“Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
Yonghua Chen ,Liang Wu,* ,Wenhui Yao ,Jiahao Wu ,Maria Serehnova ,Carsten Blawert ,Mikhail L.Zhelukevih,Yuan Yuan,Zhihui Xie,Fusheng Pan
a National Engineering Research Center for Magnesium Alloys,College of Materials Science and Engineering,Chongqing University,Chongqing 400044,China
b State Key Laboratory of Mechanical Transmission,Chongqing University,Chongqing 400044,China
c Institute of Surface Science,Helmholtz-Zentrum Hereon,Max-Planck Str.1,21502 Geesthacht,Germany
d Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province,College of Chemistry and Chemical Engineering,China West NormalUniversity,Nanchong 637002,China
Abstract Coating technologies are a commonly used way to protect metals against corrosion.However,with more and more severe service environments of materials,many protective coating systems often are not environmentally friendly or toxic as in the case of chromates.Based on the world’s abundant ideal magnesium(Mg)and its alloy,the smart self-healing anticorrosive coating can autonomously restore the damaged part of the coating according to the environmental changes,strengthen the corrosion protection ability,and prolong its service life.This paper reviews the research progress of smart self-healing coatings on Mg alloys.These coatings mostly contain suitable corrosion inhibitors encapsulated into micro/nano containers.Moreover,the different self-healing mechanisms and functionalities of micro/nano containers are discussed.The micro/nano containers range from inorganic nanocontainers such as mesoporous nanoparticles (silica (SiO2),titanium dioxide(TiO2),etc.),over inorganic clays (halloysite,hydrotalcite-like,zeolite),to organic nanocontainers such as polymer microcapsules,nanofibers,chitosan (CS) and cyclodextrin (CD),as well as,carbon materials such as graphene and carbon nanotubes and hybrids such as metal organic frameworks.The functioning of micro/nano containers can be divided in two principal groups: autonomous (based on defect filling and corrosion inhibition) and non-autonomous (based on dynamic bonds and shape memory polymers).Moreover,multi functionalities and composite applications of various micro/nano containers are summarized.At present,significant progress has been made in the preparation methods and technologies of micro/nano containers.Achieving long-term self-healing properties of coatings sensing of coating failure and early warning after self-healing function failure can be expected as the main development direction of self-healing corrosion protection coatings in the future.
Keywords: Magnesium alloy;Self-healing coating;Micro/nano containers;Mechanism;Corrosion protection.
1.Introduction
Corrosion of metals (steel,aluminum,magnesium,etc.) is a major issue related to the economy.The total annual cost of corrosion is estimated as more than $310 billion in China,or 3.34%of the country’s GDP[1,2].If the same ratio is applied to the global economy,the total annual cost will be approximately $2.5 trillion [1,3].Therefore,preventing metal corrosion is a crucial issue,and preparing protective coatings on metal surfaces is also the most common method.In terms of steel,nanocontainers or corrosion inhibitors can be encapsulated and dispersed in the coating matrix.Liu et al.[4,5]combined metal organic framework (MOF) nanocontainers loaded with corrosion inhibitors into thermosensitive polyurethane to construct a polymer composite coating on the surface of Q235 mild steel.When steel undergoes corrosion,ferrous ions were rapidly released,and color changes indicated the occurrence of corrosion.Local acidity can stimulate the decomposition of nanocontainers,and corrosion inhibitors inhibit corrosion reactions,resulting in excellent self-healing performance of the prepared coating and better service life of the steel.Unfortunately,this research strategy has limited protective effects on aluminum alloys [6,7].As far as aluminum and aluminum alloys are concerned,chromate conversion coatings and primers containing chromate pigments have been widely used in the aerospace industry in recent decades,but the new environmental protection law requires that new environmentally friendly methods must be found to replace Cr(VI) [8].Nanocontainers containing cerium and lithium salts or corrosion inhibitors in organic coatings are a good alternative.Sol-gel coatings can be used as pretreatment or primer for different alloys,but further tests are required in service conditions or accelerated corrosion tests.In addition,its high energy consumption should also be considered [8–10].
Mg and its alloys have excellent physical and chemical properties such as good recyclability,good biocompatibility and low density,and have been widely used in transportation[11],biomedicine [12,13],electronics [14],3C [3],aerospace and other fields [15–18].However,the high degradation rate of Mg alloys is the main obstacle limiting their large-scale industrial application [19,20].Traditional methods of reducing the corrosion rate of Mg alloys include electrochemical protection[21],corrosion inhibitors[22,23],and coating techniques [24,25],among others [26,27].In the early development process,corrosion protection was mainly achieved by preparation of coatings on the Mg alloy surface.The main function of these coatings was associated with barrier properties of the layer in order to prevent contact of the Mg alloys with the corrosive environments [28].Coating of metals can protect the Mg substrate from corrosion to a certain extent,but due to their inherent defects(e.g.pores,cracks),accidental damage,scratches,or use aging,the physical barrier function of the coatings will gradually weaken.Finally,the Mg substrate can corrode due to exposure to corrosive environments.Therefore,a smart self-healing anti-corrosion coating that can still protect the Mg alloy when the coating system is damaged has received extensive attention and research during the last decades [29,30].
Smart self-healing anti-corrosion coating refers to smart coating systems with “corrosion sensing”,“release of inhibitors on demand” and “self-healing” properties.As early as more than ten or twenty years ago,it has been proved that multifunctional micro/nano containers can be used to trap small molecules and introduce specific functions,and have been widely used in drug delivery,biomedicine,bioreactor,analytical applications,etc.[31].After the coating is damaged by the environment or external force,it can autonomous (via defect passivation by released corrosion inhibitors) or nonautonomous (due to shape memory effect and dynamic bonds mechanism) restore the original anti-corrosion properties under certain conditions.There are also classifications according to extrinsic (top) and intrinsic healing methods (bottom)as shown in Fig.1 [32].Extrinsic self-healing is achieved by encapsulated active agents.Mainly include active agent leakage caused by fracture,pH change (ΔpH),potential change(ΔΦ),and active agent polymerization caused by humidity,light,and temperature.Intrinsic self-healing mainly includes reversible networks driven by redox reactions,UV irradiation,and temperature.
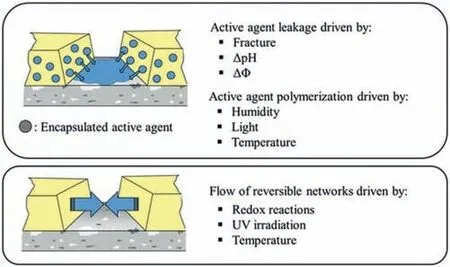
Fig.1.The common healing methods of anti-corrosion coatings [32].
In general,micro/nano containers are used to hold corrosion inhibitors,which can achieve controlled release in Mg alloy protective coating.Currently,many research and applications are implemented for the following micro/nano containers: silica (SiO2) nanoparticles [33],titanium dioxide (TiO2)nanoparticles [34],halloysite [35],hydrotalcite-like [36],zeolite [37]and other inorganic nanocontainers [38–40],polymer microcapsules [41],nanofibers [42],chitosan (CS) [43],cyclodextrin (CD) [44],polyelectrolyte self-assembled films[45]and other organic nanocontainers [46],as well as carbon nanotubes [47],graphene [48]and other carbon materials[49].Based on the wide application of Mg alloys,the micro/nano containers in smart self-healing protective coatings are divided into inorganic,organic materials,carbon materials and hybrids,and the coatings loaded with different nanocontainers are summarized.The research progress and main applications of the above mentioned different types of nanocontainers,as well as different types of self-healing mechanisms,are reviewed.As shown in Fig.2.The purpose of this paper is to provide a systematically description of the research progress of “smart” micro/nano container-based self-healing coatings on Mg alloys.

Fig.2.Research framework of “smart” micro/nano container-based self-healing coatings on Mg alloys.
2.Inorganic nanocontainers
In recent years,the use of inorganic nanocontainers as corrosion inhibitor carriers in Mg alloy self-healing coatings has received a lot of attention.Inorganic nanocontainers mainly include mesoporous nanoparticles such as SiO2[50],TiO2[51],CeO2[52],and inorganic clays such as halloysite [53],hydrotalcite-like [54],zeolite [55].Their functionality is generally based on the cavities in the inorganic nanoparticles which are used to load inorganic corrosion inhibitors (cerium salts,molybdates,tungstates,vanadates,etc.) and organic corrosion inhibitors (imidazoline (IMI),benzotriazole (1H-BTA),etc.).The corrosion inhibitor,released from the micro/nano container in response to environmental stimuli,has a physical or chemical interaction with the Mg alloy,preventing the continuation of the corrosion electrochemical reaction,thereby realizing the self-healing of the coating.Among them,mesoporous SiO2nanoparticles are the most widely used and studied,mainly because of their high porosity and stability,large specific surface,and controllable particle size,which facilitates the desired effect.In addition,the hollow halloysite can be prepared to support corrosion inhibitors.Also,the cation exchange property of zeolite can be used to support the cationic corrosion inhibitor[56],while the anion exchange property of layered double hydroxides (LDHs) can be used to support the anionic corrosion inhibitor [57].Table 1 summarizes the applications of inorganic nanocontainers loaded corrosion inhibitors in Mg alloy protective coatings in the last decade.

Table 1Applications of inorganic nanocontainers (loaded with corrosion inhibitor) coatings.
2.1.Silica nanocontainer
SiO2nanocontainers have the advantages of small size,high carrying capacity,good biocompatibility,and easy surface modification [44].As early as 2009,it was proved that they can act as corrosion inhibitor carriers,improving the chemical and mechanical properties of organic coatings [67].The preparation methods of SiO2nanocontainers loaded with corrosion inhibitors include different techniques such as prepolymer method [68],co-templates method [69]and micro emulsion polymerization [33].The loading method is mostly under vacuum conditions,where the corrosion inhibitor solution is soaked multiple times,and finally centrifugally washed and dried [33].However,the low compatibility of inorganic SiO2nanocontainers with organic coatings often compromises the protective performance of coatings.To solve this problem,the nano-mesoporous material can be used as the core,and their surface can be additionally modified by organic materials through layer-by-layer (LBL) self-assembly technology[70]or supramolecular valve technology [71],thereby improving their compatibility with coatings.At the same time,it can also realize the response of medium pH [72]and salt(NaCl) concentration [73],and stimulate the release of corrosion inhibitor.A representative example of surface modification of SiO2particles is via polymethacrylic acid (PMAA)coating offering pH triggering of the release.Li and coworkers [72]synthesized PMAA-coated SiO2nanotubes for the controlled release of pH-responsive corrosion inhibitor BTA.It maintains a contracted form under acidic conditions and swells extremely in alkaline environments due to the ionization of -COOH.This leads to a large negative charge,strong hydration,strong electrostatic interaction,and the release of corrosion inhibitor.When BTA is released to the surface of Mg alloy coating,self-healing of the damage can be achieved.
