Effects of inorganic ions,organic particles,blood cells,and cyclic loading on in vitro corrosion of Mg–Al alloys
Gunqi Liu ,Jinmin Hn ,Ying Li ,Yuzhu Guo ,Xiodong Yu ,Shenpo Yun ,Zhihu Nie,Chengwen Tn,*,Chunin Guo
aSchool of Materials Science and Engineering,Beijing Institute of Technology,Beijing 100081,China
b Department of Dental Materials,National Center of Stomatology,National Clinical Research Center for Oral Diseases,National Engineering Laboratory for Digital and Material Technology of Stomatology,Beijing Key Laboratory of Digital Stomatology,Research Center of Engineering and Technology for Computerized Dentistry Ministry of Health,NMPA Key Laboratory for Dental Materials,Peking University School and Hospital of Stomatology,Beijing 100081,China
c Department of Oral and Maxillofacial Surgery,National Center of Stomatology,National Clinical Research Center for Oral Diseases,National Engineering Laboratory for Digital and Material Technology of Stomatology,Beijing Key Laboratory of Digital Stomatology,Research Center of Engineering and Technology for Computerized Dentistry Ministry of Health,NMPA Key Laboratory for Dental Materials,Peking University School and Hospital of Stomatology,Beijing 100081,China
Abstract Recently,magnesium (Mg) alloys have attracted extensive attention as biodegradable implant materials.However,cyclic loading and the corrosive environment of the body are significant challenges for the practical use of alloys,and there are few studies on this topic.In this study,we conducted a four-point bending fatigue test for 86,400 cycles (12 h) in simulated body fluid (SBF),plasma,and whole blood with an AZ series alloy Mg-9Al-0.5Zn-0.27Mn-0.12Ag,to examine the effects of inorganic ions,organic particles,blood cells,and cyclic loading on Mg alloy corrosion.The Mg2+ concentration and solution pH were measured before and after experimentation,and the sample surfaces were characterized by 3D digital microscopy,scanning electron microscopy (SEM),energy-dispersive X-ray spectroscopy (EDS),Fourier-transform infrared (FTIR) spectroscopy,Raman spectroscopy,and X-ray photoelectron spectroscopy (XPS).Our results showed that in the non-loading condition,a porous and weak inorganic product layer (mainly Mg/Ca phosphate and carbonate)formed on the surface of the Mg alloy sample immersed in SBF,which hardly had a protective effect on Mg alloy corrosion.For the samples immersed in plasma,the organic particles promoted the formation of an organic and more compact product layer,which protected the Mg alloy from severe corrosion.For the sample immersed in whole blood,the blood cells affected organic particle deposition on the product layer and thus interfered with the formation of an organic compact product layer,which slightly accelerated the corrosion process.Furthermore,cyclic loading damaged the layer integrity and significantly increased the corrosion rates of all the studied materials compared to the samples not subjected to cyclic loading.Nonetheless,under cyclic loading,blood cells adsorbed on the Mg alloy surfaces,and formed films,which protected the Mg alloy substrate and delayed Mg alloy corrosion.
Keywords: Magnesium alloy;Cyclic loading;Corrosive environment; In vitro;Corrosion behavior.
1.Introduction
Magnesium (Mg) alloys,as biodegradable materials,have attracted much attention recently due to their suitable mechanical properties and biocompatibility [1–6],and numerous studies have been conducted to demonstrate the possibility of Mg alloys as implant materials within bones or blood vessels[7,8].However,the corrosion rate of the Mg-based implant is often too high or unpredictable for clinical applications,as it may harm tissue recovery [9,10].Therefore,a reliable evaluation system must be established for Mg alloys as degradable implants with controllable degradation rates.To establish such an evaluation system,we must first analyze the physiological factors that affect the corrosion of Mg alloys when used as biomaterials.
The human body is a complex environment with different substances,including inorganic ions (such as Na+,K+,Mg2+,Ca2+,Cl-,HCO3-,HPO4-,and SO42-),organic compounds(such as proteins and amino acids),and various cells(such as red and white blood cells).These substances can all affect Mg alloy corrosion.Many studies have investigated the effects of these substances on Mg alloy corrosion in physiological environments.It has been shown that the chloride ion(Cl-) can penetrate the product layer and accelerate Mg alloy corrosion[11–14].Organic particles such as proteins can alter Mg alloy degradation behavior due to the chelating effect or through particle deposition on the Mg alloy surface [15–20].Furthermore,the actions of the above-mentioned substances on Mg alloy corrosion may be time-dependent [21].The cell adhesion on corroding Mg alloy surfaces also needs to be considered.The products that form on Mg alloys during corrosion can also affect cell adhesion on Mg alloy surfaces [22–24].Cell adhesion layers may reduce the Mg alloy corrosion rate[25,26],but the interaction mechanism between the cells and the Mg alloy surfaces has not been clarified.Hence,a direct comparison of the results for various substance groups may not be reliable,as experiments have been conducted using different types of corrosion fluids.
