Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
Bo Hu ,Wen-Jie Zhu ,Zi-Xin Li ,Seul Bi Lee ,De-Jing Li,c,* ,Xio-Qin Zeng,c ,Yoon Suk Choi,**
aNational Engineering Research Center of Light Alloy Net Forming,School of Materials Science and Engineering,Shanghai Jiao Tong University,Shanghai 200240,China
b School of Materials Science and Engineering,Pusan National University,Busan 46241,Republic of Korea
c The State Key Laboratory of Metal Matrix Composites,Shanghai Jiao Tong University,800 Dongchuan Road,Shanghai 200240,China
Abstract The effect of Ce content (0–1.6 wt.%) on the modification of Mg2Si phase in the as-cast Mg-5Al-2Si alloy was investigated.The original Chinese script type Mg2Si phase was refined distinctly and transformed to dispersive block shape gradually by adding Ce element.The length of Chinese script type Mg2Si phase was reduced from 110 to 50 μm with increasing Ce content to 1.6 wt.%.The results calculated by Pandat software indicated that the added Ce element first combined with Si to form CeSi2 phase,which could serve as the heterogeneous nucleation of Mg2Si phase due to the small lattice mismatch of 7.97%.The modification of Mg2Si phase was mainly attributed to the facts that Ce changed the growth steps of Mg2Si phase and CeSi2 promoted the nucleation of Mg2Si phase.With increasing Ce content from 0 wt.% to 1.6 wt.%,the YS,UTS and EL at 150 °C were improved from 67.7 MPa,91.2 MPa and 1.6% to 84.2 MPa,128 MPa and 7.5%,respectively.
Keywords: Mg-Al-Si alloys;Mg2Si modification;Heterogeneous nucleation;Mechanical properties.
1.Introduction
As the lightest structural alloy,magnesium alloy has great potential for application in automotive and aerospace industries,owing to its low density,high specific strength and good castability [1–8].However,the relatively inferior elevatedtemperature strength and elongation of magnesium alloys constrain further application,compared with other commercial aluminum alloys [9–15].In recent years,researches have shown that due to the presence of thermally stable intermetallic Mg2Si phase,the Mg-Al-Si series alloys were developed as heat resistant alloys used at elevated temperatures.Mg2Si phase has high melting point,low density,high hardness,high elastic modulus and low thermal expansion coefficient [16–18].However,the undesirable,large Chinese script type Mg2Si phase is easily formed in Mg-Al-Si alloys,which would deteriorate the mechanical properties of the alloys.Therefore,the modification of Mg2Si is critical to improve the mechanical properties of Mg-Al-Si alloys [16–18].
Many researches have been carried out to investigate the modification of Mg2Si phase in Mg-Al-Si alloys by adding alloying elements.It has been reported that the Mg2Si phase in Mg-Al-Si alloys could be modified by Sr,Sb,Ca,P,Zn and RE elements [19–34].Zhang et al.[17]found that Zn could reduce the onset crystallizing temperature and increase the undercooling in Mg-Si alloys,which restricted the grain growth and promoted the nucleation of Mg2Si phase.Tang et al.[25]reported that Sr could refine Mg2Si in the Mg-4Si-2.7Al alloy.Al4Sr can theoretically act as the heterogeneous nucleus for Mg2Si due to the small lattice mismatch;beside,Sr can be adsorbed on the Mg2Si crystal plane,affecting the surface energy of Mg2Si.Ye et al.[30]investigated the effect of Gd on the modification of primary Mg2Si in the Mg-3Si alloy,the results indicated that the morphology of the primary Mg2Si was changed from coarse dendrite into fine polygon,and the size was significantly decreased.Hu et al.[31]found that Nd element could also modify the primary Mg2Si in the Mg-3Si alloy,but the primary Mg2Si become coarser again when the Nd content exceeded 3.0 wt.%.Adding RE elements is an effective way to improve the mechanical properties of Mg-Al-Si alloys,because RE elements can promote the microstructure refinement and improve the stability of an alloy at elevated temperatures.
The effect of RE elements on the modification of the main strengthening phase in Mg-Al-Si alloys,Chinese script type eutectic Mg2Si,is still to be studied.The calculations of Han et al.[35]indicated that Ce element was surface-active and could be easily absorbed onto the {100} and {111} planes of the Mg2Si crystal,thereby reducing the surface energy of Mg2Si and changing the growth steps of Mg2Si,which was called the poisoning effect.In addition,Ce element can react with Si to form CeSi2intermetallic compound before forming Mg2Si,which may promote the heterogeneous nucleation of the Mg2Si phase,further refining the microstructure of the alloys.However,excessive Ce will lead to the loss of Si content in the Mg-Al-Si alloys,which in turn reduces the fraction of the main strengthening phase Mg2Si.Therefore,adding suitable Ce content will be an effective method to modify the Chinese script type Mg2Si phase in the Mg-Al-Si alloys.