In recent years,the development of supramolecular valve technology has also provided ideas for the construction of smart micro/nano containers [74].Here,supramolecular valves act as gatekeepers to their corresponding stimuli.In this case,micro/nano containers can actively and efficiently respond to more complex environmental triggering conditions in corrosion reactions of Mg alloys,such as single or multiple responses to acids,alkali,heat,etc.[33,69,75-78].In the case of coatings targeting protection of Mg alloys,Ding et al.[33]installed supramolecular nanovalves on mesoporous SiO2nanoparticles,and added the nanoparticles as a “guest” to a“host” self-assembled nanoparticle barrier coating.Under the stimulation of alkaline environment or Mg2+,the coating can effectively release the SiO2-encapsulated corrosion inhibitor HMAP to build a self-healing super-hydrophobic coating on the surface of Mg alloy AZ31B.Once the surface is mechanically scratched,the released HMAP forms a dense molecular coating on the damaged alloy surface,inhibiting corrosion propagation and realizing self-healing function (Fig.3).Table 2 presents the stimuli-response conditions for the release of corrosion inhibitors from SiO2nanocontainers used within the coatings applied on Mg for different applications.
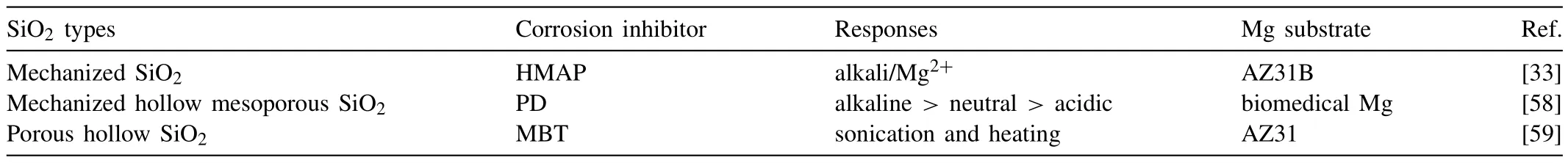
Table 2Stimulus responses of SiO2 nanocontainers under different conditions.
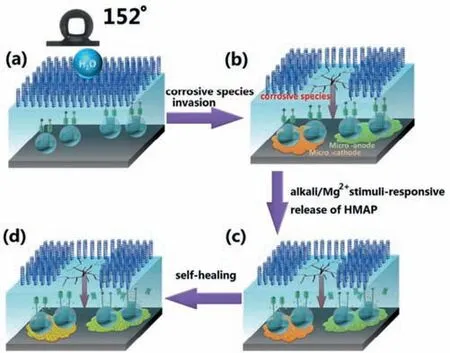
Fig.3.Working mechanism of self-healing,super-hydrophobic coating: (a)super-hydrophobic coating,(b) corrosive species invasion,micro-cathode and micro-anode formation on AZ31B surface (c) alkali/Mg2+stimuli-responsive release of HAMP,(d) HMAP forms a dense molecular coating [33].
The traditional method of preparing pH responsive nanocontainers by modifying the surface of mesoporous silica nanoparticles (MSN) with silane is costly,complex,and time-consuming,which still poses a challenge to the corrosion protection of Mg alloys.Therefore,new preparation strategies are needed to simplify the synthesis process of nanocontainers,eliminate the potential adverse effects of excessive nanovalves on coatings,and strive for widespread application in corrosion protection of metals such as Mg alloys [79].
2.2.Titanium dioxide nanocontainer
As a nanocontainer,TiO2mainly stores corrosion inhibitors in the porous TiO2containers or in nanotubes [80].After being prepared and sealed under vacuum conditions,the corrosion inhibitors could be added into sol-gel or epoxy coatings.For example,Kordas et al.[81]prepared epoxy resin coatings with 8-hydroxyquinoline (8-HQ)-loaded TiO2nanocontainers,showing enhanced corrosion resistance,and investigated the release of 8-HQ using Raman spectroscopy.The results indicated that the Mg matrix corrosion leaded to the release of 8-HQ,which reacted with the Mg to form an insoluble Mg chelate.This was the first spectroscopic evidence of selfhealing,and it can be used for self-healing of different kinds of structures.Similarly,Kartsonakis et al.[34]prepared an organic-inorganic hybrid coating on Mg alloy ZK10 by immersion process (Coat-nc-MgZK10).The hybrid coating consists of an epoxy-containing cross-linked polymer,polypyrrole,a conductive polymer (CP),and an organically modified silicate,doped with 5-ATDT-loaded TiO2nanocontainers.The addition of the nanocontainers increased the impedance value of the coating at low frequency (Fig.4).And when the specimen is exposed to corrosive environment,there is no crack on the surface of the Coat-TiO2-MgZK10,which proves that the coating improves the anti-corrosion performance of ZK10.

Fig.4.Bode plots of (a) Coat-nc-MgZK10 soaked for different times (b) different samples soaked for 288 h (12 days) in 5 mM NaCl solution [34].
Although TiO2nanocontainers have shown good performance in the field of corrosion,the preparation methods of TiO2are worth further exploration.The existing TiO2coatings still have problems such as complex preparation processes,small preparation area,poor mechanical properties,and poor resistance to oil pollutants,lacking practical application value[82].
2.3.Halloysite nanocontainer
The hollow structure material is an ideal nanocontainer for corrosion inhibitors due to its large pore volume.The natural hollow nanomaterials,HNTs,are considered as one of the most promising nanocontainer materials for encapsulating corrosion inhibitors [83–85].It has been widely studied due to its advantages of nontoxic,cheap,easy to obtain,adjustable,and large variety of corrosion inhibitors,which can be loaded.For example,organic corrosion inhibitors (BTA,2-mercaptobenzimidazole (MBI),MBT,8-HQ,etc.[53,86]) or inorganic corrosion inhibitors (such as Ce3+,Zr4+[61,87,88]) can be encapsulated into the hollow structure of HNTs to prepare self-healing coating.Fig.5 shows the process of HNT encapsulation of corrosion inhibitor [89].The healing agent infiltrates into HNT through simple mixing or vacuum conditions,and after centrifugation and drying,the corrosion inhibitor loaded HNTs are obtained.
In addition,HNTs can be modified by LBL approach to prepare pH-responsive nanocontainers to achieve controllable release of corrosion inhibitors[90].Such nanocontainers were fabricated on kaolinite surfaces using Polydiallyldimethylammonium chloride and polyacrylic acid (PAA) under different pH and salt conditions by Kamburova et al.[91].The kaolinite particles are derived from Zettliz kaolin.The BTA inhibitor was encapsulated in the polyelectrolyte multilayer during the assembly step.Halloysite nanomaterials were encapsulated in polymers shell and used as nanocontainers,which could release the encapsulated BTA under neutral conditions.This is because the solubility of BTA in water decreases as it approaches neutral conditions [92].The amount of BTA embedded in the kaolinite nanocontainers is sufficient to stop the corrosion process that protects the coating defect areas under neutral ambient conditions.Taking advantage of the pH-dependent electrostatic interactions between HNTs and L-valine (L-Val),Dong et al.[93]prepared pHresponsive anti-corrosion materials and compared them with the preparation of MBT-loaded HNTs by LBL.Both methods achieved self-healing properties and controlled release of corrosion inhibitors,while the carrying capacity of HNTs for L-Val (12%) was higher than MBT (7%).The pH-responsive release properties were evaluated by UV–Vis spectrophotometry.The results showed that at pH 10,HNTs released 98%of L-Val-inhibitor molecules in 300 min,while release of MBT required 120 h.In addition,the pH-responsive anticorrosion material prepared in this study could release corrosion inhibitor when the protective coating is mechanically scratched,and form a new protective film through self-healing response.
In some HNTs applications,the ability and capacity of loaded corrosion inhibitors to enter HNTs are influenced by HNTs morphology,solution pH,solvent,and HNTs size.Further research is needed to investigate the impact of loading parameters on loading efficiency,as well as the availability of several corrosion inhibitors that can be loaded into the inner cavity of halloysite [94].
2.4.Hydrotalcite-like nanocontainer
Hydrotalcite-like materials are anionic clays,also known as LDHs.Due to their excellent properties such as structure and composition tunability,anion exchangeability,etc.,they are widely used as a corrosion inhibitor carrier in the field of corrosion protection of Mg alloys [80,95].LDHs are generally used as pigments in protective coatings or as conversion layers.In contrast,conversion coatings are more widely used in corrosion protection of Mg alloys.Therefore,LDHs conversion coatings are introduced below.Inorganic corrosion inhibitors as well as organic species can be loaded into the LDHs interlayer via the anion exchange process.When chloride ions appear in the environment,they act as triggers and smart capture of chloride ions and controlled release of corrosion inhibitors can be realized.The release mechanisms show great research and application potential in the field of corrosion protection [16,35,96-100].
Additionally,the way how to load cationic corrosion inhibitors in LDHs host layers to further broaden the inhibition ability of LDHs has also attracted the attention of many researchers.For example,Wu et al.[36]fabricated in situ Mg-Al-Ce-V2O74-LDH films on anodized Mg alloy AZ31 by the hydrothermal method.The corrosion resistance of the coatings was characterized by immersion experiment (Fig.6a),hydrogen evolution experiment (Fig.6b) and weight loss experiment (Fig.6c) in 3.5 wt.% NaCl solution.The results all prove that LDH–CeV has the best corrosion resistance(LDH–CeV>LDH-V>LDH–Ce>LDH).The intercalation of Ce3+and vanadate changed the crystal structure of Mg-Al LDH,making the LDH–CeV films uniform and dense.Ce3+and vanadate anions significantly improved the corrosion resistance of the LDH film,and the active double-doped LDH film (LDH–CeV) effectively protected the Mg alloy from corrosion.