Furthermore,the human body is not only a static liquid environment but also a harsh environment with complex cyclic loading,which will accelerate the corrosion of Mg alloys.For example,Mg alloy bone-fixation screws may be subjected to loading when the patient moves.Likewise,vascular closure devices and stents are subjected to large loads during initial deployment and subsequent contraction of the heartbeat [27].This combined action in a corrosive environment,with cyclic loading,is called corrosion fatigue,which will lead to premature failure of metals by cracking.The final fracture as the result of corrosion fatigue usually occurs in the form of sudden failure,which may have serious consequences,such as secondary fracture in orthopedics and restenosis in cardiovascular diseases[28].Some relevant studies on biomedical Mg and its alloys have been conducted.For example,Gu et al.[29]studied the corrosion fatigue behavior of AZ91D and WE42 Mg alloys in simulated body fluid (SBF) and found that both alloys were susceptible to corrosion fatigue,and pits served as crack initiation sites.This phenomenon was also observed by Jafari et al.[30]in their study on Mg alloys in modified SBF under fully reserved cyclic loading.Harandi and Singh Raman [31]found that bovine serum albumin (BSA) affected the corrosion-fatigue crack propagation of an Mg alloy when it was tested in BSA containing Hanks’ solution using the three-point cyclic bending test.However,there is still a critical knowledge gap regarding the corrosion behavior of Mg alloys from the viewpoint of biomedical applications.For example,no specific or systematic study has been conducted on the effects of cyclic loading and various substances in the body such as inorganic ions,organic particles,and cells,on Mg alloy corrosion.Therefore,a comprehensive study is needed to assess the influence of these factors.
In this study,the Mg alloy corrosion behavior in three corrosive liquids (SBF,plasma,and whole blood) with and without cyclic loading (four-point bend model) was investigated.The design of the three corrosion components allowed us to systematically investigate the respective effects of the inorganic ions,organic components,and blood cells on Mg alloy corrosion.In addition,the coupling effect of cyclic loading on the corrosion process was observed.An AZ series Mg-9Al-0.5Zn-0.27Mn-0.12Ag alloy,which we investigated as a degradable biomaterial in a previous preliminary study,was chosen for this test.In the present study,we found that within our experimental timeframe,the blood cells may affect organic component deposition on the product layer and interfere with the formation of a compact product layer in the non-loading group.Under cyclic loading,the cells may adsorb on the surface and form a protective membrane on the Mg alloy substrate.This study further clarifies the Mg alloy corrosion behavior and mechanism in physiological environments and contributes to establishing anin vitroevaluation method for the degradation of Mg and its alloys.
2.Materials and methods
2.1.Preparation of materials
The elemental composition of the alloy,denoted by Mg-9Al-0.5Zn-0.27Mn-0.12Ag,is presented in Table 1.The wrought alloy was cut into ingots of 30 mm × 110 mm × 70 mm in size,homogenized at 410 °C for 12 h,and aged at 175 °C for 18 h [32–33].Then,the test sample was cut along the extrusion direction and formed into 2 mm × 4 mm × 60 mm samples to fit the test system.

Table 1Chemical composition of the experimental alloy (wt.%).
2.2.Four-point bending corrosive fatigue test and static corrosion test
The experiments consisted of three solutions,with and without four-point bending fatigue testing.The test solutions were: standard SBF,blood plasma,and whole blood.The standard SBF solution was prepared according to Loos et al.,which involved dissolving an appropriate amount of relevant reagent-grade chemicals in deionized water [34].The whole blood was obtained from healthy adult male New Zealand rabbits,and our study protocol was approved by the Animal Care and Use Committee of Peking University.The plasma was obtained by centrifuging the whole blood,twice,at 4000 rpm for 20 min.
The main components of the three solutions are presented in Table 2.The SBF mainly contained inorganic ions,while the plasma contained inorganic ions and multiple organic particles such as proteins (main component),amino acids,glucose,and cholesterol.The whole blood,besides the components in the plasma,also contained red and white blood cells,along with few other various cells.Through a comparison of the three liquids,the effects of inorganic ions,organic matter,and blood cells on Mg alloy corrosion could be determined.

Table 2Content of SBF,plasma,and whole blood [35–37].
The four-point bending fatigue test was conducted using a computer-controlled servo-hydraulic testing machine (Instron 8874,Instron,High Wycombe,Bucks,United Kingdom) with an applied vertical sinusoidal load.The test platform was supported by two stainless steel rollers which were 3 mm in diameter and 20 mm in length and remained in contact with the lower surface of the sample at a distance of 40 mm.The upper surface of the sample was also in contact with the two rollers,separated by a distance of 20 mm.A diagram of the device is displayed in Fig.1a.Before the experiment,the roll surfaces were coated with epoxy resin AB glue to prevent electrochemical reactions with the alloy.To simulate actual service conditions,the cyclic loading of the sample was set to 5–70 N (37.5 N ± 32.5 N),at 2 Hz and 86,400 cycles(12 h).
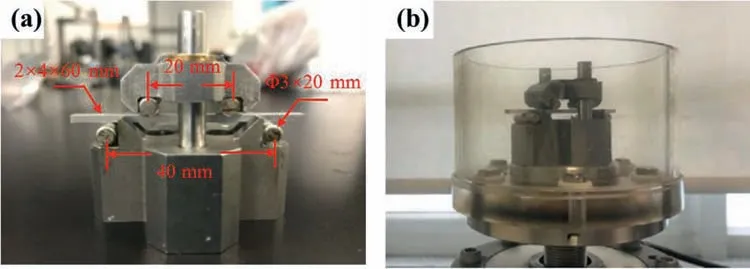
Fig.1.(a) Device setup and diagram of the four-point bending fatigue test,and (b) device placed in a plastic container,ready for the addition of solutions for corrosion fatigue testing.
The device was placed in a plastic container (Fig.1b),and 100 mL of solution was added,as the corrosive environment.In addition,static-immersion tests (non-loading group) were conducted for comparison.The immersion solution volume and duration time of the static-immersion test were identical to the corrosion fatigue test counterparts,which were 100 mL and 12 h,respectively.After testing each group,the samples were washed with absolute ethanol and collected for morphology and composition analysis.
