Differential expression of antimicrobial peptides in human fungal keratitis
Jia-Song Wang, Xi Peng, Zhao Zhao, Chao Wang, Hua-Tao Xie, Ming-Chang Zhang
Department of Ophthalmology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei Province, China
Abstract
● KEYWORDS: antimicrobial peptides; fungal keratitis;Aspergillus; Fusarium
INTRODUCTION
Fungal keratitis (FK) is a serious infection of the cornea that can lead to irreversible vision loss, with approximately 100 000 eyeballs were removed worldwide per year[1-5].The current challenges are poor treatment response due to a number of factors, such as difficulty in microbiological identification, inadequate efficacy and permeability of antifungal drugs, and extremely wide drug sensitivity of existing drugs[6-9].Therefore, the main alternative therapy is to study the human immune response to fungal infection.
Antimicrobial peptides (AMPs) are host defense protein peptides secreted naturally by epithelial cells and immune cells, playing an important role in the primitive immune system and adaptive immune system, and having a wide range of activities against pathogens such as viruses, bacteria, and fungi[10-13].Several articles have described the presence of AMPs, such as human beta-defensin (HBD) and LL-37 on the surface of the human ocular surface (OS) and their expression in corneal infections[14-17].
In recent years, a large number of studies have confirmed the antifungal activity of AMPs in animals[18-21], in corneal epithelial cells[22-23]and in humans[24-25].However, the expression of AMPs was divergent, and the response profile of human AMPs to fungal infections has not been completely elucidated.We therefore analyzed gene expression of human AMPs common in corneal specimens during active fungal infection and after healing cases.At the same time,we compared the gene expression of AMPs inFusariumkeratitis andAspergilluskeratitis, two of the most common strains.

Table 1 Demographic data, diagnosis and laboratory results
SUBJECTS AND METHODS
Ethical ApprovalIn accordance with the principles of the Declaration of Helsinki, this comparative and retrospective study was approved by the Ethics Committee of Union Hospital, Tongji Medical College and Huazhong University of Science and Technology (UHCT230035).All patients received informed consent prior to corneal collection.
Inclusion criteria were: 1) active FK with typical clinical symptoms and positive fungal culture; 2) active FK that did not respond to treatment or did not receive any treatment; 3)corneal scar was FK ulcer healing after antifungal treatment.Exclusion criteria were as follows: 1) mixed keratitis or other types of keratitis; 2) eye inflammation not caused by infectious agents; 3) systemic or local steroid therapy; 4) those with known immunosuppression or undergoing immunosuppression therapy.
The experimental group received deep anterior lamellar keratoplasty (DALK) for corneal buttons (CB) in patients with active FK, and the control group received DALK for corneal scar.
Isolation and Reverse Transcription of Total Ribonucleic AcidCorneal tissue was homogenized in an RLT buffer using TissueRuptor (Qiagen, Germany), iced for 60s, total ribonucleic acid (RNA) was isolated and reverse-transcribed.RNeasy mini kit (catalog number 74104; Total RNA was isolated from CB samples using Qiagen, Germany), following the instructions provided by the manufacturer, including the optional DNase step.The isolated RNA was quantified using a biological spectrophotometer (Eppendorf, Germany).Totally 200 nanograms of total RNA was reverse-transcribed (RT)into complementary deoxyribonucleic acid (cDNA) using the Eurogentec reverse transcription core kit (RT-RTCK-03,Eurogentec, Belgium).In addition, no RT-enzyme control samples were available.
Real-Time Quantitative Polymerase Chain ReactionTaqMan probe chemistry was used to analyze the selected AMPs by real-time quantitative polymerase chain reaction (qPCR).The decision to select only AMPs was influenced by the small amounts of RNA that could be extracted from tiny tissue samples.TaqMan tests were repeated for AMPs, subxanthineguanine phosphate ribose transferase and appropriate controls on a 96-well plate equipped with an Mx3005p real-time PCR instrument (Agilent technologies, Milton Keynes, UK).Simply put, the cDNA template is first diluted 1:5 in nucleasefree water.According to the instructions for TaqMan gene expression Master Mixture (Applied Biosystems, Waltham,MA, USA), 20 mL of reaction mixture was prepared per well.The 10 mL of 2 master stock, 1 mL of 20 TaqMan assay, 5 mL of diluted cDNA, and 4 mL of nuclease-free water were added to each reaction mixture.This study used only one template per probe.However, appropriate non-RT controls also include probes for each gene to exclude any genome amplification.
Statistical AnalysisAll aggregated data were expressed as mean±standard deviation (SD).Statistical analysis of qPCR data was performed by GraphPad Prism 8.1 software(GraphPad software, San Diego, CA, USA), and the alpha value was set asP≤0.05.Due to differences in group size, we used the unpaired Welch unequal variancet-test.