Currently,the significant disadvantages of LDHs formation on Mg-based alloys can be associated with the necessity of autoclave conductions use (this approach significantly limits the possible industrial application of LDHs based surfaces)and/or with addition of carbonates in the treatment bath.Unfortunately,only very stable LDHs-CO3,which are difficult for further functionalization and can act only as barrier layer,can be formed in the presence of carbonates in the surroundings.In order to facilitate the formation of LDHs,containing nitrate and/or hydroxide cations,on the surface of Mg alloys,the approach of chelating agents use was recently suggested and developed [101–103].These chelating agents change the solubility of cations from the substrate and allow to form LDHs under relatively mild conditions,suitable for industrial needs.
The discussions about the release kinetics of corrosion inhibitors from LDHs have attracted the attention of researchers in recent years [104–109].It is believed that the ion exchange kinetics of LDHs for monovalent anions is diffusioncontrolled,while for divalent anions is surface reactioncontrolled [97].Moreover,the properties of LDHs,such as the structure and interlayer spacing,also have a certain influence on the release behavior of corrosion inhibitors.Bendinelli et al.[107]explored the effect of the remodeling process of Mg-Al LDHs on the release kinetics and anion exchange properties of corrosion inhibitors.After calcination,LDHs were reconstituted by two methods: (1) reconstituted in terephthalic acid and then replaced terephthalic acid with corrosion inhibitor imidazole (HTCTe-Im).(2) Direct reconstitution in imidazole solution (HTC-Im).The results showed that there was a synergistic effect between HTCTe-Im and HTC-Im,and the presence of both can minimize the corrosion rate.
Although LDHs self-healing coatings have been extensively studied,current methods still have limitations.The LDHs coatings can only load small molecule anionic corrosion inhibitors,and the embedding ability of organic macromolecular corrosion inhibitors is still lacking.To achieve the loading of macromolecular corrosion inhibitors,foreign substances can only be relied on [110].
2.5.Other inorganic nanocontainers
In order to explore the application potential of various nanocontainers for corrosion inhibitor loading,many other porous nanoparticles and inorganic clay-based nanoparticles have received additional attention.Following materials can be mentioned: cerium molybdate [111,112],boehmite[113,114],calcium carbonate microspheres [64,115,116],hollow manganese oxide microspheres [40,117,118],cerium oxide [52,65,119,120],montmorillonite [39,121-123],zeolites[55,124,125],metal organic framework-based compounds[126–131].For example,Snihirova et al.[64]modified calcium carbonate microspheres with different corrosion inhibitors,such as cerium nitrate,SAL and DMTD.Then dispersed them into epoxy resin coatings and coated on the surface of AZ91.Calcium carbonate microspheres are not stable under acidic pH and dissolve,releasing the inhibitors,when corrosion process starts (Fig.7).Hosseini et al.[65]prepared MBT-supported CeO2particles nanocontainers.Different contents of CeO2nanocontainers were dispersed into epoxy resin coatings and then applied to AZ31 alloy.The experiment proved that the release amount of MBT inhibitor was different at different pH.The coating showed better self-healing performance in acidic medium.
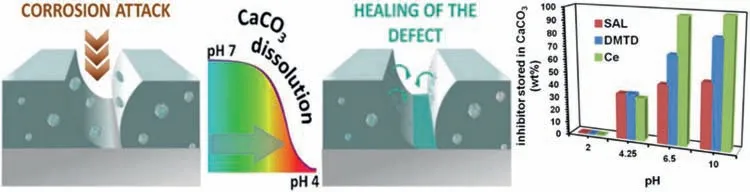
Fig.7.Conceptual schematic diagram of smart self-healing by releasing corrosion inhibitors from CaCO3 nanospheres in response to pH [64].
Zeolite molecular sieve refers to aluminosilicate molecular sieve.On the one hand,due to the ion exchange properties of cations (such as Na+),it can be used to support cationic corrosion inhibitors.On the other hand,the porosity of molecular sieves and large specific surface area can be used to adsorb and load various corrosion inhibitors.Zhang et al.[37]investigated pH,Mg2+and Na+release stimulation of Ce3+from modified zeolites.The PEO-pretreated AZ31 specimens were covered with CeNaX-doped epoxy resin,and the results showed that the modified AZ31 specimens reached good corrosion resistance [132,133].Rassouli et al.[124]doped NaX zeolite nanoparticles with Zn2+and MBT as inorganic and organic inhibitors,respectively,to improve the protective activity of silane sol-gel film on metal matrix in NaCl solution.The synergistic inhibition effect was demonstrated for such a system and effective corrosion protection was achieved.
In summary,inorganic nanocontainers have the advantages of uniform pore size,tunable pore structure,high mechanical stability,high loading rate,easy functionalization,and high surface area.Table 3 presents common inorganic nanocontainers.The nanocontainers can be modified (such as hydrogen bond,electrostatic interaction,chemical bond interaction and covalent bond grafting modification) to release corrosion inhibitors in a controlled stimulus response.However,the content of inhibitor is a subject to be determined.
Summarizing the corrosion inhibitor loading methods of various micro/nano containers mentioned above,it can be concluded that most inorganic micro/nano containers can be immersed in different corrosion inhibitor solutions (solvents such as water,acetone,ethanol,etc.),mechanically stirred under vacuum conditions,and then centrifugally dried to obtain corrosion inhibitor loaded micro/nano containers.While,The corrosion inhibitor loading of LDHs (hydrotalcite-like nanocontainer) is obtained through ion exchange characteristics.In many cases,the inhibitor content in the coating is too small to provide any inhibition effect because the inhibitor concentration in the defect does not reach the critical value.The compatibility of some inorganic nanocontainers with the surface coating is poor,and excessive addition of nanocontainers will lead to a rapid decline in the protective performance of the composite protective coating.Therefore,the use of inorganic nanocontainers depends not only on their compatibility,but also on the amount of inhibitors required to ensure inhibition.Proper addition amount and suitable surface modification can play its role to a greater extent.Inorganic nanocontainers show great application potential in the encapsulation and release of corrosion inhibitors,which facilitate the preparation of self-healing coatings.
3.Organic nanocontainers

Organic nanocontainers are also widely investigated in the coating design,mainly including urea-formaldehyde (UF)resin microcapsules,polymer microcapsules,CS microcapsules and other microcapsules,as well as nanofibers and CD nanocontainers.The corrosion protection mechanism is generally based on the encapsulation of inorganic corrosion inhibitors(cerium salts,molybdates,tungstates,vanadates,etc.),organic corrosion inhibitors (IMI,BTA,etc.) or other substances in organic nanocontainers.When the active surface is subjected to external impact or the local environment changes,the organic nanocontainers respond to this signal and release the encapsulated corrosion inhibitors to repair the defect.Among others,UF-based microcapsules are the most widely used and studied,mainly because of their simple synthesis process,cheap raw materials,and good mechanical properties,which can be used in many fields such as energy storage,self-healing,self-lubrication,and self-cleaning.In addition,the self-healing function can also be achieved by adding corrosion inhibitor-loaded organic nanofibers to the coating to build a network structure.
3.1.Polymer microcapsules
Polymer microcapsules (including phenolic,polyurethane(PU),polyaniline (PANI),etc.) are commonly used micro/nano containers for supporting corrosion inhibitors.UF is a rigid slow-degrading polymer that can be prepared by in-situ polymerization in aqueous solution.When the UF material is used as the shell material,a long-term leakage and diffusion of the coated corrosion inhibitor can be avoided,so it has been widely studied by various researchers [135–137].Mahmoudi et al.[138]packed Pr3+ions into the capsule cavity and coated UF to form UF microcapsules,and prepared a capsule-type silane coating for Mg alloy AZ31.Encapsulation of Pr3+was achieved by using a saturated corrosion inhibitor Pr(NO3)3·6H2O solution,vacuum loading in the inner space of the capsule,washing with water and acetone to remove Pr3+ions from the outer surface of the capsule,and drying in an air atmosphere.Fig.8a and b shows the HRTEM and FESEM images of the microcapsules containing the corrosion inhibitor,respectively.The Pr-encapsulated microcapsules were dispersed in a silane hybrid matrix and exhibited long-lasting active corrosion protection in 3.5% NaCl solution.The study found that the surface polarization resistance of the coating containing the microcapsules increased.Fig.8c shows the result of the scratched sample after 120 h of salt spray test.The experiment found that at the scratched site,the release of Pr3+cations generated a protective film of Pr oxide/hydroxide to prevent the electrolyte from entering the metal coating,achieving self-healing of damage and protecting Mg alloys from corrosion.
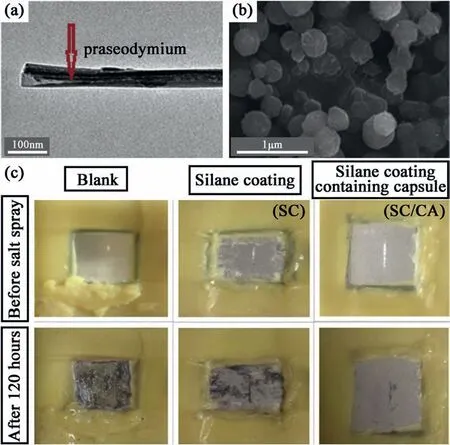
Fig.8.(a) HRTEM,(b) FESEM images of microcapsules containing corrosion inhibitor,(c) salt spray test results of scratched samples [138].
PU microcapsules prepared by interfacial polymerization had the advantage of being highly reproducible[139,140],and the diameter,weight fraction and coating thickness of the microcapsules significantly affected the self-healing properties of the coating [141].Vimalanandan et al.[41]prepared redox responsive CP-based nanocapsules with 3-nitrososalicylic acid inhibitor as core material and redox sensitive PANI as shell material.To avoid the occurrence of Fermi-level dislocations and direct contact between metal and CP,the surface of nanocapsules was sprayed with gold nanoparticles to ensure stable electronic contact.When the coating was intact,the PANI shell prevented 3-nitrososalicylic acid release.When the Mg alloy corrosion began,the corrosion inhibitors was released to protect the Mg matrix and complete self-healing.When the metal was passivated,the PANI shell again prevented release.