2.3.pH and Mg ion concentration measurements
The pH values of the fluids were measured before and after immersion testing.Mg ion concentrations in the solution were measured after testing via inductively coupled plasma–atomic emission spectrometry (PerkinElmer,Optima 7300DV,Houston,TX,USA),and each fluid was tested three times.
2.4.Morphology analysis of the corrosion product layer
After immersion testing,the overall sample appearance was characterized using a 3D digital microscope (DVM6A,Leica,Wetzlar,Germany)at 100×magnification.Surface and cross-section microstructures were characterized using fieldemission scanning electron microscopy (FE-SEM,QUANTA 200F,FEI,Hillsboro,OR,USA/ HITACHI SU8220,Tokyo,Japan),with an acceleration voltage of 10 kV.The working distance varied between 10 and 12 mm.
2.5.Composition analysis of the corrosion product layer
The surface and cross-section compositions of the corrosion product layer were analyzed using an energy-dispersive X-ray spectrometer and a Bruker FlatQuad X-ray spectrometer.Furthermore,the deposited matter on the sample surfaces was characterized via Fourier-transform infrared (FTIR)spectroscopy,Raman spectroscopy,and X-ray photoelectron spectroscopy (XPS).
FTIR was conducted using an infrared spectrometer (Nicolet 170SX,Madison,WI,USA),with a diamond reference crystal for the immersion parameters.Measurements were conducted in a wavenumber range of 1200–4000 cm-1,with a penetration depth of 2 μm.Raman spectra between 400 and 1800 cm-1were collected using a laser confocal Raman microscope (Labor Raman series,HR-800,Jobin Yvon,Paris,France).Raman scattering was obtained by exciting the sample with 785 nm radiation from a diode laser,and a 50 ×objective lens was used to collect the Raman data.In addition,X-ray photoelectron spectroscopy (XPS,PHI QUANTERA-II SXM,ULVAC-PHI,Kanagawa,Japan) was used to characterize the surface chemical states of the samples after immersion.The XPS spectra were recorded using Al Kαradiation(1486.6 eV) as the excitation source.The binding energy of the C1s signal was used for spectral charge correction.Background subtraction was carried out using the Shirley method for all spectra.For each immersion parameter,a survey spectrum and high-resolution spectra for C1s,O1s,N1s,Mg2p,Ca2p,and P2p signals were obtained,and the C1s narrow scans were fitted using MultiPak Software (Gaussian multipeak fitting).
3.Results and discussions
3.1.pH and Mg2+ concentration of solutions before/after immersion test
Fig.2a shows the variations of the pH value in different immersion tests.Before immersion testing,the pH values of the three solutions were similar:~7.40 for SBF and~7.21 for plasma and whole blood.After static-immersion testing for 12 h,the pH values increased to~8.44 for SBF and~9.38 for plasma.However,the pH of the whole blood group increased to~7.63,indicating that this group had the smallest pH increase.In plasma,the serum albumin has a region that strongly binds to mono-or bi-valent ions,such as Ag+,Zn2+,Ca2+,and Mg2+[38].The Ca2+and Mg2+ions share some of the same binding sites and exhibit competitive reactions.While Ca2+binds better at basic pH conditions,and the number of Mg ions bounds increases as pH increases [38].Since Mg degradation releases OH-,which increases pH,the Mg2+is taken out of the "normal" chemical reaction cycle due to the above-mentioned binding effect,and the electrochemical corrosion of magnesium will be promoted.This will release more OH-into the solution,which also results in higher pH values.For the whole blood,the increase in pH was not significant,which was due to the buffer effect of the blood cells[39].Because blood cell metabolism produces acid,the generated H+products combined with the OH-products produced by Mg alloy corrosion,thus the pH value of the solution decreased.Furthermore,cyclic loading intensified the increase in pH value.The discrepancy in pH values between the three solutions also increased (~10.12 for SBF,~10.99 for plasma,and~9.48 for whole blood),as cyclic loading accelerated the corrosion process.
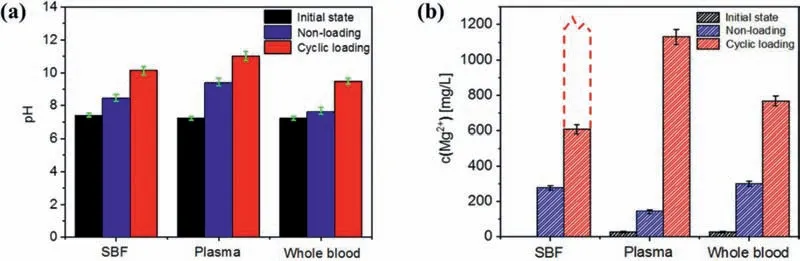
Fig.2.(a) pH values and (b) detected Mg ion (Mg2+) concentration values after 12 h immersion testing: non-loading group,cyclic loading group after 86,400 cycles and 2 Hz.