RESULTS
DemographicsA total of 12 samples were collected, as shown in Table 1.We examinedn=4 CB specimens from the healed group andn=8 in active FK group.Final diagnosis was based on the positive growth of fungal in cultures from corneal scrapings.In active FK cases, all samples showed well growth on culture,Fusariumsolani(5 of 8, 62.5%) andAspergillus flavus(3 of 8, 37.5%) were identified, respectively.
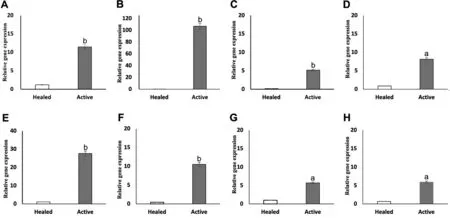
Figure 1 Gene expression of AMPs in corneal tissue from patients with FK Relative fold change of HBD-1 (A), HBD-2 (B), HBD-3 (C), HBD-9 (D), LL-37 (E), S100A7 (F), S100A8 (G), S100A9 (H) in active FK and healed groups.Student’s t-test with Welch’s correction was performed to compare active FK versus healed with P≤0.05 denoting statistical significance.aP≤0.05, bP≤0.01.AMPs: Antimicrobial peptides; FK: Fungal keratitis.
Differential Expression of AMPs Between Active FK and Corneal ScarHere, we reported the expression of several AMPs in patients with FK as determined by qPCR.As shown in Figure 1, AMPs were differentially expressed in patients with FK.HBD-1, -9, S100A8, 9 and LL-37 were shown to be constitutively expressed in all healed samples, whereas mRNAs for HBD-2, -3, S100A7 were expressed at a very low level.
As shown in Figure 1, all AMPs were increased in active FK.HBD-1, -2 -3, S100A7 and LL-37 mRNAs were significantly increased in all active FK samples.HBD-1 was upregulated by 11.49±0.56-fold (P=0.00033), HBD-2 was elevated by 106.78±11.12-fold (P=0.0036), HBD-3 was increased by 5.16±0.56-fold (P=0.0038), S100A7 was increased by 10.50±1.01-fold (P=0.0039) and LL-37 was upregulated by 27.63±3.14-fold (P=0.0046) in active FK compared with controls.
The levels of HBD-9, S100A8 and S100A9 mRNAs were moderately upregulated in all active FK samples.HBD-9 was increased by 8.15±1.18-fold (P=0.011), S100A8 was elevated by 5.70±1.03-fold (P=0.025) and S100A9 mRNA expression was shown to be increased by 5.93±1.44-fold (P=0.027) in active FK samples compared with controls.
Variable Expression of AMPs BetweenFusariumandAspergillusKeratitisAs shown in Figure 2, subgroup comparison shown that HBD-2 mRNAs were significantly elevated inFusariumkeratitis, while LL-37 mRNAs were significantly increased inAspergilluskeratitis samples.HBD-2 was upregulated by 111.79±6.25-fold inFusariumkeratitis compared with 95.91±2.72-fold inAspergilluskeratitis samples (P=0.041).LL-37 was elevated by 29.77±1.28-fold inAspergilluskeratitis compared with 23.38±2.72-fold inFusariumkeratitis samples (P=0.028).
Whereas, there were no significantly increased of HBD-1,-3, -9, S100A7, 8, 9 (P>0.05) mRNA inAspergilluskeratitis compared withFusariumkeratitis samples.
DISCUSSION
FK is a serious public health problem affecting the agricultural poor and therefore requires special attention.High rates of corneal perforation have been reported in cases of FK,although the progression of FK is slower compared to bacterial keratitis[3-5].Due to the limited available treatments, there is an urgent need to study the mechanism of host immune response to fungal infections in order to find new treatments.Compared with existing antibacterial agents, AMPs have unique mode of action, so they have received special attention as a potential treatment for microbial infections[26].As numerous studies over the past two decades have shown, human AMPs already play a crucial role in microbial keratitis[11,15-17,24-26].

Figure 2 Gene expression of AMPs in corneal tissue between patients with Fusarium keratitis samples and Aspergillus keratitis samples Relative fold change of HBD-1 (A), HBD-2 (B), HBD-3 (C), HBD-9 (D), LL-37 (E), S100A7 (F), S100A8 (G), S100A9 (H) in Fusarium keratitis samples and Aspergillus keratitis samples.Student’s t-test with Welch’s correction was performed to compare Fusarium keratitis samples versus Aspergillus keratitis samples with P≤0.05 denoting statistical significance.aP≤0.05.AMPs: Antimicrobial peptides; FK: Fungal keratitis.