The exogenous self-healing system that combines microcapsules into a polymer matrix can release the healing agent to the target area (damaged area) once the microcapsule ruptures.However,traditional polymer self-healing microcapsules have complex manufacturing processes,typically ranging from 10 to 400 μm to adapt to different applications[142].When using this microcapsule method,self-healing components must have low viscosity so that they can be stored in microcapsules and released when needed.In addition,the microcapsule must be strong enough to be processed and dispersed in the polymer,but it is easy to break when the matrix ruptures [143].
3.2.Chitosan microcapsules
CS is a green macromolecule produced by deacetylation of natural compound chitin in nature,which is easy to recover and has excellent film-forming ability.Due to its good biocompatibility,safety,non-toxicity,easy degradation and others,it has been widely studied.Zheng et al.[43]investigated self-healing coatings with synergistic effects under UV light and moisture.First,a repair agent containing CH2=C(CH3)-COO-and -SiOCH3groups was synthesized and encapsulated in CS microcapsules with dense CS-rGO(graphene oxide).Furthermore,the GO flakes were assembled and stacked to form a dense CS-rGO shell.Then,CSrGO microcapsules were mixed into silicone resins and coated on the surface of Mg alloy to achieve efficient self-healing.The interaction between CS and GO helped the microcapsule core to maintain the stability.The experimental results showed that the CS-rGO microcapsules were embedded in the resin,which improved the anti-corrosion and mechanical properties,and endowed the self-healing ability of the Mg matrix.Liu et al.[144]prepared CS microspheres (containing green corrosion inhibitor sodium phytate) by reduced pressure impregnation.Environmental mesoporous CS microspheres were loaded with sodium phytate to prepare self-healing waterborne polyacrylate coatings.The release of sodium phytate in response to pH was verified by EIS (electrochemical impedance spectroscopy).The experimental results showed that the CS microspheres provided good anti-corrosion performance and self-healing ability to the protective coating due to the release of sodium phytate in the zones of artificial defects in the coating.
Jia et al.[145]prepared a double-layer self-healing coating structure to prevent biocorrosion of Mg-1Ca.The preparation process is shown in Fig.9a.The inner layer was a micro/nano-porous ceramic precoating,and the outer layer was CSCe (CS loaded with nano-sized cerium oxide).The preparation process of CSCe was as follows: 1 wt.%Ce(NO3)3·6H2O was added to the pre-prepared CS solution(1 wt.% CS+0.4% acetic acid),sonicated for 1 hour,and stirred overnight to obtain CSCe.The precoating acted as a barrier layer to improve corrosion properties and gain a complete structure (Fig.9b).The self-healing behavior of the M-CSCe hybrid coating is as follows (Fig.9c): environmental friendly CS utilized the complex chemistry of Ce-NH2to capture more Ce3+.It was also considered that coordination was the main reason for strong immobilization ability of CS and the prolonged release time of Ce3+.After the barrier layer is defective,it will be disintegrated by corrosion,resulting in a local pH increase.The pH triggered the formation of CeO2precipitates in double-layer coatings,enhanced the pH-buffering activity and swelling capacity of CS macromolecules,suppressed anode activity of Mg-1Ca and healed dynamic crack/defect propagation.

Fig.9.(a) Preparation process,(b) structure,(c) self-healing behavior of M-CSCe hybrid coatings [145].
The membrane of CS microcapsules is formed by the composite reaction of CS and negatively charged macromolecules(such as gelatin)through polyelectrolyte.There are tiny pores,which can only allow a certain amount of substances to enter and exit.And the yield of the reactants between CS and gelatin is affected by temperature and ionic strength,so it is necessary to select appropriate experimental conditions to optimize the CS microcapsule system [146].
3.3.Nanofibers
Adding corrosion inhibitor-loaded nanofibers to construct a network structure in polymer coatings (10–20 μm) can not only improve the mechanical properties of the Mg alloy,but also obtain self-healing behavior of the protective coating.By coaxial electrospinning,Dong et al.[42]prepared polyvinyl alcohol/PVDF core-shell nanofibers,and loaded them with MBT corrosion inhibitor.The MBT-loaded coaxial nanofibers reduced the corrosion activity and improved the barrier properties of the Mg alloy protective coatings.The addition of inhibitor-loaded coaxial nanofibers into epoxy coatings can effectively improve the anti-corrosion performance and ensure self-healing ability.Nawaz et al.[147]synthesized cellulose microfibers (CMFs),which were used as carriers to load dodecylamine (DOC) and polyethyleneimine (PEI).During the loading process,DOC was dissolved in ethanol and synthesized CMFs were added,and incubated at room temperature for 24 h.PEI followed the same steps.Centrifuged the two samples and dried at room temperature for 24 h to obtain CMF/DOC and CMF/PEI.The loaded-CMFs were completely dispersed into the epoxy resin coating,and a smart self-healing coating was formed on the surface of Mg alloy.UV–Vis spectroscopy demonstrated that the release of DOC and PEI was time-dependent and pH-sensitive.In addition,the impedance value of smart coating was higher than the reference coating (epoxy resin coating with CMFs only),confirming the effective corrosion behavior of Mg alloy coating.This was explained in a way that corrosion inhibitor (DOC and PEI) was efficiently released and formed a stable film to protect the Mg substrate.The synergistic effect of PEI and DOC contributed to the self-healing ability of the epoxy resin coatings.
Usually,electrospinning is applied to the preparation of nanofibers due to its simple operation.However,it is worth noting that electrospinning is difficult to obtain separated nanofiber filaments or short fibers,and the spinning efficiency is low.Some electrospinning systems need to be carried out in highly corrosive or highly toxic solvents.These all limit the further industrial application of solution electrospinning[148].
3.4.Cyclodextrin nanocontainer
CD is a cyclic oligosaccharide composed of several glucuronic acid units.It is an efficient complexing agent with a truncated cone-shaped structure.It has hydrophobic inner cavity and hydrophilic outer surface,thus,it can form complexes with different organic molecules suitable for the CD cavity size.Yang et al.[44]modified HMSN with cysteine and CD to form pH/glutathione/Mg(II) responsive nanocontainers.The pH/glutathione responsiveness utilized the charged amino acid functional groups and the thiol group of cysteine.Mg(II) also can form complexes with CD and has been confirmed to be pH-dependent.Its release behavior is shown in Fig.10.Further studies showed that the size of the drug or macrocyclic molecule as well as the thickness of the HMSN can affect the effective release of the corrosion inhibitor.The researchers also loaded a corrosion inhibitor in the carrier,which can mitigate the corrosion of AZ31 in 3.5% NaCl solution.Liu et al.[149]synthesized a novel GO/β-CD-based supramolecular nanocontainer with high impermeability and strong encapsulation ability.BTA is a corrosion inhibitor that was loaded into rGO–CD containers based on host guest interactions.This process was carried out under reduced pressure to ensure loading efficiency.Firstly,mixed the synthesized container with an ethanol solution containing BTA and stirred in vacuum for 5 h.And then,centrifuged (5000 rpm)and dried in a vacuum oven (-0.1 MPa) at 50 °C to obtain a container loaded with BTA.Doping the BTA-loaded nanocontainer made the coatings have efficient and durable self-healing and corrosion resistance.Local EIS was used to characterize the achieved self-healing behavior.The selfhealing function of the coating relied on the released BTA to form an adsorption layer on exposed surface.The impermeable GO nanosheets hindered the penetration of electrolyte and the extension of corrosion,additionally improving the corrosion performance.
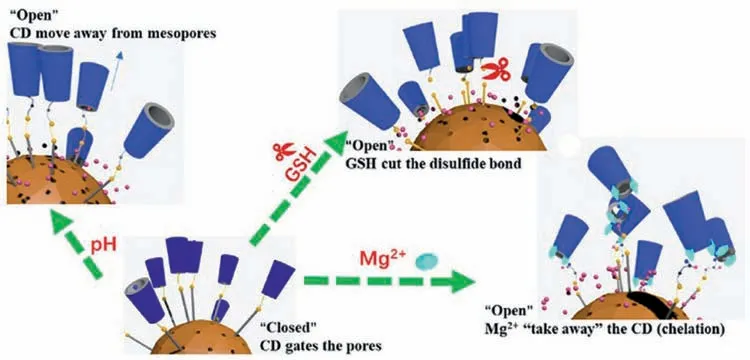
Fig.10.Schematic diagram of release behavior under different stimuli [44].
The interaction between the guest and CD host molecules is directly related to the adhesion strength.Therefore,the glass transition temperature (Tg) of the selected material should be lower than the ambient temperature to achieve sufficient chain mobility and appropriate interaction between the guest molecule and the CD ring [150].
3.5.Other organic nanocontainers
Micro-gels are micron-sized gel particles that combine solids and liquids as the active part of self-healing coatings.The particle morphology provided by the polymer network ensures mechanical stability of the coating,while the liquid corrosion inhibitor in the matrix maintains its fluidity,and the interaction enables the coating to have a self-healing function.Stankiewicz et al.[46]prepared gelatin micro-gels by the water-in-oil emulsion method as storage containers for environmentally friendly corrosion inhibitors.The effects of temperature,stirring speed,ionic and nonionic surfactants on the quality of the micro-gels were investigated.It was found that at 80 °C,micro-gels obtained from nonionic surfactant solutions with concentrations below their critical micelle concentration were the most stable and less polydisperse.A Ni-P-gelatin micro-gels hybrid coating was prepared by chemical method,which proved the feasibility of the method,it could be applied to Mg alloy surface to protect Mg alloy from corrosion.The corrosion inhibitors used were sodium dodecyl sulfate,dodecyl trimethyl ammonium bromide and tetraethylene glycol dodecyl.
In summary,organic nanocontainers have the characteristics of biodegradability,reproducibility,low cost,simple synthesis,good rigidity,eco-friendliness,high flexibility,and large specific surface area.Moreover,some organic nanocontainers are easy to form films,have excellent adhesion to Mg alloys and many organic polymers can reversibly form complexes with certain corrosion inhibitors.However,some microcapsules have unsatisfactory mechanical strength and can be easily broken during use.Therefore,it is very important to control the properties and chemical structure of the microcapsule shell to meet practical needs.
