One-step hydrothermal synthesis of 2,5-PDCA-containing MgAl-LDHs three-layer composite coating with high corrosion resistance on AZ31
Fn Shi ,Jingmo Zho,b,* ,Mohmmd Tbish ,Jingbo Wng ,Pu Liu ,Jiyu Chng
a College of Materials Science and Engineering,Beijing University of Chemical Technology,Beijing 100029,China
b Beijing Key Laboratory of Electrochemical Process and Technology for Materials,Beijing 100029,China
Abstract The addition of 2,5-pyridinedicarboxylic acid (2,5-PDCA) to the Mg-Al LDH coating,which was prepared by one-step hydrothermal synthesis,had extremely enhanced the corrosion protection of AZ31 Mg alloy,although the 2,5-PDCA could not be intercalated into the interlayer spacing.The corrosion current density of 0.05 mol L-1 2,5-PDCA LDH containing LDH coating is 3.18 nA cm-2,reduced by two orders of magnitude compared to the LDH coating without inhibitor,and the corrosion inhibition efficiency of the coating is 98.05%.The coating formed on the surface of AZ31 was peeled off from the substrate by using a mechanical method and SEM observation of the cross-section showed that the coating consisted of three different layers.The innermost layer is a thick layer that consists of Mg(OH)2 and the intermediate layer is LDH,which is vertical to the substrate and the outmost layer is a thin but very dense deposit layer of LDH agglomerates with complexes of 2,5-PDCA and Mg.This kind of sediment/LDH/Mg(OH)2 three-layer composite structure was accountable for the increase in the corrosion resistance of AZ31 Mg alloy.
Keywords: Three-layer composite coating;Hydrothermal synthesis;2,5-PDCA;MgAl-LDHs;Peeling.
1.Introduction
Magnesium and its alloys are reflected as the lightest metallic material and have immense usage in various fields i.e.computer industry,communication industry,consumer electronics products,aerospace and automotive industries [1–5].However,Mg alloys have high chemical activity and poor corrosion resistance,which limits their application [6–10].In recent years,a variety of Mg alloy corrosion and protection methods have been proposed,including various protective films [11–16]and corrosion inhibitors [17–22].Apart from others,the addition of corrosion inhibitors is one of the most effective Mg alloy protection methods.A large number of corrosion inhibitors have been proposed,including quinaldinic acid [23],2,5-pyridinedicarboxylic acid (2,5-PDCA) [17],8-hydroxyquinoline (8-HQ) [24,25],malic acid and 5-aminosalicylic acid [17],etc.
Layered double hydroxide (LDH) has been widely studied due to its unique anion intercalation structure.LDHs,also known as hydrotalcite-like (HT) compounds,are an anionic clay with a brucite structure with tunable interlayer spacing and various anions in the laminate [26].The general molecular formula of LDHs is [M2+1-xM3+x(OH)2]x+(Am-)x/m·nH2O]x,where M2+and M3+represent divalent metal cations (e.g.Mg2+,Ca2+,Zn2+) and trivalent metal cations (e.g.Al3+,Fe3+),respectively [27,28].Am-is the interlayer anion(e.g.NO3-,Cl-,CO32-,PO43-),mis the charge of the interlayer anion,whereas,xrepresents the molar ratio of M3+/(M2++M3+),ranging from 0.20 to 0.33 [29,30].The corrosion inhibitor anion can be inserted between layers and exchanged with Cl-in the solution to be released owing to the anion-loaded structure and ion exchange characteristics of LDH [31].Therefore,LDH has great potential in the field of corrosion protection [32].In addition to the research of LDH powder,there are also more and more researches focus on the preparation of LDH by thein-situreaction.Zeng et al.[33]synthesized a molybdate intercalated hydrotalcite(MgAl-MoO42-LDH) coating with a nanoplatelet structure and divulges excellent corrosion protection for 144 h on AZ31.Anjum et al.[34]formed an 8-HQ intercalated MgAl-LDH coating on AZ31.The addition of 8-HQ has a significant effect on the morphology of MgAl-LDH,which enhanced the corrosion resistance properties due to the formulation of a chelate complex between Mg2+and 8-HQ.Lately,Chen et al.[35]prepared an aspartic acid intercalated MgAl-LDH coating on the surface of AZ31,and reduction of corrosion current density represents the excellent protection ability of LDH.In general,the corrosion resistance of magnesium alloys is significantly improved by inserting corrosion inhibitor anions into the LDH interlayer spacing.
LDH coatings are mainly composed of metal hydroxides,which are environmentally friendly and benign to the human body.LDH coatings loaded with green corrosion inhibitors have potential applications in neutral and alkaline conditions.In biomedical materials,Mg alloys are susceptible to corrosion and cannot be directly used as human implants [36,37].While the LDH coatings enable the Mg alloy matrix to be used in the human body by protecting the surface from corrosion.In addition,as compared to Ti alloys and high entropy alloys,Mg alloys are inexpensive.In the field of transportation,Mg alloys with LDH coatings loaded with corrosion inhibitors can meet the corrosion resistance requirements and replace Al alloy and steel for the structural materials of automobiles and airplanes,which can reduce the overall weight and thus greatly reduce energy loss.