The Mg ion concentrations of the solutions before and after immersion testing are illustrated in Fig.2b.The initial Mg ion concentration was nearly 0 mg/L in SBF and~27.0 mg/L in plasma and whole blood.In the non-loading group,the detected Mg ion concentration in plasma (~144 mg/L) was lower than that in SBF (~280 mg/L),as the binding effect of the serum albumin with Mg ions resulted in a decrease in dissociative Mg ions.In addition,the product layer that formed in plasma was possibly more protective than the one that formed in SBF,which would slow down Mg ion release during corrosion.Mg ion concentrations were the highest in whole blood (~301 mg/L),as the cells possibly interfered with corrosion product formation,thus more Mg ions were released from the Mg alloy matrix.Furthermore,Mg ions from the cells were also likely present in the solutions.The Mg ion concentration in the cyclic-loading group was much higher than that in the non-loading group.This was attributed to cyclic loading,which damaged the product layer,exposing the Mg alloy substrate and releasing more Mg ions into the solution.In addition,the Mg ion concentration in whole blood(~766 mg/L) was lower than that in plasma (~1133 mg/L),which was because a more protective product layer formed that hindered Mg ion release.The detected Mg ion concentration in the SBF (~608 mg/L) was possibly lower than the actual released free Mg ions as a result of corrosion.In this group,some products which were bound to the released Mg ions may form during corrosion.They were likely filtered out from the solution during Mg ion concentration detection.Thus,the expected Mg ion concentration value was possibly higher.
3.2.Surface morphology and composition of product layer
The morphologies of the samples after immersion testing are shown in Fig.3,and the difference between the peak and valley heights (donated as roughness) was detected.Before testing,the surface roughness was about 12.65 μm(not shown in the figure).After immersion in the solution,the roughness increased.Fig.3a–c present the morphologies of the nonloading group samples.The sample immersed in SBF showed pitting corrosion,and the roughness reached~99.07 μm.The sample immersed in plasma exhibited a more uniform corrosion surface,and the roughness was~26.25 μm.This was possibly due to the formation of a more protective layer,which was induced by organic matter.The roughness of the sample immersed in whole blood (~98.88 μm) was similar to that of the sample immersed in SBF.This may be due to the interference of the cells with alloy corrosion.

Fig.3.Surface morphologies of different samples after 12 h immersion testing in SBF,plasma,and whole blood without cyclic loading (a,b,and c,respectively),and with cyclic loading for 86,400 cycles (d,e,and f,respectively).The value at the top of each figure is the maximum height.
Fig.3d–f shows the roughness of the samples in the cyclic-loading group.The roughness values were higher than those in the non-loading group,as cyclic loading promoted corrosion.The roughness values of the samples in SBF(~906.0 μm) and plasma (~244.7 μm) under cyclic loading were approximately nine times higher than those of the samples under the non-loading condition.However,the roughness of the sample in whole blood under cyclic loading(~289.1 μm) was only approximately three times higher than that of the sample without cyclic loading.The smaller increase in roughness in this sample was due to the protective effects of cell adsorption on the sample surface.
Fig.4a–c present the SEM images of the sample surfaces after immersion in the three solutions without cyclic loading.Overall corrosion with some residual Mg alloy corrosion products occurred on the surfaces of all three samples.Furthermore,local pitting corrosion occurred on the surface of the SBF immersion sample (Fig.4a).Studies have shown that local pitting is caused by chloride ions [40,41],which can penetrate the corrosion layer and cause further corrosion of the substrate.The sample immersed in plasma exhibited a uniform corrosion surface,with micro-cracks along the grain boundaries,which were due to the anodic dissolution of the Mg matrix by electrochemical corrosion.The sample immersed in whole blood showed an indistinct morphology of micro-cracks on the surface,which was due to the contact of the cells in the blood.However,no cellular membrane was observed on the sample surface.
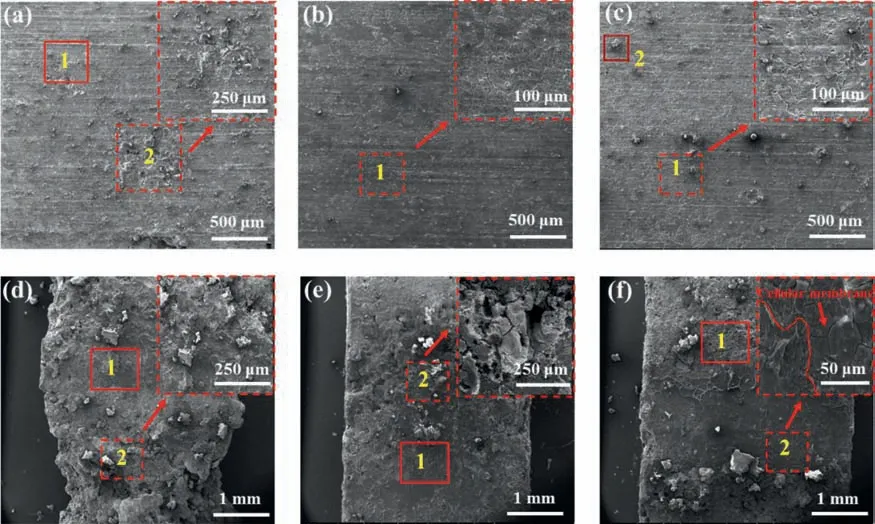
Fig.4.SEM surface images of the surface of samples after 12 h immersion in SBF,plasma,and whole blood without cyclic loading (a,b,and c,respectively)and with cyclic loading for 86,400 cycles (d,e,and f,respectively).