In this work,efforts have been made to investigate the effect of Ce content (0,0.4,0.8 and 1.6 wt.%) on the Mg2Si modification and mechanical properties of the Mg-5Al-2Si(AS52) alloy.Combining with the evolution of microstructure,the phase diagrams and solidification paths calculated by Pandat software based on Scheil’s model [36]were used to analyze the mechanism of Mg2Si modification.
2.Experimental procedure
Commercial high-purity Mg (99.99%),Al (99.99%),Mg-10Si (99.99%) and Mg-90Ce (99.99%) were used to prepare the Mg-5Al-2Si-xCe (x=0,0.4,0.8,1.6 wt.%) alloy ingots.Pure magnesium was melted under a protective gas (mixture of CO2and SF6)in a steel crucible coated with the mixture of sodium silicate and chalky powder.The Mg-10Si master alloy and pure aluminum were added into the melt at 680 °C.The melt was held at 780 °C for 20~30 min after adding the Mg-90Ce master alloy into it at 750 °C.Then the melt was stirred for 10 min at 740°C and held at 750°C for 20~30 min to ensure homogenization.Finally,it was poured into a preheated(200 °C) steel mold at 750 °C,and cooled naturally to room temperature to prepare as-cast Mg-5Al-2Si-xCe alloy ingots.The chemical compositions of the specimens were determined by inductively coupled plasma atomic emission spectroscopy(ICP-AES) and the results were listed in Table 1.
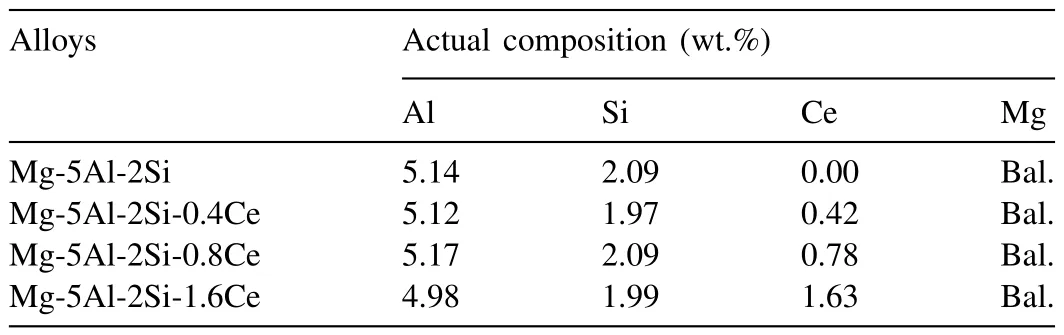
Table 1Chemical compositions of the experimental alloys.
Metallographic samples were cut from the middle segment of the alloy ingots.The specimens were etched using Aceticpicral reagent (5 mL acetic acid,6 g picric acid,10 mL H2O,and 100 mL ethanol) after grinding and polishing.The microstructure was characterized using ZEISS optical microscope (OM),electron probe micro-analyzer (EPMA-1600,JEOL,Japan) and MIEA field emission scanning electron microscope(FE-SEM)equipped with an energy dispersive X-ray spectroscopy(EDS).The sizes of phases were evaluated based on 10 OM images for each alloy using Image-Pro-Plus software.Phase identification was conducted by Rigaku X-ray diffraction (XRD),which was performed using monochromatic Cu Kα1 radiation at 40 kV and 40 mA.The hardness of the sample was characterized by the Vickers hardness tester(XHVT-10Z)with a loading force of 5 kg and a loading time of 20 s.The hardness value of each alloy was an average of 10 individual measurements.The tensile samples (54.5 mm × 15.0 mm × 2.0 mm) cut from the middle segment of alloy ingotswere tested on a tensiletesting machine (Zwick Z100) under astrainrate of 1 × 10-4s-1at 150 °C.The 0.2% yield strength (YS),ultimate tensile strength (UTS) and the fracture elongation (EL) were obtained based on the average of 5 tests.The tensile fracture morphology was observed by FE-SEM.
3.Results
3.1.Phase diagram of Mg-5Al-2Si-xCe alloys calculated by Pandat software
Fig.1 shows the phase diagrams of Mg-5Al-xSi and Mg-5Al-2Si-xCe alloy systems calculated by Pandat software.The phase diagram of the Mg-5Al-xSi alloy system can be considered as a pseudo-binary eutectic system that varies with Si content.It indicates that the Mg-5Al-2Si alloy can be regarded as a pseudo-binary hypereutectic alloy.While cooling,the primary Mg2Si is generated from the liquid first.When the composition of the liquid phase reaches the binary eutectic point,the eutectic Mg+Mg2Si structure is formed from the liquid until the Si element is completely consumed.Since the maximum solid solubility of Al element in the Mg matrix is 12.7 wt.%,which is greater than 5 wt.%,the formation of Mg-Al intermetallic compound during solidification cannot be seen from the equilibrium phase diagram.