4.Nanocontainers based on carbon nanomaterials
Common carbon nanomaterials mainly include carbon nanotubes,fullerenes,and graphene.Carbon nanomaterials have many excellent physical and chemical properties and are widely used in various fields.In corrosion protection,the incorporation of carbon nanotubes into the coating can enhance the mechanical properties of Mg alloys [151],while graphene can build a composite coating with excellent corrosion protection.Combining the excellent physical properties of carbon nanomaterials with the active protection function of corrosion inhibitors can greatly improve the protection performance of Mg alloy composite coatings.Montemor et al.[47]used multi-walled carbon nanotubes to adsorb cerium or lanthanum nitrates,which were further mixed with bis-[triethoxy amino]silane and applied them on the surface of AZ31.It was found that carbon nanotubes can be uniformly dispersed in the silane coating and act as a carrier for corrosion inhibitors.The corrosion resistance of composite silane was improved compared to directly doped cerium or lanthanum nitrate in silane.
Wang et al.[152]prepared a magnetic poly-UF microcapsule with magnetic benzotriazole (MBTA) microcapsules as the core and magnetic multi-walled carbon nanotubes (MWCNTs) as the magnetic target.MBTA microcapsules were prepared using modified in-situ polymerization method.The MBTA microcapsules can be separated from the aqueous solution by magnetic separation technology (Fig.11).In short,urea,resorcinol,and ammonium chloride were dissolved in water,stirred,and sodium dodecyl benzene sulfonate aqueous solution was added to the mixture.Then BTA was dissolved in castor oil as a solvent and slowly added into the mixture to form a lotion of oil and water.After manual grinding,MWCNTs were added to the lotion (step 4),and hydrochloric acid was dropped to slowly adjust it to pH 3.5(step 5).Added formaldehyde aqueous solution and then ultrasonic treatment (step 6).Then raised the temperature to 60°C (step 7),stirred continuously for 2 h (step 8) to recover the polymerized microcapsules.The thermogravimetric analysis test results showed that the BTA microcapsules contained 12.8% magnetic carbon nanotubes.Magnetic MWCNTs participated in the formation of self-healing magnetic gradient(SMG) coatings.The MBTA microcapsules readily migrated in epoxy coatings due to an external magnetic field.Electrochemical tests showed that the corrosion inhibition rate of the coating can reach 91.2% after immersion in 0.6 M NaCl solution for 4 h.SMG coating shortened the migration distance of BTA and accelerated the formation of passivation layer.The SMG coating significantly improved the self-healing efficiency.
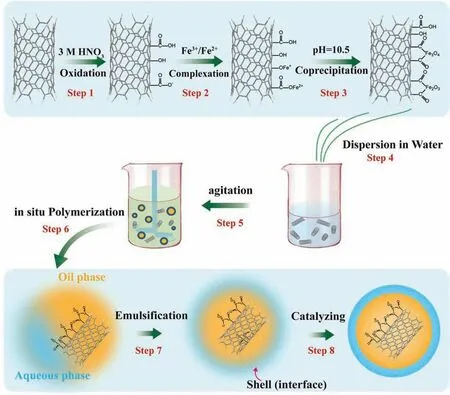
Fig.11.The process of MBTA microencapsulation [152].
In recent years,graphene coatings have been widely used to prevent Mg alloy corrosion since they can prevent the penetration of corrosive media.Johari et al.[48]reviewed the nanocontainer and inhibitor containing coatings with selfhealing ability,super hydrophobicity and biocompatibility,including GO coatings.Mg0.5Zr and Mg0.5ZrxZn (x=1~5 wt.%) matrix nanocomposites reinforced with different contents of graphene nanosheets were prepared by Shahin using powder metallurgy technique,demonstrating that graphene nanosheets were effective nano-enhancing particles for the improvement of mechanical and corrosion properties of Mg-Zr Zn matrix,and can endow Mg alloys with self-healing ability[153].Nikpour et al.[154]investigated the adsorption behavior of the corrosion inhibitor (nettle leaf extract) on GO nanosheets by experiments and density functional theory.It was found that the interaction force between GO and the active substances of nettle leaf extract was strong,and the corrosion inhibitor was easier to release under acidic conditions.In addition,when Zn2+was added,the corrosion inhibitor can form precipitates with Zn2+,thereby protecting the Mg matrix.
In summary,the nanocontainers based on carbon materials have the advantages of small size and large interfacial area,which can improve the mechanical properties of coatings.However,the preparation cost of some carbon nanocontainers is high,and it is difficult to achieve large-scale preparation under the current technical conditions.Meanwhile,some carbon nanocontainers have poor compatibility with the polymer materials.Unfortunately,the way how to solve their reduced protective effect on Mg substrates due to galvanic corrosion and aggregation remains a challenge [155].Table 4 presents common organic nanocontainers and carbon materials nanocontainers.

Table 4Common organic nanocontainers and carbon materials.
5.Synergistic inhibition of different nanocontainers
It can be seen from above that different nanocontainers have different response modes and coating capabilities.However,the self-healing and protection capabilities of a standalone nanocontainers are relatively limited.If the advantages of different nanocontainers are combined,and the nanocontainers are reasonably designed in coating application,the coating can be endowed with multiple self-healing properties and multiple effective protection mechanisms.The synergistic inhibition of different nanocontainers is of great significance for the development of high-performance smart self-healing protective coatings.Some researchers have studied the synergistic effect of different nanocontainers [159–161].
Using ZIF-8 as pH-responsive valve for a self-sacrificial template,Zhou et al.[162]successfully synthesized HMSN,and encapsulated the corrosion inhibitor BTA in the cavity (HMSN-BTA) and pores to fabricate a novel corrosion inhibitor-coated nanocontainer (HMSN-BTA@ZIF-8)(Fig.12).ZIF-8 was used as a multifunctional gatekeeper for HMSN-BTA.After adding HMSN-BTA@ZIF-8 to the epoxy resin coating on the surface of Mg alloy,the coating exhibited excellent anti-corrosion and smart self-healing properties.This was mainly attributed to the good compatibility between ZIF-8 and SiO2and between HMSN-BTA@ZIF-8 and epoxy resin coating,the hydrophobicity of ZIF-8,high cross-linking density and the controlled release of BTA.
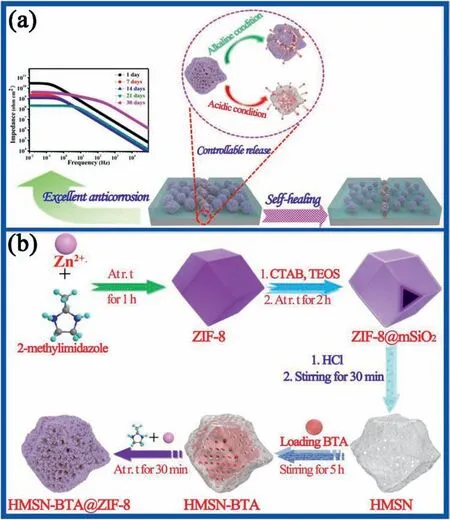
Fig.12.Schematic diagram of (a) the protection mechanism of smart anti-corrosion coatings and (b) pH-responsive synthesis of HMSN-BTA@ZIF-8 (The experiments demonstrated that the introduction of BTA@ZIF-8 not only blocked the micropores in the epoxy coating,but also increased the crosslinking density,resulting in a tortuous diffusion path of the electrolyte in the coating,showing good corrosion resistance.In addition,HMSN-BTA@ZIF-8 had obvious pH-triggering activity under both acidic and basic conditions.) [162].
Liang et al.[163]synthesized SiO2-IMI nanocomposites with high loading of the 1-hexadecyl-3-methylimidazolium bromide.The composite material had special acid/base dual stimulation to accelerate release of corrosion inhibitor.SiO2-IMI demonstrated high good compatibility with sol-gel coating.Once the Mg alloy was locally corroded,SiO2-IMI will quickly respond to the pH change in the corrosion area,release the corrosion inhibitor to form a dense film,inhibiting corrosion diffusion and achieving self-healing function.In addition,the super hydrophobic properties of the film enabled it to maintain good protective properties even after immersion in NaCl solution for 35 days,far superior to traditional sol-gel coatings.
Mirabedini et al.[164]prepared various nanocomposite epoxy resin coatings including synthetic UF-based microcapsules (5 wt.%) and purchased clay nanoparticles (1,2,3,5 wt.%).Linseed-oil (LO)-loaded PUformaldehyde-based microcapsules were prepared by in-situ polymerization,and then were surface-modified with 3-aminopropyltrimethoxysilane (APS) using the sol-gel method at pH below 7.5.Both the tensile strength and corrosion resistance of the APS-treated microcapsule and nanoclay particle samples were improved,mainly due to the interaction between the microcapsule shell and the epoxy resin coating,as well as the barrier of clay nanosheets.Test results showed that samples containing APS-treated microcapsules and nanoclay particles performed the best of all samples.And the APS-treated microcapsules were more easily broken during the scratching process,and had better corrosion resistance and self-healing properties.Yan et al.[165]designed a novel smart self-healing epoxy resin coating by introducing aminofunctionalized Ti3C2Tx-loaded ZIF-8@BTA composite fillers.The coating self-healed at the active corrosion site of Mg alloy.This was ascribed to the fact,that the pH change caused by electrochemical corrosion can induce BTA release,which then formed a corrosion inhibitor film at the corrosion site,thereby protecting Mg substrate.
Li et al.[38]combined PDA-functionalized Cu2+-doped GO,octadecylamine (ODA) and polydimethylsiloxane(PDMS).A POPG-Cu2+(PDMS/ODA/PDA/rGO–Cu2+) selfhealing superhydrophobic coating was prepared on the surface of AZ31B,which could restore the superhydrophobic properties and microstructure of the coating after chemical/physical damage (Fig.13).In addition,the prepared self-healing coatings also exhibited good mechanical stability,chemical durability and weather resistance.
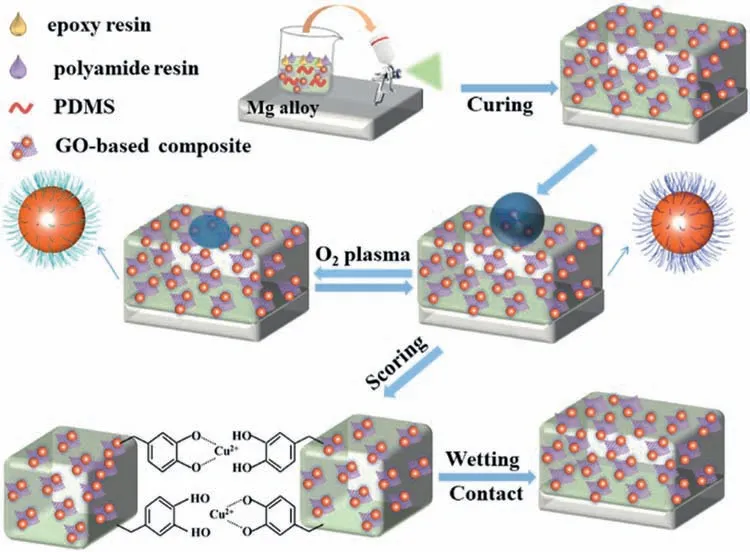
Fig.13.Preparation of self-healing superhydrophobic coatings and self-healing after chemical/physical damage [38].