The surface morphologies of the ravines and their surroundings areas of the samples subjected to cyclic loading are shown in Fig.4d–f.The resulting ravines were 1–2 mm and formed next to the contact site between the sample and the roll bar,as these sections were the most affected areas and subjected to both stress and corrosion factors.Under cyclic loading,the alloys corroded more quickly,as the Mg alloy corrosion product became larger in both quantity and size.The sample immersed in SBF corroded the most and contained a ravine that was~2 μm wide,which was twice as wide as the other two samples.The sample immersed in plasma displayed less corrosion than the sample in SBF,which could be due to the protective effects of the proteins or other organic substances.The ravine of the sample immersed in whole blood had a smoother morphology,possibly because of cell adhesion on the surface.Area 2 in Fig.4f shows that the sample surface from the whole blood cyclic loading group contained a cellular membrane.
Table 3 presents the chemical compositions of the general corrosion products that formed on the sample surfaces after immersion testing,and the test areas are marked in Fig.4.For all samples,the predominant surface compositions were O and Mg,suggesting that numerous Mg oxides were deposited on the sample surfaces.
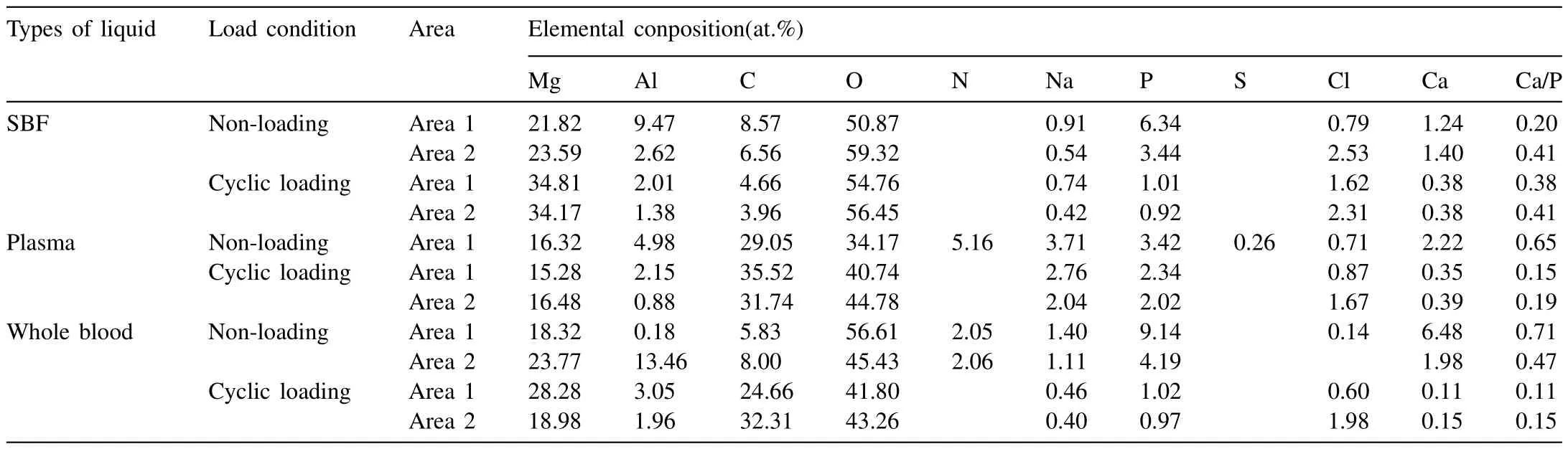
Table 3Chemical compositions of the corrosion products formed on sample surfaces after immersion testing (at.%).
The sample immersed in SBF without cyclic loading featured more Cl content in the pitting (area 2) than in the bulk(area 1),which proved that Cl-caused pitting corrosion.The increase in O content in the pits was due to the formation of more oxygenated compounds.The occurrence of Ca and P demonstrated the formation of calcium phosphates on the alloy surface.This assumption has been reported by multiple studies,which described the formation of calcium phosphates on Mg surfaces after immersion in SBF or other solutions supporting cell cultures [42–45].The Ca/P ratio was calculated for each area to estimate the calcium phosphate formation process.Typically,a Ca/P atomic ratio can be used to determine the mature degree of a particular calcium phosphate phase,where anhydrous calcium phosphate (CaHPO4) and calcium phosphate dehydrate (CaHPO4·2H2O)=1.0 [46],octacalcium phosphate (Ca8H2(PH4)6)·5H2O=1.33 [47],tricalcium phosphate (Ca3(PO4)2)=1.5,and hydroxyapatite(Ca10(PO4)6(OH)2,main component of mature bone)=1.67[48].The Ca/P ratios in areas 1 and 2 of the sample immersed in SBF (Fig.5a) were approximately 0.20 and 0.41,respectively.These values were less than half of the ratios corresponding to hydroxyapatite,indicating that the calcium phosphates were in the initial stages of formation.

Fig.5.(a) Raman and (b) FTIR spectra of the sample surfaces after 12 h immersion in SBF,plasma,and whole blood without cyclic loading.
The surface of the sample immersed in plasma exhibited lower O content than the sample immersed in SBF,however the C content on the plasma immersion sample was over three times higher than the SBF immersion sample surface.Moreover,N and S were found on the surface of the sample immersed in plasma.These results indicated that proteins or other organic matter were deposited on the sample surface.The Ca/P was 0.65 on the sample surface in plasma,which was higher than the sample immersed in SBF,indicating the further formation of calcium phosphate compounds.
Area 1 of the sample immersed in whole blood (Fig.4c)displayed higher Ca,P,and O content than the other samples,and the Ca/P ratio was 0.71,which indicated the further accumulation of calcium phosphates on the surface layer.Another interesting phenomenon was that the C content on the whole blood sample surface was not as high as the plasma sample,thus indicating that blood cells affected the organic matter deposition on the product layer.Furthermore,the Al content on the surface (area 2 in Fig.4c) rose to 13.46%,suggesting that more Al-containing compounds,such as hydrotalcite-like compounds (HTs) [49–50],possibly developed on the sample surface.