Fig.1.Phase diagrams of the (a) Mg-5Al-xSi alloy system and (b) Mg-5Al-2Si-xCe alloy system calculated by Pandat software.
As shown in Fig.1(b),the phase diagram of the Mg-5Al-2Si-xCe alloy system can be considered as a pseudo-binary system that varies with Ce content.In the Mg-5Al-2Si-0.4Ce alloy,when the temperature of the liquid reaches the liquidus,the Ce element will react with Si to form CeSi2first.Then the structure Mg2Si+CeSi2will be generated from the liquid when the liquid composition reaches the binary eutectic point.When the liquid composition reaches the ternary eutectic point,the eutectic structure Mg+Mg2Si+CeSi2will be formed from the liquid until the Si element is completely consumed.Similarly,there is no Mg-Al intermetallic compound formed during solidification according to the equilibrium phase diagram.When the Ce content increases to 0.8 and 1.6 wt.%,the solidification sequences of the phases are the same as that of the Mg-5Al-2Si-0.4Ce alloy.Differently,the fraction of each phase changes.With increasing Ce content,the increasing CeSi2phase may change the microstructure a lot.
3.2.The effect of Ce content on the phase composition of the Mg-5Al-2Si-xCe alloys
Fig.2 displays the XRD patterns of Mg-5Al-2Si-xCe(x=0,0.4,0.8,1.6 wt.%) alloys.The results show that the Mg-5Al-2Si alloy consists ofα-Mg,Mg2Si and Mg17Al12phases.When Ce was added,CeSi2phase showed up.As shown in Fig.2,with increasing Ce content,the fraction of CeSi2phase increases slightly.As for the fraction of Mg17Al12phase,there is no obvious changes.It is worth noting that Mg2Si phase is always the main intermetallic compound.
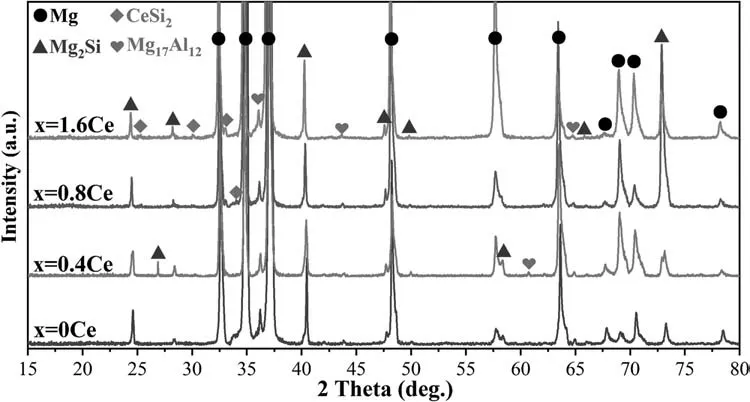
Fig.2.XRD results of Mg-5Al-2Si-(0,0.4,0.8,1.6) Ce alloys.
EPMA was used to analyze the distribution of elements(Mg,Al,Si and Ce) in the Mg-5Al-2Si-xCe (x=0,0.4,0.8 wt.%) alloys.As shown in Fig.3(a),in the Mg-5Al-2Si alloy,the Al element is distributed in the Mg17Al12phase,and the Si element is concentrated in the Mg2Si phase.As shown in Fig.3(b,c),after adding Ce element,the Al element is distributed in the Mg17Al12phase,a large amount of Si element exists in the form of Mg2Si phase,and the Ce element exists in the form of CeSi2phase.In addition,the formation of CeSi2phase has an influence on the morphology of the Chinese script type Mg2Si,to some extent.

Fig.3.EPMA maps of the (a) Mg-5Al-2Si,(b) Mg-5Al-2Si-0.4Ce and (c) Mg-5Al-2Si-0.8Ce alloys.
There are two types of Mg2Si phases in Mg-5Al-2Si alloy,polygonal type and Chinese script type,as shown in Fig.3(a).The polygonal type phase is primary Mg2Si,and the Chinese script type phase is eutectic Mg2Si [27].The primary Mg2Si phase is surrounded byα-Mg and Chinese script type Mg2Si.Due to the relatively high cooling rate,its solidification deviates from the equilibrium phase diagram.During solidification,the Mg2Si is formed primarily,resulting in an enrichment of magnesium around the primary Mg2Si.As the temperature decreases to the eutectic point,the eutectic structure ofα-Mg and Chinese script type Mg2Si is formed around the primary Mg2Si [25,27].For the Ce-modified AS52 alloy,as shown in Fig.3(b),Ce element exists in the form of CeSi2phase.The CeSi2phase appears around the Mg2Si phase,which may have a great effect on the modification of Mg2Si phase.As shown in Fig.3,the size of Chinese script type Mg2Si phase decreases with increasing Ce content.