The smart compound design of different nanocontainers can offer them better dispersibility and compatibility with Mg alloy coatings,and at the same time improve the loading efficiency of corrosion inhibitors.They can contain different stimuli-responsive release behavior.Through the complementary advantages of different nanocontainers,the Mg alloy protective coatings can be endowed with multiple self-healing properties and multiple effective protection mechanisms,but the complex process of compound design also hinders its large-scale production.
6.Autonomous self-healing mechanisms
Generally,metal corrosion can provoke the release of inhibitive species containing in coatings,which delay the further propagation of corrosion [166–168].So it can be considered as “active protection”.In other words,active protection is the passivation of the defective area by active species,so that the defective area can be fully or partially recovered(i.e.,protect the bare metal against further corrosion) [169].In general,two autonomous self-healing mechanisms can be involved: (1) the formation of a protective layer mainly due to the oxidation of the healing agent or other related reactions to fill defects,and (2) the formation of chelates/precipitates related to corrosion inhibitors to block the corrosion path [48].
6.1.Defect-filling effect by polymerization healing
Mechanical damage can stimulate the release of corrosion inhibitors and is often used in coatings with autonomous selfhealing mechanisms based on crack filling [170].To achieve self-healing effect in the defect,this section mainly focuses on a self-healing mechanism,that is the protection of Mg alloy surfaces by preloading microcapsules containing corrosion inhibitors (Fig.14).A good self-healing capability mainly depends on a good balance between the size,the number,and the chemical/mechanical properties of microcapsules [140].It is believed that an ideal microcapsule should have walls that are rigid enough to maintain an intact capsule and the sufficient coating of the substrate with the capsules embedded.In addition,the microcapsules also need to have good adhesion with the Mg alloy protective coating.In order to ensure the long-term effectiveness of the self-healing ability,the capsule material also needs to have good stability to chemical substances such as water,oxygen and others.As shown in Fig.15.
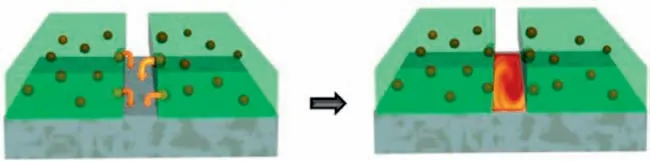
Fig.14.The corrosion inhibitors passivate the metal surface and fill defects [171].
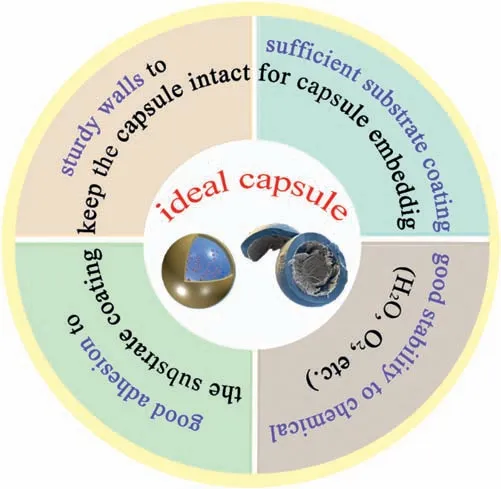
Fig.15.Requirements of ideal microcapsule.
6.2.Corrosion inhibition effect by blocking corrosion path
Numerous researchers have prepared anti-corrosion coatings containing loaded active species (corrosion inhibitors)in micro/nano containers.The purpose of these works was to obtain coatings,which are able to autonomously protect Mg alloy at the onset of corrosion by responding to environmental changes,enabling self-healing behavior of coatings[170–174].The schematic diagram of self-healing process from the nanocontainer loaded with corrosion inhibitor is shown in Fig.16.
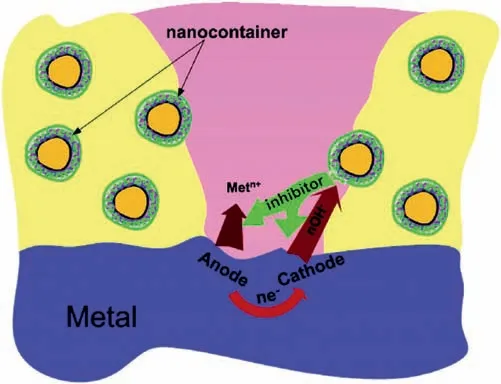
Fig.16.The corrosion inhibitor reacts with the ions generated by the metal matrix to fill defects [175].
For the SiO2inorganic nanocontainers,Qiao et al.[176]doped mesoporous SiO2loaded with MBT in epoxy sol-gel coatings.When Cl-migrate to the Mg/epoxy coating interface,the formation of Mg(OH)2releases H2(reaction 6),raising the local pH,the SiO2nanocontainers break up,allowing MBT to be released and filled into the damaged part.The self-healing mechanism of the coating is shown in Fig.17.
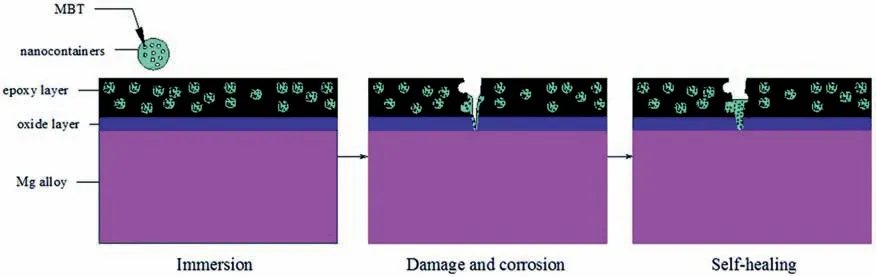
Fig.17.The self-healing mechanism of the coating [176].
HNTs inorganic nanocontainers can be described as an example of particles,with defect-filling effect,when applied from the coatings.Shaa et al.[53]loaded different corrosion inhibitors,Ce3+/Zr4+,MBT and 8-HQ,into HNTs and performed acid-assisted etching on the nanotubes to increase the lumen diameter,thereby increasing the loading of corrosion inhibitors.Cerium nitrate and zirconium-n-propoxide were used as sources of cationic corrosion inhibitors,while HQ and MBT are directly used as organic corrosion inhibitors.Polymer microcapsules were loaded with HNTs,which were subsequently dispersed in a sol-gel SiO2mixed matrix applied on AZ91D.In 3.5 wt.% NaCl buffer solutions with various pH,the kinetic mechanism of corrosion inhibitor released from HNT cavity was verified.Compared with other corrosion inhibitors,when the corrosion inhibitor Ce3+/Zr4+was loaded into HNTs,it has a more effective anticorrosion effect after being exposed to neutral or alkaline corrosive medium for 120 h.With the increase of the release amount of the cationic inhibitor,a stable passive film was formed at the defect,which filled the defect,avoided further corrosion of the Mg substrate and realized self-healing.Stability of zeolites loaded with corrosion inhibitor and their self-healing abilities from the doped coatings are largely related to the pH changes[177].
Furthermore,HNTs loaded with corrosion inhibitor can also be used as an additive for PEO electrolyte,assisting and participating in the formation of PEO coating on Mg alloy surface.As a PEO protective coating with autonomous self-healing ability,it can significantly improve the corrosion resistance of Mg alloys [93,178].Sun et al.[35]added BTA to HNTs,and then added it as an additive to PEO electrolyte to obtain a PEO coating with self-healing function on the surface of Mg alloy AM50.The results showed that,in addition to the important role of HNTs in promoting the mechanical properties of PEO coatings,the increase in pH at the corrosion site promoted BTA-mediated Mg(OH)2nucleation[179].Therefore,the formation of a dense Mg(OH)2layer at the corrosion site realized the self-healing of the damage and hindered pitting corrosion.
The other example can be based on Ce-related coatings.Song et al.[180,181]prepared La(NO3)3/Ce(NO3)3-containing gelatin-CS microcapsules by complex coacervation.And it was doped into the epoxy resin coating and coated on the surface of AZ91D to obtain a cerium-based conversion coating with a self-healing properties.These microcapsules were mainly distributed in the tiny cracks of the Mg alloy coating,which can effectively avoid the traditional porous state,that is,the active repair material (Ce3+or La3+)was released immediately after the coating integrity changes.Similarly,Wang et al.[182]prepared epoxy coatings containing MCM-22 zeolite and Ce-MCM-22 zeolite on the surface of Mg-Li alloys.It was demonstrated that the cathodic site plays an important role,and its pH values can be higher than 8.PH is the relevant trigger in the case of corrosion of Mg since a strong alkalinization occurs in the active defects at both nominally cathodic and anodic sites [183].At the cathode the oxygen and water reduction (reactions 1 and 2 respectively) lead to generation of hydroxyls [184–186],while at the nominally anodic side water is also reduced as a result of negative difference effect.As soon as corrosion starts,the cation-exchange properties of zeolite are enabled and a large amount of Ce3+can be released for further inhibition process.Then,Ce3+interacted with the hydroxide ions initiated by the cathodic redox reaction [187]to generate Ce(OH)3(reaction 3).The formed Ce(OH)3can act as a protective layer (the red position in Fig.18),providing a slight self-healing effect on the damaged area of origin.Furthermore,the Ce (III) can be further oxidized in alkaline environment to Ce (IV) by the oxygen present in the electrolyte (reaction 4).The stable and dense CeO2precipitation significantly delays corrosion process and partially hinders the cathodic reaction,especially the oxygen reduction process.On the other hand,during the anodic process,Mg and Li are dissolved (reactions 5 and 6)[188].
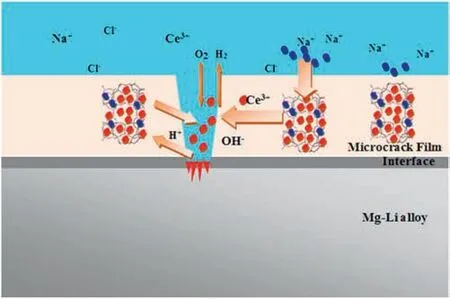
Fig.18.Response release process of Ce3+ [182].