Moreover,in the whole blood sample,the surface exhibited significantly higher C content in the cyclic loading group than in the group without cycling loading.As a result,we concluded that more organic matter was deposited on the sample surface.In addition,for all the samples immersed in the three solutions with cyclic loading,the O amount in the ravines(area 2) was slightly higher compared to areas farther away(area 1).This was because cyclic loading intensified the corrosion process,resulting in the formation of more oxides on the Mg alloy.
Raman and FTIR spectroscopy measurements were conducted to analyze the bonding types of the samples immersed in the three solutions.Because the results of the cyclic-loading and the non-loading groups were similar,we chose the nonloading group to assess the bonding types.Fig.5a shows the Raman spectra of the samples immersed in the three solutions.The baseline correction was performed via piecewise polynomial fitting combined with the Savitzky-Golay smoothing method [51,52].As shown in Fig.5,a peak at 417 cm-1was present in the Raman spectra of all three samples,which was associated with the PO4shift [53]owing to the formation of phosphates.The peak at 456 cm-1in the spectra of the plasma sample group corresponded to cholesterol [54].The sample immersed in whole blood showed the presence of leucocytes on the surface,as indicated by peaks at 500,1376,and 1571 cm-1[55],and protein components,as indicated by peaks at 560,742,828,938,1003,1125,1311,and 1466 cm-1[56,57],and the peak at 1671 cm-1was associated with cholesterol [58].The Raman spectra peaks of the whole blood sample were almost entirely derived from the vibrations of hemoglobin (Hb) [59,60].Since all blood samples contain Hb,the number and location of peaks should be consistent,regardless of whether the spectra belonged to blood from humans or animals,provided that the donor was healthy.Thus,this result has served as a reference for human clinical research.
Samples from the non-loading group were also analyzed via FTIR spectroscopy,and the results are shown in Fig.5b.The band positions of all three spectra were nearly identical,and only differences were in proportion.The results of the peak positions showed a broad and strong absorption peak that appeared at 3400–3200 cm-1,which corresponded to the stretching vibration absorption peak of O–H.This peak suggested the presence of H2O molecules or CH2COOH,which remained embedded in the oxidized surface.The peak at 2960–2830 cm-1was attributed to the stretching vibrations of the C–H groups from the organic products or due to atmospheric contamination [61].The peak at 1900–1650 cm-1was due to C=O stretching vibrations,which was often the strongest absorption signals in the FTIR spectrum.The C=O region was assigned to organic components such as amide groups and acids that deposited on the sample surface [44],and the band at 1400–1300 cm-1and the weak peak at approximately 850 cm-1were assigned to the asymmetrical stretching mode and out-of-plane bending of CO32-[62].Due to the high concentration of Mg2+near the sample surfaces,MgCO3·3H2O or more complex carbonates possibly formed on the surfaces [38].Two peaks were observed in the 1400–1300 cm-1wavenumber range,which confirmed that the collection area was comprised of crystalline and amorphous fractions.The peak at 1070 cm-1was attributed to the PO3symmetrical expansion in HPO42-,which indicated the formation of the anhydrous calcium phosphate (CaHPO4)and calcium phosphate dehydrate (CaHPO4·2H2O).In addition,the peak at approximately 600 cm-1corresponded to the out-of-plane bending of C–OH with alcohol,suggesting that the remaining ethanol was embedded on the sample surface.The spectra of the three samples also showed a weak band around 420 cm-1,which corresponded well to the wavenumber of PO4bending,indicating the presence of phosphates.This peak was probably due to the hydroxyapatite precursor Ca5(PO4)3(OH),which is the main mineral component of human bones and teeth.This also demonstrated that Mg-9Al-0.5Zn-0.27Mn-0.12Ag can promote osteogenesis (mineralization).Considering the increase in Mg2+concentration near the sample surfaces,and the lowkspof magnesium and calcium phosphate,the MgxCay(PO4)zcompound could also form on the surface [38].Considering the proportion of bond types,from the FTIR spectra,the sample immersed in the plasma exhibited the highest C=O quantity,which demonstrated the presence of more amide groups and acid deposited on the sample surface.However,the sample immersed in whole blood displayed a weaker C=O peak than the sample immersed in plasma.This also verified that the blood cells interfered with organic substance deposition on the alloy.
The XPS survey spectra of the samples after immersion in different solutions with and without cyclic loading are presented in Fig.6.The data were corrected according to the C 1s spectrum at 284.8 eV,and the spectral results showed that Mg,O,and C were the main contents of the six samples.Peaks corresponding to Al 2p,N 1s,Ca 2p,P 2p,Na 1s,and Cl 2s were also detected.The Na 1s and Cl 2s were attributed to the adhesion of Na2+and Cl-from the solution.Furthermore,the presence of the N1s element confirmed the formation of protein-containing species on the samples.In the static-immersion group,the proportion of N 1s in SBF was too low to be detected,while the N 1s proportion in the plasma group was the highest,followed by the whole blood group.This once again confirmed that blood cells may affect the deposition of organic particles,such as proteins,on the sample surface and disrupt the formation of the organic layer.Under cyclic loading,the stress damaged product layer on the sample surfaces,and as a result Mg KLL proportions in the three solutions were more than those without cyclic loading,while C 1s,N 1s,Ca 2p,and P 2p proportions were opposite.In addition,the N 1s proportion in the whole blood was slightly higher than in the plasma.Thus,we concluded that under cyclic loading,more protein-containing substances were deposited on the sample surfaces.