3.3.The effect of Ce content on the size of Mg2Si phase
Fig.4 shows the low magnification OM images of the present alloys.For all alloys,two types of Mg2Si phases(Chinese script type and polygonal type) can be easily observed.It can be found from Fig.4(a) that the Mg2Si in the unmodified AS52 alloy exhibits large Chinese script type morphology.After adding a small amount of Ce to the AS52 alloy,although the Chinese script type Mg2Si phase is still obvious,it becomes relatively fine as shown in Fig.4(b,c).At the same time,the polygonal Mg2Si phase has also been refined.Furthermore,as shown in Fig.4(d),adding more Ce(1.6 wt.%) has a better refinement effect.And its morphology changes from initial Chinese script type to irregular block shape.These indicate better mechanical properties can be obtained in the Mg-5Al-2Si-1.6Ce alloy.
In order to quantify the ability of Ce to refine the Mg2Si phase,the sizes of Chinese script type and polygonal type Mg2Si phases were evaluated based 10 OM images of each alloy.The results are presented in Fig.5.The length of the Chinese script type Mg2Si phase is around 110 μm and the size of the polygonal type Mg2Si phase is about 23 μm in the unmodified Mg-5Al-2Si alloy.With addition of Ce,the sizes of Chinese script type and polygonal type Mg2Si phases are decreased gradually.Specially,when the Ce content is 1.6 wt.%,the length of Chinese script type Mg2Si phase is reduced to 50 μm,and the size of polygonal type Mg2Si phase is reduced to 8 μm.
3.4.Mechanical properties of Mg-5Al-2Si-xCe alloys
The mechanical properties of the Mg-5Al-2Si alloy are improved by adding Ce element.As shown in Fig.6(a),the hardness of the Mg-5Al-2Si alloy is 53.9 HV;when the added Ce content is 0.4 wt.%,0.8 wt.% and 1.6 wt.%,the hardness increases to 54.6 HV,55.9 HV and 56.2 HV,respectively.
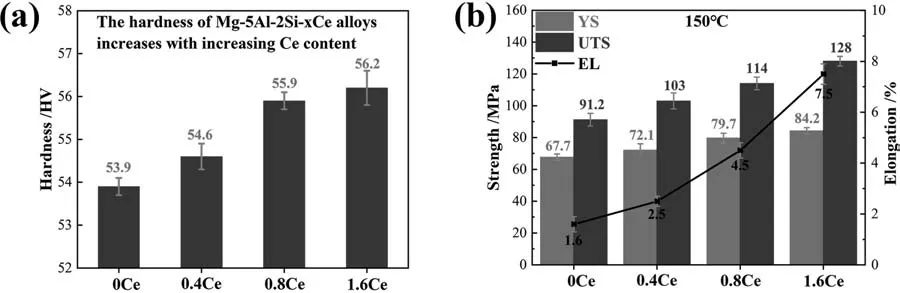
Fig.6.The (a) hardness and (b) tensile properties increase with increasing Ce content in Mg-5Al-2Si-xCe alloys.
Tensile properties of Mg-5Al-2Si-xCe alloys at 150 °C with varying Ce contents are shown in Fig.6(b).The values of YS,UTS and EL of the modified AS52 alloys,are obviously higher than that of the unmodified AS52 alloy.The results confirm the expectation based on the metallographic results.The YS,UTS,EL values of AS52 alloy at 150 °C are 67.7 MPa,91.2 MPa,1.6%,while for the Mg-5Al-2Si-1.6Ce alloy are 84.2 MPa,128 MPa,7.5%,respectively.The increasing in tensile strength and elongation is attributed to the finer Mg2Si phase,to most extent.According to the Orowan mechanism,the stress required to force dislocations to bypass the second phase particles is inversely proportional to the distance between particles,which explains why the refinement of Mg2Si increases the strength of the Mg-5Al-2Si alloy.
Fig.7 shows the typical SEM images of the tensile fracture surfaces of Mg-5Al-2Si-xCe alloys at 150°C.Many fracture facets are present,some dimples and lacerated ridges can also be observed in the tensile fracture surfaces,indicating that all the tensile fracture surfaces have mixed characteristics of cleavage and quasi-cleavage fractures [26].The fracture surface of the Mg-5Al-2Si alloy exhibits large cleavage type facets,which can reach 230 μm,as shown by the yellow arrows in Fig.7(a).This phenomenon is mainly attributed to the large Chinese script type Mg2Si phase in the Mg-5Al-2Si alloy.The microcracks will initiate at the Chinese script type Mg2Si and propagate along the interface of Mg2Si/Mg to form large fracture facets due to the concentration of tensile stress.Fortunately,the cleavage type facets in the fracture surface of the Ce containing Mg-5Al-2Si-xCe alloys are much smaller,as shown by the yellow arrows in Fig.7(b–d).There are more dimples and lacerated ridges on the fracture surface of the Mg-5Al-2Si-1.6Ce alloy shown in Fig.7(d).Therefore,as the Ce content increases,the refinement of Mg2Si increases the plasticity of the Mg-5Al-2Si alloy significantly.