The relevant reactions involved are shown below.The cathodic processes:
Chemical reactions:
The anodic processes:
For LDHs inorganic nanocontainers,it can exchange the inserted anions with the electrolyte,so Cl-exchanges with the inserted anions and is trapped between the interlayers of LDH,increasing the corrosion resistance of the Mg substrate[189].In addition,the inserted anions react with the Mg2+ions produced by prolonged immersion to form chelates.These chelates,which have self-healing ability,will deposit on the LDH coating surface to seal defects,block the active sites,and hinder the growth of defects [190–192].Zeng et al.[193]synthesized LDH coating containing molybdate anion intercalation (LDH-MoO42-) on the surface of AZ31,which had self-healing ability.As an inhibitor,MoO42-reacted with Mg2+to form stable compounds at pitting or crack sites,successfully preventing the penetration of aggressive ions,preventing Mg alloys from being exposed to the atmosphere,and showing good corrosion resistance.
Chen et al.[110]successfully synthesized 8-HQ@GO composites by ring-opening reaction.Without introducing any salts,based on the pre-grown PEO coating on AZ31,8-HQ@GO was doped into LDHs via a hydrothermal process(HG).A PEO-LDHs/HG composite coating with excellent anti-corrosion performance and self-healing ability was obtained [194].Its anti-corrosion mechanism was suggested as follows: (1) the LDHs nanosheets were dense and can hinder the intrusion of corrosive media through the coating until into the substrate,thus protecting the AZ31.(2) The high aspect ratio and permeation resistance of GO increased the tortuosity of conductive path of the corrosive medium in the composite coating.That allows the composite coating to have a complex structure sufficient to impede the penetration and transmission of corrosive media.(3) When a corrosive medium (such as Cl-) diffused into the damaged area,8-HQ was released,and the matrix dissolved to form Mg2+.Mg2+combined with 8-HQ to form a Mg(HQ)2protective film,achieving self-healing performance of damaged area.
For the GO nanocontainers,Chen et al.[195]prepared GO,PO43--intercalated hydrotalcite (PIH),and GO@PIH coatings on the surface of biomedical Mg alloys.Due to the superior barrier properties of GO,the prepared GO@PIH coatings had strong anticorrosion properties.Its self-healing mechanism can be attributed to the continuous ion exchange between Cl-and PO43-,which enabled the release of the corrosion inhibitor PO43-at the metal/coating interface.The released ions adsorbed and deposited a product layer at the corrosion site,thereby limiting the anodic reaction rate,and finally a phosphate film was formed on the Mg substrate surface for self-healing reaction.In addition,some studies have also proved that GO itself can be used as a corrosion inhibitor[49,196-198],so GO also plays an important role in the anticorrosion and self-healing of Mg alloy coatings.Fan et al.[199]added GO as a corrosion inhibitor on the cerium-based conversion layer,and provided the self-healing capability of the Mg alloy AZ31 coating system by coating PEI/PAA multilayers.The test results show that the proposed coating possesses rapid self-healing ability,and the remarkable result of the healing ability may be attributed to GO acting as a kind of barrier layer by preventing the penetration of corrosive electrolytes.Also,the pH values of PEI (9.5) and PAA (4.5) were adjusted,which allowed higher molecular mobility,favoring rapid self-healing of the layered coating.
In summary,autonomous self-healing coatings can achieve smart self-healing of coating defects without external energy supply,and it is hoped that it can be applied to practical industrial applications in the near future.However,as can be seen from the above,the corrosion inhibitor can suppress the corrosion reaction of Mg alloy for a period of time,but cannot completely restore the physical barrier.And the variety of nanocontainers for coating is still limited.The kinetics of crack repair and catalyst stabilization are for further investigated.Therefore,the choice of corrosion inhibitor is crucial for developing Mg alloy self-healing coatings.Desirable synergies may arise between different inhibitors.
7.Non-autonomous self-healing mechanisms
Non-autonomous self-healing coatings require an external stimulus,most commonly heat and light.In contrast to autonomous self-healing,the healing process can be repeated.Stimuli responses in these coatings can also be promoted by complementary corrosion-sensitive components that detects pH changes in the early stages of corrosion [200].These coatings are healed by restoring the physical conformation and/or inherent chemical bonds of the polymer network in the coating matrix.The effect increases the chemical stability and provides a self-healing option to the Mg alloy protective coating.The reaction mechanisms include dynamic bonds and shape memory effects,which are explained below [48].
7.1.Based on dynamic bonds
Supramolecular interactions of materials (hydrogen bonds,charge interactions,host-guest recognition,etc.) and dynamic chemical bonds interactions (imine bonds,acylhydrazone bonds,oximes,boronate esters,Diels-Alder reactions,disulfide bonds,coordination,etc.)have made them widely used in the field of smart self-healing coating [201].Common chemical compositions that can be used for reversible bonds in selfhealing polymers coatings have been comprehensively commented by different research groups [202,203].
Liu et al.[204]designed a disulfide bond-modified PU based on the corrosion inhibitor M-16 embedded in a PEO coating and self-healing PU.A smart dual-acting self-healing composite coating was then obtained for corrosion protection of Mg alloys.The scratched coating exhibited excellent anticorrosion performance recovery after heat treatment,and its self-healing mechanism relied on the following reasons(Fig.19):first,when corrosion products were pre-formed within the scratches of the composite coating,the PEO coating (embedded M-16) as an intermediate layer can effectively improve the repairability of the PU polymer coating by separating the polymer coating from the corrosion products.Second,the corrosion inhibitor M-16 can be released from the porous PEO coating to the scratched area for self-healing.Finally,the physical damage of the PU coating can be repaired by the synergistic effect of dynamic disulfide exchange reaction and shape memory effect after heat treatment.

Fig.19.Self-healing mechanism of smart double-acting self-healing composite coating [204].
Hydrogel is an organic material with an extremely high water content,composed of cross-linked polymers.Selfhealing hydrogels refer to hydrogels that can restore their integrity after damage [205–207].The self-healing hydrogels,constructed from dynamic chemical links,demonstrate fast self-healing ability,high link strength and mechanical strength.Cui et al.[208]constructed biocompatible Janus composite membranes with dual self-healing capabilities on Mg alloys surfaces.The corrosion inhibitor paeonol-doped poly(ε-caprolactone) as the bottom hydrophobic layer.PDA with better adhesion as the connecting layer.4-formylbenzoic acid-PEG-4-formylbenzoic acid (DFPEG) and PGD (imide-bond based glycol CS hydrogels) as the surface hydrophilic layer.The PGD of the hydrophilic layer was responsive to pH,and its imide-bond was formed by the reaction between the carbonyl group of DFPEG and the amino group of glycol CS,which can prevent the permeation of water,showing the strong anti-permeability ability.When the surface of the Mg substrate corroded,the imide-bond in PGD responded to the pH change caused by corrosion.
Under the acid condition given by the outside,the imidebond can be dissociated (Fig.20a3,pH=2,methylene blue turns yellow,and yellow liquid appeared at the bottom of the bottle),and re-formed under the alkaline condition(Fig.20a1,pH=10,methyl orange turned yellow,and no substance appeared at the bottom of the bottle).Therefore,the damage can be actively repaired and self-healing can be achieved(Fig.20b).Paeonol in the hydrophobic layer can delay the contact between Mg and corrosion medium and reduce the corrosion rate of Mg alloys.When the Mg substrate was degraded to generate Mg2+ions,paeonol and Mg2+undergo a complex reaction to form a chelating layer to repair the damage (Fig.20c).The dual self-healing synergistic effect between the hydrophilic and hydrophobic layers enabled the effective protection of Mg alloys.
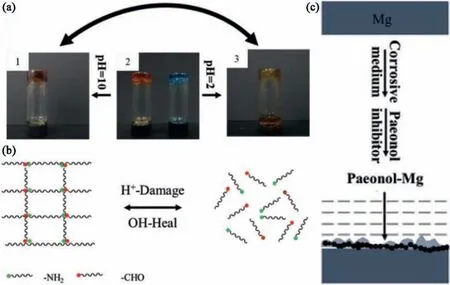
Fig.20.(a) Response of hydrogel to different pH values,(b) self-healing mechanism of hydrogel,(c) the formation of chelate layer on Mg [208].
7.2.Based on shape memory polymers
Shape memory polymers (SMPs) are a new type of smart polymer material with stimulus-response.It can perceive changes in the external environment and respond to external stimuli,thereby spontaneously adjusting its own state parameters and recovering from a temporarily fixed deformation to a pre-designed state [209,210].During the healing process of SMPs and polymer blends,it follows the molecular diffusion theory based on chain entanglement without reverse covalent/non-covalent bond formation and breakage[211].When the heating temperature is higher than Tgof the polymer or melting point of the thermoplastic polymer (Tm),the polymer molecular chains diffuse,resulting in physical sealing at the coating defects [212].
Zhang et al.[213]successfully developed fluorinated polysiloxane-modified multi-walled carbon nanotubes(SMP/PF-POS@MWCNTs) super-hydrophobic coatings with photo thermal self-healing property on Mg alloy AZ31B surfaces.The coating consisted of an SMP primer and an upper super-hydrophobic coating PF-POS@MWCNTs with good super-hydrophobicity and photothermal effect.At the same time,microcrystalline wax and BTA were added to the SMP primer.Microcrystalline wax can enhance the selfhealing properties of SMP coatings and help seal scratches.BTA helped avoid corrosion before self-healing.The coating exhibited good super-hydrophobicity,photo thermal effect and corrosion resistance to Mg alloys.The coating had good self-healing properties against chemical and microstructural damage (Fig.21).