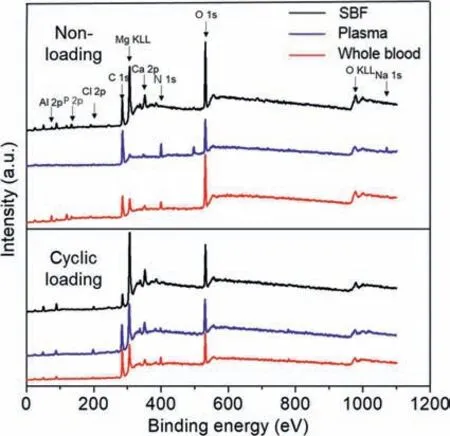
Fig.6.X-ray photoelectron spectra of the sample surfaces (away from ravines) after 12 h immersion in SBF,plasma,and whole blood with cyclic loading (86,400 cycles) and without cyclic loading.
Fig.7 shows the high-resolution spectra and peak fitting of C 1s for the six parameters.All of the sample surfaces were assigned to the peak at 284.8 eV,which was attributed to atmospheric contamination (C–H) or the carboxyl or carbonate groups (C–C) [63].The C–O/C–N peak was assigned to the protein backbone,and the O=C–N peak was assigned to the peptide bonds in the proteins.The O=C–O or C=O groups likely originated from CO32-,which can form Mg carbonate on the sample surfaces [64–66].The bonds of the samples varied according to the immersion solution and the presence/absence of cyclic loading.Given the more complicated compositions of SBF,plasma,and whole blood,the deposition products on the sample surfaces were more complex.The peak relative intensity represented the relative content.In the non-loading group,the peak intensity proportions of protein-related bonds such as C–O/C–N and O=C–N in the spectrum of the sample immersed in whole blood were lower than the spectrum of the plasma immersed sample.In the cyclic-loading group,the situation was reversed.Thus,these results were consistent with the above-mentioned results and confirmed the different behaviors of blood cells with and without cyclic loading.

Fig.7.High-resolution C 1s X-ray photoelectron spectra of the alloy surface after 12 h immersion in SBF,plasma,and whole blood without cyclic loading(a,b,and c,respectively) and with cyclic loading for 86,400 cycles (d,e,and f,respectively).
3.3.Cross-sectional morphology and composition of product layer
The cross-section mappings of the six samples are shown in Fig.8.In the non-loading group(Fig.8a–c),the sample immersed in SBF showed corrosion pits with a depth of approximately 500 μm.By contrast,the samples immersed in plasma and whole blood showed uniform corrosion,with depths of 10–20 μm for plasma and 20–30 μm for whole blood.Cavities were also detected between the product layer and the Mg alloy substrate in plasma and whole blood immersed samples.These cavities formed during immersion,when H2gas was produced on the surface and rapidly dissolved,leading to a dynamic interface,which may have directly hindered the formation of the cell/organic content–surface bonds [67].The EDS mapping results showed the deposition of Ca and P on the three sample surfaces.In the whole blood samples,Al and O accumulated on the corrosion layer,demonstrating that the cell interfered with the formation of the compact corrosion layer,allowing the Mg–Al alloy substrate to corrode.In the cyclic-loading group (Fig.8d–f),ravines formed on the surface,while the depths of the ravines on the samples immersed in SBF,plasma,and whole blood were approximately 800 μm,200–300 μm,and 150–250 μm,respectively.Furthermore,Ca and P deposition was disturbed (except for the whole blood sample),and Al,O,and Mg distributions were more dispersed than those in the non-loading group.Thus,we concluded that the cyclic loading destroyed the integrity of the product layer and accelerate the corrosion process.The sample immersed in whole blood exhibited less severe damage than the sample immersed in plasma.This is because the cells may form a film on the samples,providing secondary protection in addition to the organic product layer,thus preventing further corrosion of the Mg alloy substrate.
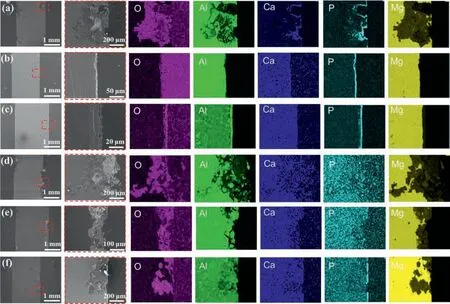
Fig.8.Energy-dispersive X-ray mapping data of the cross-sections of corrosion products after immersion testing for 12 h in SBF,plasma,and whole blood without cyclic loading (a,b,and c,respectively) and with cyclic loading for 86,400 cycles (d,e,and f,respectively).
We also conducted line scans of the cross-sections of the six samples,as shown in Fig.9,and found that the Mg/Al ratio in all samples was relatively stable,suggesting that the Mg–Al compound [Mg0.833Al0.167(OH)2](CO3)0.083·0.75H2O may form on the surface.In addition,this compound has also been found in Mg–Al alloy corrosion in alkaline environments with a sufficient amount of HCO3-[68].Moreover,we also found that Ca and P content was higher on the outer surface of the product layer,especially in the non-loading group.These results were consistent with Fig.8 and have also been reported by other studies [69–72].