4.Discussion
4.1.Ce modifies the morphology of Mg2Si phase
Fig.8 shows the end morphology of the Chinese script type Mg2Si phase in the microstructure.As shown in Fig.8(a),in Mg-5Al-2Si alloy,the end of Chinese script type Mg2Si phase is relatively sharp.When the Ce content is 0.4 wt.%,as shown in Fig.8(b),the end of Chinese script type Mg2Si phase changes and the fraction of sharp end decreases.With increasing Ce content to 0.8 and 1.6 wt.%,as shown in Fig.8(c,d),the end of Chinese script type Mg2Si phase is relatively smooth.
According to the theory of surface energy [30,31],alloying elements can reduce the surface energy of Mg2Si phase,thereby inhibiting the growth of Mg2Si phase and optimizing its morphology.Since Ce is a surface-active element,whose maximum solid solubility in Mg matrix is very low (only 0.74 wt.%),it is easy to enrich in the front of liquid-solid interface.Han et al.[35]reported that Ce atoms would be adsorbed onto the {100} and {111} planes of the Mg2Si crystals,which reduced their surface energy by lattice distortion and thereby inhibited the growth on the<100>and<111>crystal orientations.In addition,<100>and<111>are the preferred orientations of Mg2Si with a face-centered cubic structure.Therefore,due to the poison effect of Ce,the preferred growth manner of the Mg2Si is suppressed,while another type of growth,isotropic growth type,is accelerated [35,37–40].Consequently,with increasing Ce content,the morphology of Chinese script type Mg2Si phase becomes smoother.
4.2.Ce promotes the nucleation of Mg2Si phase
As shown in Fig.9(a),there are three kinds of second phases including Mg17Al12and two types of Mg2Si in the unmodified AS52 alloy.When Ce is added,CeSi2phase appears in the modified AS52 alloys.As shown in Fig.9(b–d),the white CeSi2particles are often intertwined with the gray Mg2Si particles,and part of the CeSi2particle is wrapped by the Mg2Si particle,which indicates that there is a certain nucleation relationship between these two phases.In addition,according to the calculation result of the phase diagram shown in Fig.1(b),the CeSi2phase is formed earlier than the Mg2Si phase.Therefore,the CeSi2phase may be the nucleation site of Mg2Si phase,thereby promoting the nucleation and refinement of Mg2Si.
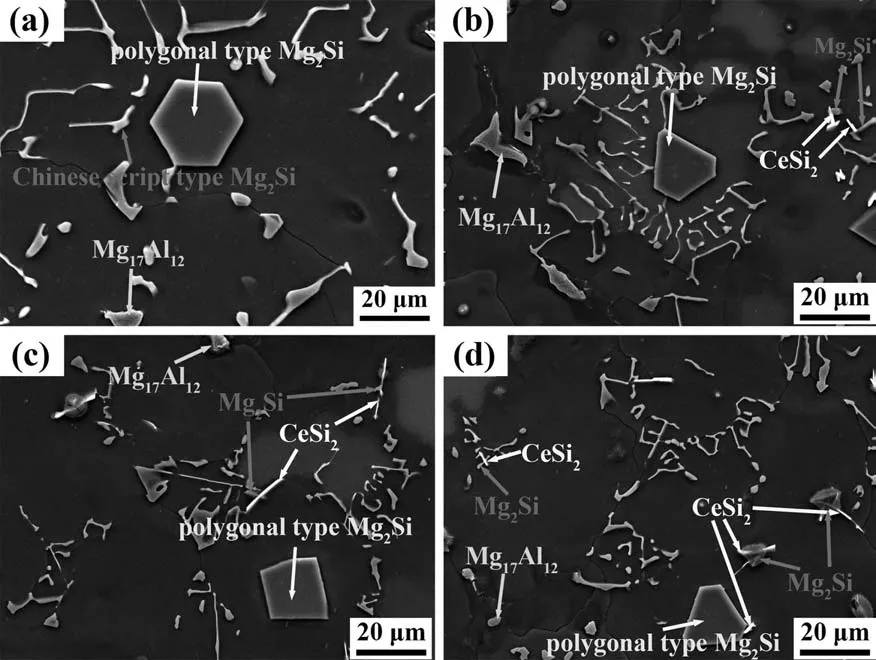
Fig.9.SEM images of (a) Mg-5Al-2Si,(b) Mg-5Al-2Si-0.4Ce,(c) Mg-5Al-2Si-0.8Ce and (d) Mg-5Al-2Si-1.6Ce alloys.
