence of the author’s contributions and the contributions of the journals.This means that the results should be interpreted with caution and within the context of this research.Moreover, only English-language articles can be analyzed by VOSviewer and CiteSpace, which wouldn’t affect the results of annual global output and predominant actors’ output.However, the results of research hotspots and research frontiers might be subject to language bias.To address this issue, future scientometric research should focus on the landscape, hotspots, and frontiers of genetic research on pediatric cataracts in different regions and languages to provide a more refined analysis.It should be acknowledged that there may be a delay in the updating of information in the database, resulting in the potential omission of newly indexed articles during the data collection process.Conducting literature searches at a more recently updated time would likely yield a larger volume of data.For this study,the search was conducted until November 27, 2022.One limitation of this study is that the publication volume for 2022 does not encompass the entire year and is limited to a specific cut-off date of November 27, 2022.As a result, the annual publication volume for 2022 is underestimated in this study.However, given that our study covered a substantial majority of articles published between 2013 and 2022, it is unlikely that the inclusion of newly published articles would significantly alter our conclusions about the research hotspots and frontiers.Additionally, the results of this study may not comprehensively represent the barriers encountered by academics working in this field.This suggests that further research, such as a scoping review, may be necessary to fully understand these barriers.
In summary, researchers from different regions and countries paid continuous attention to genetic research on pediatric cataracts in the past decade.This field focused on the roles of key structural proteins in lens development and transparency maintenance, and on the molecular mechanism of ocular or systemic syndromes that presents with lens opacity.The application of next-generation sequencing and other new technologies, as well as multidisciplinary cooperation, played an important role in deepening and advancing the research in genes associated with pediatric cataracts.
ACKNOWLEDGEMENTS
Foundations:Supported by the National Natural Science Foundation of China (No.81900841); the Science and Technology Program of Guangzhou, China (No.202201011815);the Guangdong Basic and Applied Basic Research Foundation(No.2022A1515011181); the Teaching Reform Research Program of Sun Yat-sen University (No.JX3030604024); the Youth Project of State Key Laboratory of Ophthalmology(No.2021QN02); the Construction Project of High-Level Hospitals in Guangdong Province (No.303020102).
Conflicts of Interest: Tan Y,None;Jiang W,None;Hu LY,None;Shen YY,None;Chen H,None;Zou YS,None;Luo LX,None;Jin GM,None;Liu ZZ,None.
 International Journal of Ophthalmology2023年10期
International Journal of Ophthalmology2023年10期
- International Journal of Ophthalmology的其它文章
- A novel approach for 25-gauge transconjunctival sutureless vitrectomy to evaluate vitreous substitutes in rabbits
- Visual resolution under photopic and mesopic conditions in patients with Sjögren's syndrome
- Effects of obstructive sleep apnea on retinal microvasculature
- Bibliometric analysis of research relating to refractive cataract surgery over a 20-year period: from 2003 to 2022
- Three-dimensional bioprinting in ophthalmic care
- Agreement of intraocular pressure measurement with Corvis ST, non-contact tonometer, and Goldmann applanation tonometer in children with ocular hypertension and related factors