In this study, we observed an increased pattern of AMPs(HBD-1, -2, -3, -9, S100A7, 8, 9, and LL-37) expression in corneal specimen during the active phase of infection and healed corneal scar.Notably, all AMPs’ mRNA was found to be at a basal level in healed specimens.The increased levels of AMPs varied in active FK specimens.HBD-1, -2,-3, S100A7, and LL-37 mRNAs were significantly increased,while the levels of HBD-9, S100A8, and S100A9 mRNAs were moderately upregulated in all active FK.Among them,HBD-1, -2, and LL-37 increased in the 3 most significant proportions.Subgroup comparison between the two most common FK, HBD-2 was significantly increased inFusariumkeratitis samples and LL-37 was significantly increased inAspergilluskeratitis samples.The other AMPs were not significantly increased inAspergilluskeratitis compared withFusariumkeratitis.As a result, active FK samples showed an elevated pattern of CAMP and β-defensins expression in the current study.This raised the possibility that the human AMPs,particularly HBD-1, -2, and LL-37, might be key players in FK.Our study, similar to previous studies, confirmed that mRNA levels of the HBD family, class S100A, and LL-37 in human OS specimens were significantly higher during active FK than after healing[24].In response toFusarium spp.[22]andC.albicans[23], corneal epithelial cells also expressed LL-37,hBD-2, and hBD-3 more frequently.In addition, cathelicidin related antimicrobial peptides (CRAMP) and β-defensins have shown variable expression at the onset of disease in an animal model ofC.albicanskeratitis, but they returned to their normal levels by healing on day 7 after infection[21].In a mouseFusariumkeratitis model, the expressions of endogenous mBD-3, -4, -14, and CRAMP were significantly increased on day 3 after infection and returned to baseline levels after ulcer healing[20].Nevertheless, Mallelaet al[25]found that β-defensin expression was decreased and LL-37, S100A12, and RNase 7 expression was significantly increased in corneal scrapes in patients withAspergilluskeratitis.However, the consistency of the results would be affected by the diversity of AMPs, various materials and different samples.We could speculate that the human AMPs, particularly β-defensins might play a significant role inFusariumkeratitis and LL-37 might be the key player inAspergilluskeratitis.
AMPs have been shown to induce adaptive immunity and is an effective chemical attractant[26].Thus, in addition to directly killing microorganisms, increased level of AMPs during FK may potentially increase neutrophil infiltration and other unknown antifungal mechanisms.LL-37 and β-defensins have fungicidal activity againstCandidain vitroby binding to cell wall β-1, 3-exoglucaseviasecreted glycosylated exodomain protease Msb2[27]and reducing their ability to infect the host.The yeast cell membrane is penetrated by human LL-37 and β-defensin, resulting in fungal death[28].Psoriasis protein(S100A7) in linear peptide-reducing form killsAspergillus spp.and induces fungal apoptosis by chelating zinc metals[11].Calprotectin (S100A8/A9) inhibited the growth ofAspergillus spp., inhibited hyphal growth, and exhibited antifungal activity.In a mouse model of FK[29], zinc and manganese transport were prevented by fungi.
The limitation of this study was that it only involved assessment of gene expression of AMPs in active and healed groups.A subsequent study should focus on neutrophil infiltration and understanding the mechanisms of immune activation of AMPs during FK.
In conclusion, our results corroborate that AMPs expression increased in active FK.HBD-2 and LL-37 expression levels were the highest, showing some specificity of AMP expression related to FK.Subgroup comparison showed that HBD-2 mRNAs were significantly elevated inFusariumkeratitis, while LL-37 mRNAs were significantly increased inAspergilluskeratitis samples.We could speculate that the human AMPs, particularly HBD-2 might play a significant role inFusariumkeratitis and LL-37 might be the key player inAspergilluskeratitis.This further implicates a potential pivotal role for AMPs in OS defense against fungal pathogens.
ACKNOWLEDGEMENTS
Authors’ contributions:Data curation, Wang JS and Peng X;Funding acquisition, Xie HT and Zhang MC; Investigation,Wang JS and Zhao Z; Methodology, Wang JS and Wang C;Writing - original draft, Wang JS; Writing - review & editing,Xie HT and Zhang MC.All authors have read and agreed to the published version of the manuscript.
Foundations:Supported by the National Natural Science Foundation of China (No.82171025; No.82070934); the Fundamental Research Funds for the Central Universities(HUST: No.2019kfyXMBZ065); the Key Research and Development Program of Hubei Province (No.2021BCA146);the Clinical Research Foundation of Wuhan Union Hospital(No.2021xhlcyj03).
Conflicts of Interest:Wang JS,None;Peng X,None;Zhao Z,None;Wang C,None;Xie HT,None;Zhang MC,None.
 International Journal of Ophthalmology2023年10期
International Journal of Ophthalmology2023年10期
- International Journal of Ophthalmology的其它文章
- A novel approach for 25-gauge transconjunctival sutureless vitrectomy to evaluate vitreous substitutes in rabbits
- Visual resolution under photopic and mesopic conditions in patients with Sjögren's syndrome
- Effects of obstructive sleep apnea on retinal microvasculature
- Bibliometric analysis of research relating to refractive cataract surgery over a 20-year period: from 2003 to 2022
- Three-dimensional bioprinting in ophthalmic care
- Agreement of intraocular pressure measurement with Corvis ST, non-contact tonometer, and Goldmann applanation tonometer in children with ocular hypertension and related factors