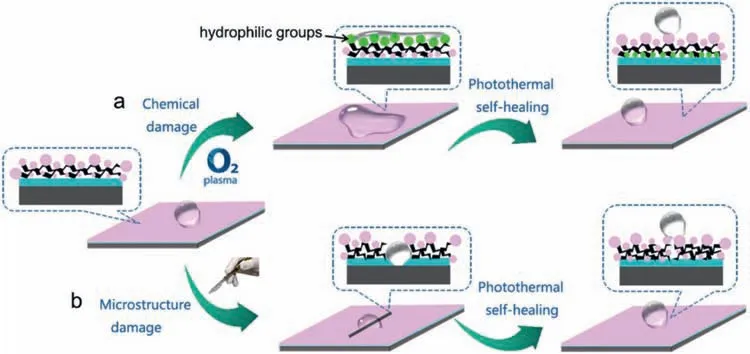
Fig.21.Self-healing of SMP/PF-POS@MWCNTs coatings under sunlight irradiation: (a) chemical damage (b) microstructural damage [213].
To investigate the self-healing properties of the SMP/PFPOS@MWCNTs coating against chemical damage,the coating was chemically damaged by O2plasma for 3 min(Fig.21a).The study found that the damaged coating can completely heal itself after 4 h of natural sunlight or 10 min of strong sunlight exposure.The stronger the light was applied,the faster the self-healing speed was achieved.In order to study the self-healing properties of SMP/PF-POS@MWCNTs coating on microstructure damage,the Mg alloy coating was scraped with a scalpel to introduce microstructure damage(Fig.21b).The damaged coating self-healed for a period of time under simulated sunlight.The impedance modulus and phase angle curves of the self-healing specimen were overlaid on the curves of the intact specimen,indicating complete self-healing for complex microstructural damage.These excellent properties were due to the synergistic effect of the PF-POS@MWCNTs coating and the SMP primer.
In summary,non-autonomous self-healing process relies on reversible physical or chemical polymer networks that can reduce coating defects,but these defects will always be exposed to corrosive environments before responding to artificial external stimulation.The performance of non-autonomous smart self-healing anti-corrosion coatings is mainly determined by polymer coatings.Being triggered by light,heat,etc.,the polymer coating can be repaired directly,greatly reducing the reliance on repair agents.In theory,the coating can be repaired an infinite number of times.Compared with autonomous self-healing coatings,non-autonomous healing coatings are more promising in terms of rapid crack healing,and can even repair large-area cracks.However,some protective coatings have high cost,limited use,potentially contaminate the environment and offer poor mechanical properties and weak fracture resistance.
8.Multiple self-healing mechanisms
Stimulating factors for the release of active substances in smart coatings include pH gradients,mechanical fragmentation,availability of aggressive ions,and mixed triggering in multi-action systems [171].In fact,more than 15 years ago,many researches have proved that smart coatings can provide multi-level active corrosion protection for Mg alloys[214–216].In terms of the specific stimulus responses,pHresponsive micro/nano containers that utilize pH changes to trigger the release of corrosion inhibitors have been developed (Fig.22a).The mechanical damage-responsive container is developed by encapsulating the inhibitor in microcapsules,which are then added to the coating and activate by mechanical impact (Fig.22b).The availability of aggressive ions is also used to release active species in self-healing coatings.In the coating design,the inhibitor is immobilized in the ionexchanged species.In such a case,corrosive substances (Clor other substances) trigger the release of inhibitors.The release mechanism relies on an exchange process between the inhibitor and the invasive species (Fig.22c).
Additionally,the realization of multiple repairs by designing and encapsulating of multiple functional repair substances has become a research trend in recent years.The POPGCu2+self-healing coating prepared on the surface of AZ31B by Li et al.[38]had good water repellency,which made the Mg alloy have good anticorrosion performance.Once the coating was scratched,the locally damaged structure was automatically repaired by chelation of catechol and Cu2+.The self-healing function originated from the rearrangement of ODA/PDMS molecules and the dynamic coordination of catechol-Cu2+.In addition,a large number of carboxyl and hydroxyl functional groups existed on the surface of GO,and these hydrogen bonds were beneficial to the self-healing behavior of the coating.After the coating was etched by O2plasma,its superhydrophobicity could be rapidly recovered even under solar irradiation.Furthermore,the as-prepared Mg alloys self-healing coatings still exhibited good corrosion resistance after immersion in 3.5% NaCl solution for 30 days.This was attributed to the efficient repair of GO defects by PDA throughπ-πinteractions and the inherent chemical inertia of PDMS (Fig.23).

Fig.23.Self-healing mechanism of POPG-Cu2+ coatings [38].
For the UF microcapsules organic nanocontainers,the selfhealing mechanism is to release the corrosion inhibitor encapsulated in the microcapsules,complete the self-healing at the coating defect,and protect the Mg alloy.Leal et al.[45]prepared encapsulated LO in the inner layer of UF microcapsules and embedded BTA into the polyelectrolyte in the outer layer of the microcapsules by LBL.The results showed that the epoxy resin smart coating containing microcapsules had good anti-corrosion performance and dual stimulus response.When stimulated by external stress,the microcapsules ruptured,releasing LO to heal the cracks.If the solidified film formed by LO at the defective area was not fully covered or had too low thickness,the solution will penetrate into the Mg matrix through coating cracks.The Mg alloy will be corroded,resulting in a pH change around the microcapsules in the coating.This leads to changes in the structure and properties of the microcapsule wall material,ultimately leading to the BTA release [217].The released BTA adhered to the Mg alloy surface,preventing electron transfer between the alloy and the solution,thereby inhibiting corrosion.Once corrosion was suspended,the pH returned to neutral,the release channel of BTA was reduced,and retained BTA could provide the long-term self-healing effect of the coating.This further reflected the multi-level active protection of smart coatings.
Zhang et al.[25]prepared a composite coating of nitrogendoped carbon dots (N–CDs) and PDA on Mg alloy AZ91D surfaces by a combination of electrodeposition and immersion plating.The preparation process is shown in Fig.24.The experimental results show that with the increase of particle size,the corrosion performance of N–CDs coating is enhanced.Moreover,the surface of the N–CDs/PDA composite coating exhibited obvious self-healing properties.The specific realization was that the PDA covered the cracks at the scratches.The cracks became shallow,indicating that the scratched coating had achieved the effect of self-healing.This self-healing effect originated from the diffusion and extension of PDA from surrounding surfaces [218].While the N–CDs coating acted as a transition layer to chemically bond with the Mg substrate and the PDA coating,while protecting the Mg substrate by forming a planar graphene structure to isolate the corrosive medium.

Fig.24.Preparation process of N–CDs/PDA composite coating on AZ91D [25].
Table 5 presents the applications of nanocontainers in coatings.It can be seen from it that there are still some shortcomings to be improved.To sum up,the combination of two coatings can result in a new generation of smart anti-corrosion self-healing coatings with wider adaptability and better performance.For instance,the addition of inhibitors-containing microcapsules to SMP coatings allows to achieve the dual repair of damaged coatings and provide longer-term stable protection for Mg substrates [219].

Table 5Coatings for nanocontainers applications.
Overall,the external self-healing mechanism only allows for one-time healing,while the internal self-healing mechanism allows for repeated healing.Therefore,the internal self-healing mechanism is more advantageous as it not only increases the service life of the material,but also provides longer corrosion protection.However,there are still many challenges to develop an ideal self-healing system.In the future,more new polymer materials and different self-healing mechanisms will be explored and studied to develop efficient self-healing materials [143].
9.Conclusions and future trends
Coating corrosion prevention is a very attractive method because it is one of the most effective,flexible,economical,and direct methods.“Smart” micro/nano container-based selfhealing coatings have been developed to protect metals and alloys,but industrial applications still require coatings with better performance and environmental benefits.In order to determine research needs,we reviewed relevant research in this field and reached the following main conclusions:
(a) Surface treatment has a significant impact on the durability of coatings.The common corrosion protection of anti-corrosion coatings is based on barrier performance,self-healing,active corrosion inhibition,anodic passivation,and cathodic protection.
(b) Doping micro/nano containers into the coating can significantly improve the barrier performance by reducing the porosity of the coating and increasing the tortuosity of corrosion media (such as H2O,O2,and Cl-) invading the alloy.Micro/nano containers can improve the chemical stability,uniformity and biocompatibility of self-healing protective coating on Mg alloy surface,as well as its adhesion to the substrate.
(c) The development of environmentally friendly polymers and micro/nano containers is a promising solution for creating more environmentally friendly nanocomposite“smart” self-healing coatings.
(d) Further research should examine the causal relationship between surface properties,water penetration,and corrosion rate of Mg alloys to evaluate the anti-corrosion behavior and durability of“smart”self-healing coatings.
The above-mentioned methods of corrosion inhibitors encapsulation in coatings based on nanocontainers can noticeably restore the anti-corrosion ability of damaged coatings to varying degrees.However,the effect of corrosion inhibitor only inhibits the corrosion reaction for a certain period of time,and cannot completely repair the physical shielding effect of the coating.Therefore,in the future development,in order to apply intelligent self-healing coatings of micro/nano containers (containing corrosion inhibitors) to Mg alloys,more in-depth and specific research is needed in the following aspects:
(1) The long-term self-healing of the Mg alloy protective coating can be improved through the strict selection of corrosion inhibitor types and the rational design and formulation of the micro/nano containers.Their selfhealing effect can be assessed by long-term corrosion tests in simulated or actual service environments.
(2) At present,the preparation process of most of the smart micro/nano containers loaded with suitable corrosion inhibitors is complicated and demanding,making it difficult to achieve large-scale industrial production.The structural properties of some materials (such as the sensitivity of LDHs to ambient Cl-) should be fully exploited to reduce complicated surface modification or post-treatment steps.
(3) There are still many challenges in size control of micro/nano containers,effective loading and controlled release of corrosion inhibitors,and uniform distribution within coatings.These issues should be solved for successful application of smart self-healing coatings.
(4) It is of great research significance to endow self-healing coating with corrosion self-warning function.When the corrosion inhibitor is exhausted and the self-healing function of the coating fails,timely self-diagnosis and early warning is necessary to perform the maintenance on the damaged site of the coating and ensure the service safety of the equipment.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Liang Wu,Mikhail L.Zheludkevich,Yuan Yuan and Fusheng Pan are editorial board member/editor-in-chief for Journal of Magnesium and Alloys and were not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (51971040,52171101),the Natural Science Foundation of Chongqing (cstc2021jcyj-msxmX0613),the National Natural Science Foundation of China(52001036,51971044)and the Independent Research Project of State Key Laboratory of Mechanical Transmissions (SKLMT-ZZKT-2022M12).
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
- Formation and growth of precipitates in a Mg-7Gd-5Y-1Nd-2Zn-0.5Zr alloy aged at 200 °C