As shown in Fig.10,a small particle existed in the polygonal Mg2Si phase was proved as the nucleation site of the polygonal Mg2Si phase in our previous work [41].The concentration profiles of elements show that the particle inside the polygonal type Mg2Si,is enriched in Ce element(Ce:Si ≈1:2,atomic ratio),its stoichiometry is about CeSi2.It can also be found that CeSi2particles aggregate around the Chinese script type Mg2Si phase.In addition,Han et al.[35]reported that the Ce atoms could be absorbed on the surface of Mg2Si crystal,which reduced the surface energy and poisoned the growth steps of Mg2Si.As a result,the isotropic growth type would be accelerated resulting in smoother Mg2Si particles.

Fig.10.CeSi2 acted as the nucleation site of Mg2Si in Mg-5Al-2Si-0.4Ce alloy [41].
Further analysis is needed to confirm that the CeSi2phase can act as the nucleation site for the formation of Mg2Si phase.Generally,the nucleation ability of one phase on another phase can be expressed by the lattice mismatch of interface between two phases.The lattice mismatch of interface between two phases can be calculated by Bramfitt’s lattice mismatch equation [42]as displayed in Eq.(1):
where(hkl)sis a low-index plane of the substrate,(hkl)nis a low-index plane of the nucleated solid,[uvw]sis a low-index direction in(hkl)s,[uvw]nis a low-index direction in(hkl)n,d[uvw]sis the interatomic spacing along[uvw]s,d[uvw]nis the interatomic spacing along[uvw]n,andθis the angle between[uvw]sand[uvw]n.The Bramfitt’s theory[42]indicates that if the mismatch between two planes is smaller than 15%,one phase can act as the heterogeneous nucleation site for another.
The tetragonal CeSi2(lattice parameters:a=4.156,c=13.840) belongs to I41/amd (141) space group.Mg2Si has a face-centered cubic structure with a lattice constant ofa=6.391and belongs to Fmm (225) space group.As the substrate,(001) plane of CeSi2is selected as the lowindex plane.As the nucleated solid,(100) plane of Mg2Si is selected as the low-index plane.The atomic arrangement of CeSi2on (001) lattice plane is displayed in Fig.11(a),and that of Mg2Si on(100)lattice plane is displayed in Fig.11(b).The matching relationship between (001) plane of CeSi2and(100) plane of Mg2Si is displayed in Fig.11(c).Then the lattice mismatch between the (001) CeSi2// (100) Mg2Si planes can be calculated to be 7.97%,which is considerably less than 15%.Therefore,CeSi2is an effective heterogeneous nucleation substrate for the Mg2Si phase.The formation of small CeSi2particles promotes the nucleation of Mg2Si phase,which modifies the morphology of Mg2Si phase and reduces its size.
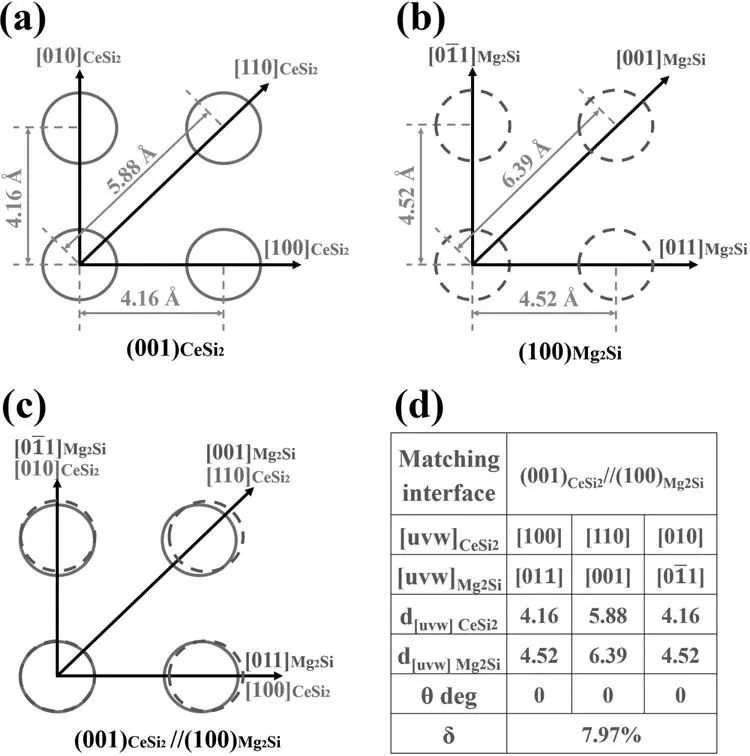
Fig.11.Schematic of (a) the atomic arrangement of CeSi2 on (001) lattice plane and (b) the atomic arrangement of MgSi2 on (100) lattice plane;(c) matching relationship between atoms of CeSi2 on (001) lattice plane and Mg2Si on (100) lattice plane;(d) the lattice mismatch between the (001) CeSi2// (100) Mg2Si planes is 7.97%.