Fig.9.Energy-dispersive X-ray line scan data of the cross-sections of the corrosion products after 12 h of immersion testing in SBF,plasma,and whole blood without cyclic loading (a,b,and c,respectively) and with cyclic loading for 86,400 cycles (d,e,and f,respectively).
3.4.Summary of Mg alloy corrosion in vitro
We conducted this study mainly to obtain detailed information on corrosion product layers.A comprehensive understanding of the growth of corrosion layers can clarify the corrosion behavior of Mg alloys under different conditions.According to the above results,we can summarize the corrosion behavior of Mg–Al alloys under the various conditions assessed in this study,and a corrosion behavior diagram is presented in Fig.10.

Fig.10.Schematic depicting the corrosion behavior of the Mg alloy in SBF,plasma,and whole blood without cyclic loading (a,b,and c,respectively) and with cyclic loading (d,e,and f,respectively).
After the Mg alloys were immersed in the various aqueous solutions,galvanic corrosion between the Mg matrix and the precipitates proceeded,following the reaction:
As corrosion progressed,the pH value increased near the Mg alloy surface,and numerous corrosion products formed on the sample surface.
In the non-loading condition,in SBF,an inorganic product layer formed on the Mg alloy surface,and the main compositions possibly consisted of [Mg0.833Al0.167(OH)2](CO3)0.083·0.75H2O,MgxCay(PO4)z,CaHPO4or CaHPO4·2H2O,and MgCO3·3H2O or more complex carbonates [35,49,50,68].The inorganic product layer was weak and pits easily formed on the surface due to the presence of aggressive ions (mainly Cl-),which resulted in the rapid corrosion of the Mg alloy substrates.Inorganic ions and organic particles(such as amino acids,protein,and glucose) exist simultaneously in plasma.There are some controversies in the literature about the effects of organic particles on Mg alloy corrosion.Wagener et al.[73]found that BSA can either inhibit or accelerate metal corrosion,and the different adsorption behaviors may be due to the various electrostatic interactions between the charged organic particles and the metal oxide/hydroxide product layers of the different surface charges.In addition,the pH value may affect the interactions and enhance or diminish protein adsorption.Under the conditions assessed in this study,specifically in the alkaline environment,the presence of organic particles on the sample surface may result in the product layer becoming denser and more compact,thus preventing aggressive ions from entering the Mg alloy substrate.This may occur as a result of the organic particles,which can increase the density of the product layer,as the organic particles can bond with the oxygen atoms through acid/base interactions or other inorganic ions such as Ca2+and PO43-during the hydroxyapatite mineralization process,as shown by other researchers [74,75].In the whole blood condition,the situation is even more complicated.The blood cells may hinder the deposition of organic particles and hence interfere with product layer formation on the sample surface,resulting in a faster corrosion rate than the sample immersed in plasma.
After cyclic loading,the areas near the contact points had the largest stress concentrations and were the main crack initiation points.As corrosion progressed,the surface product layer was damaged,and galvanic corrosion occurred between the matrix and the precipitate phase,which exposed the Mg alloy substrate (serving as the anode) and the product layer (serving as the cathode),forming another galvanic cell,which accelerated the Mg alloy degradation process.Therefore,during the electrochemical reaction,more cathodes generated more H2,and H2diffused rapidly through dislocation migration and was easily enriched at defect sites such as dislocations,grains,and phase boundaries.This process increased the brittleness of these defects,and fatigue crack nucleation occurred more easily.In this four-point bending model,the origin of the crack eventually expanded into a ravine.
Under cyclic loading,the reactions of various substances in the solution were also more intense.The corrosive ion effect in SBF was significant,and the ravines were deep.By contrast,in the plasma condition,the organic particles protected the matrix from corrosion and the ravine was shallower.In the whole blood condition,the cells formed an adhesive membrane on the product layer,which hindered further corrosion of the Mg alloy substrate.
Both substance exchange and pH on the Mg alloy surfaces were highly dynamic and may have changed dramatically during the experiment.Magnesium alloys are very different from highly corrosion-resistant biomaterials,such as Ti alloys,whose surface morphology and chemical composition change very little during corrosion experiments.Therefore,in future studies,long-term and continuous-monitoring corrosion experiments need to be conducted.
4.Conclusions
This study investigated the effects of inorganic ions,organic particles,blood cells,and cyclic loading on Mg alloy degradationin vitrousing four-point bending and static immersion tests in different corrosive environments (SBF,plasma,and whole blood).From our results,the following fundamental conclusions can be drawn:
(1) Inorganic ions may promote the galvanic corrosion of Mg alloy and form a porous and weak layer (mainly Mg/Ca phosphate and carbonate) on the surface.This layer can hardly protect the Mg matrix,thus the corrosion rate was high.
(2) Organic substances,such as proteins,will deposit on the sample surface and contribute to the formation of a denser and more protective organic product layer,which could decrease the corrosion rate of Mg alloys.
(3) Cells behave differently depending on the application of cyclic loading.Under static-immersion conditions,cells may interfere with the product layer formation process and accelerate corrosion.However,under cyclic loading,cells can form an adhesive membrane on the sample surface to protect the Mg alloy matrix from severe corrosion.
(4) Cyclic loading destroys the integrity of the product layer and accelerates the alloy corrosion process.
Declaration of competing interest
The authors declare no competing financial interests.
Acknowledgments
This work is financially supported by the National Natural Science Foundation of China (Grant No.81771119),and the National Key Research and Development Project (Governmental International S&T Innovation Cooperation Projects,Grant No.2019YFE0101100).The authors thank LetPub(www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- “Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