Two key points are responsible for the modification of Mg2Si phase.On the one hand,Ce atoms reduce the surface energy of the Mg2Si crystal,which poisons the growth steps of Mg2Si and promotes it to be smooth [35].On the other hand,CeSi2phase can act as the nucleation site for the Mg2Si phase,which promotes Mg2Si to be fine and dispersive.This trend is more pronounced as the increasing of Ce content.
4.3.Ce changes the solidification sequence then modifies Mg2Si phase
As shown in Figs.1–3,the experimental results are different from that predicted by equilibrium phase diagram,due to the severe solute segregation caused by the high cooling rate.During the solidification process in this experiment,there is little diffusion behavior in the solid phase,while the liquid phase diffusion is relatively sufficient.Compared with the result of equilibrium solidification prediction,our experimental results are more consistent with the prediction results by Scheil’s model.It assumes that there is no diffusion in the solid phase during the solidification process,while the liquid phase is completely diffused.
Fig.12(a) shows the solidification paths of Mg-5Al-2SixCe alloys calculated by Pandat software based on Scheil’s model.In the Mg-5Al-2Si alloy,there are three kinds of structures,primary Mg2Si,binary eutectic Mg+Mg2Si and ternary eutectic Mg17Al12+Mg+Mg2Si.After adding Ce element,four types of structures will be generated,primary CeSi2,binary eutectic Mg2Si+CeSi2,ternary eutectic Mg+Mg2Si+CeSi2and quaternary eutectic Mg17Al12+Mg+Mg2Si+CeSi2.As shown in Fig.12(b),the formation temperature and the fraction of each phase can also help to understand the solidification process of Mg-5Al-2Si-1.6Ce alloy.Different Ce contents mainly change the formation temperature of CeSi2.Obviously,the fraction of CeSi2will also change slightly.
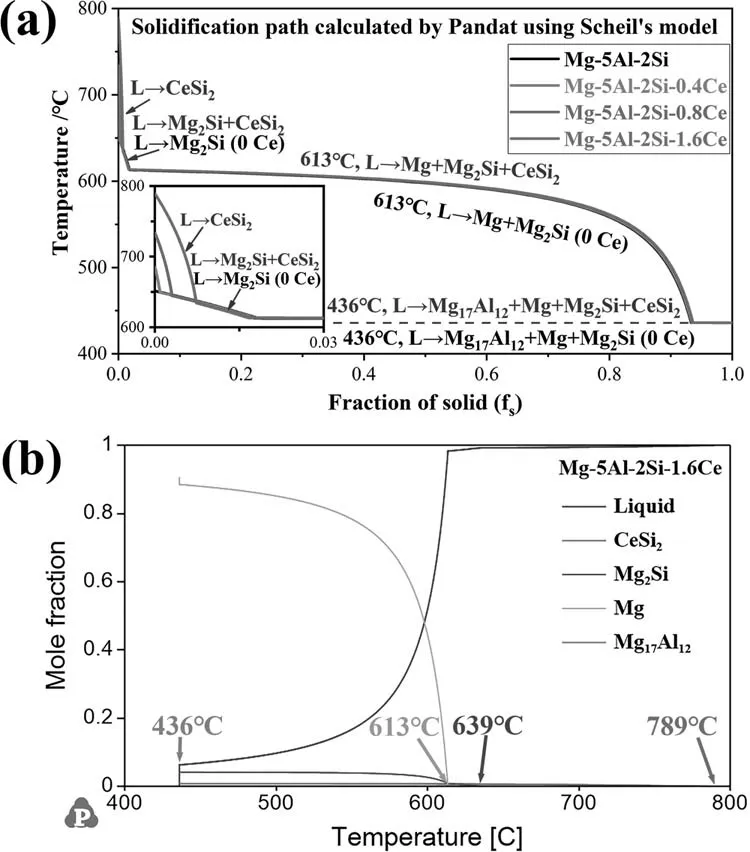
Fig.12.(a) Solidification paths of Mg-5Al-2Si-xCe alloys calculated by Pandat software based on Scheil’s model;(b) mole fraction of each phase in Mg-5Al-2Si-1.6Ce alloy calculated by Pandat software based on Scheil’s model.
The possibility of forming a compound between elements generally depends on the value of electronegativity difference.A larger value means a higher formation possibility [43,44].The electronegativities of Mg,Al,Si and Ce are 1.31,1.61,1.98 and 1.12 [45,46],respectively.As shown in Table 2,the electronegativity difference between Si and Ce(0.86)is larger than that between Si and Mg (0.67),Al and Ce (0.49),Al and Si (0.37) or Mg and Ce (0.19),which means that Ce element preferentially reacts with Si rather than Mg and Al.In the unmodified Mg-5Al-2Si alloy,the electronegativity difference of Mg/Si is largest,so the Mg2Si intermetallic compound will be preferentially formed.Since there is no Al-Si intermetallic compound,Mg17Al12will be formed last.In the modified Mg-5Al-2Si-xCe alloys,the electronegativity difference of Ce/Si is largest,so the CeSi2intermetallic compound will be preferentially formed.Since most Ce atoms will be consumed by the formation of CeSi2phase,there is no Ce atoms used to form Al-Ce and Mg-Ce compounds.As that of the unmodified Mg-5Al-2Si alloy,the Mg2Si phase and Mg17Al12phase will be formed later.Therefore,adding different contents of Ce element mainly affects the fraction of CeSi2.

Table 2Intermetallic compounds formed in this work.
According to the experimental results and the calculation results,the microstructure evolution can be described by the schematic diagram shown in Fig.13.As shown in Fig.13(a),in the unmodified Mg-5Al-2Si alloy,there are three types of second phases,polygonal type Mg2Si primarily formed between 651 and 613 °C,Chinese script type Mg2Si generated between 613 and 436 °C and the irregular porous Mg17Al12formed at 436 °C.The sizes of two types of Mg2Si phases are large,and their ends are sharp.As shown in Fig.13(b),in the modified alloy with 0.4 wt.% Ce,the second phase first formed between 685 and 649 °C is CeSi2.Subsequently,the polygonal Mg2Si is generated between 649 and 613 °C.Finally,as that of the unmodified Mg-5Al-2Si alloy,the Chinese script type Mg2Si and the irregular porous Mg17Al12are formed.Differently,the CeSi2phase can act as the nucleation site of polygonal type Mg2Si and Chinese script type Mg2Si.Therefore,their particles are refined by CeSi2.Due to the poisoning effect of Ce element [35],the Mg2Si particles become smooth.With increasing Ce content to 0.8 and 1.6 wt.%,CeSi2will be formed at higher temperatures.Since the formation of CeSi2consumes some Si content,the formation temperature of polygonal Mg2Si is further reduced.In addition,more CeSi2particles enhance the refinement of Mg2Si.As shown in Fig.13(d),in the Mg-5Al-2Si-1.6Ce alloy,the Mg2Si particles become smaller and smoother.
It is worth noting that,due to the relatively low Ce content,the formation of CeSi2phase requires a concentration enrichment process.Therefore,Mg2Si will be formed before all Ce atoms are consumed by the formation of CeSi2phase.Because the Ce element has a strong ability of surface enrichment,the low content of Ce can be enriched to achieve the composition condition for the formation of CeSi2.At the same time,Ce atoms can be adsorbed on the surface of the Mg2Si crystal along with the formation of Mg2Si,thereby changing the surface energy of the Mg2Si crystal,poisoning its growth steps and promoting it to be smooth [35].The two processes of generating CeSi2phase and poisoning Mg2Si phase can be carried out simultaneously,so the Mg2Si phase will be refined due to the increase in the number of nuclei,and its morphology will also become smooth.
5.Conclusion
Effects of Ce content on the Mg2Si modification and mechanical properties of Mg-5Al-2Si-xCe (x=0,0.4,0.8,1.6 wt.%) alloys have been investigated.The main results are summarized as follows:
(1) The length of Chinese script type Mg2Si phase decreased significantly from 110 to 50 μm with increasing Ce content to 1.6 wt.%.The morphology of the Mg2Si phase transformed from large and sharp Chinese script type to small and smooth block shape.
(2) The mechanical properties of Mg-5Al-2Si-xCe alloys at 150 °C were improved due to the refinement of Mg2Si phase.Especially,after adding 1.6 wt.% Ce,the YS,UTS and EL of the Mg-5Al-2Si alloy were improved from 67.7 MPa,91.2 MPa and 1.6% to 84.2 MPa,128 MPa and 7.5%,respectively.
(3) The modification of the Mg2Si phase is mainly attributed to two aspects: on the one hand,Ce atoms reduce the surface energy of Mg2Si crystal,poison the growth steps and then promote the isotropic growth of Mg2Si.On the other hand,the preferential formation of CeSi2compound promotes the nucleation of Mg2Si phase due to the small lattice mismatch of 7.97%.
Acknowledgements
This research was supported by the Major Science and Technology projects in Qinghai province (2018-GX-A1) and Global Frontier Program through the Global Frontier Hybrid Interface Materials (GFHIM) of the National Research Foundation of Korea (NRF) funded by the Ministry of Science,ICT &Future Planning (Grant No.2013M3A6B1078874).This work was also funded by Shanghai Science and Technology Committee(Grant No.18511109302)and the National Natural Science Foundation of China (Grant No.51825101).
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- “Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
- Formation and growth of precipitates in a Mg-7Gd-5Y-1Nd-2Zn-0.5Zr alloy aged at 200 °C