The key to studying the structure of the LDH coating is the characterization of the cross-section.In the past research,there are two main research methods for the characterization of the cross-section.One of the methods is to seal the crosssection with epoxy resin and then sand it with sandpaper[33,38–40].The disadvantage of this method is that powder particles have been generated to fill the gaps during the grinding process,while the LDH layer is usually porous.Owing to this drawback,the porous cross-sectional coating face of LDH is difficult to observe which further accounts for the deviations in the judgment of its thickness and structure.The other method is TEM characterization [41].The internal structure and lattice information can be obtained by this method.However,the coating on the substrate needs to be ultra-thinly sectioned until the sample thickness is less than 200 nm before observation,which is costly and inconvenient for extensive testing.These factors limit the research on the structure of LDH.At present,most researches only discuss the thickness of LDH coating.The thickness of the coating will influence by lots of factors including different conditions,preparation methods,and the addition of different anions.Takahiro et al.[42]prepared a MgAl-CO32-LDH coating with a thickness of 80 μm on the AZ31 substrate using the method of steam coating.Wang et al.[43]prepared MgAl-CO32-LDH coating with a thickness of 25 μm on the AZ91D substrate by hydrothermal treatment.Lin et al.[40]prepared an LDH coating with a thickness of 5–8 μm on the AZ91D substrate via onestepin situgrowth method.However,only a few studies have observed loose and porous LDH structures on cross-sections.In these studies,the observed LDH multilayer structure is also reported.Vahdat et al.[41]used TEM to observe the doublelayer structure of the MgCe-LDH coating prepared by the hydrothermal synthesis method,indicating that both a dense layer and a porous layer exist in the coating.Zeng et al.[33]also observed a dense layer and a porous layer in the MgAl-MoO42-LDH prepared by the hydrothermal method using the epoxy resin sealing method.However,the explanations of the components and causes of each part are insufficient.In order to explore the composition structure and formation reasons of LDH coatings,it is very important and essential to explore a new method.
In this work,the high-efficiency inhibitor 2,5-PDCA was added during the preparation of the LDH coating by the onestep hydrothermal synthesis method.The influence of concentration on the surface morphology and corrosion resistance of the coating was explored.A new peeling method that causes brittle fracture was developed to peel off the LDH coating from the substrate for characterization.Moreover,the crosssectional structure of the coating was observed and the elemental analysis was used to determine the different layers of the composite structure.The formation process of the coating was also examined by observing the surface morphology under different reaction times.
2.Experimental methods
2.1.Preparation of MgAl-LDH coatings
Commercial AZ31 Mg alloy was used as the substrate and cut into pieces of 25 × 25 × 1 mm3size.The samples were ground with SiC waterproof abrasive paper with the grit size of 400,800,1000,1200,1500,and 2000,respectively.The polished samples were rinsed with deionized water,wiped with alcohol cotton balls,and dried with warm air.All samples were prepared by a one-stepin-situhydrothermal synthesis method.
MgAl-CO32-LDH was used as a control group.As shown in Fig.1,Mg(NO3)2·6H2O and Al(NO3)3·9H2O were dissolved in 75 mL of deionized water at a ratio of [Mg2+]:[Al3+]=3.0 to form solution A.The concentrations of Mg(NO3)2·6H2O and Al(NO3)3·9H2O were 0.15 mol L-1and 0.05 mol L-1,respectively.The anhydrous Na2CO3and NaOH were dissolved in 75 mL of deionized water to form solution B according to the ratio of[OH-]:[Mg2++Al3+]=1.6,[CO32-]:[Al3+]=2.0.The concentrations of Na2CO3and NaOH were 0.10 mol L-1and 0.32 mol L-1,respectively.The B solution,contained in the cylindrical separatory funnel,was slowly dropped into the three-necked flask containing solution A.The three-neck flask was stirred in an oil bath at 80 °C,under the N2atmosphere,and a condenser tube was used to prevent evaporation.NaOH(2 mol L-1) was used to adjust the pH of the mixed solution to 10.The AZ31 substrate was hanged into the Teflon-lined autoclave with a capacity of 200 mL.The mixed solution was then poured into the autoclave and placed in an oven at 125 °C for 24 h.After the reaction,the autoclave was normalized at an ambient condition for 4 h.The coated samples were rinsed with deionized water,wiped with alcohol cotton balls,and dried for characterization.The LDH solution was suction filtered,dried and ground to obtain LDH powder.
Compared with the control group,the preparation method of LDH containing 2,5-PDCA only changed the composition of solution B while the other processes were completely the same.Solution B of LDH containing 2,5-PDCA was prepared by dissolving different concentrations of 2,5-PDCA and 0.32 mol L-1NaOH in 75 ml deionized water.The corrosion inhibitor 2,5-PDCA in solution B was tested at three different concentrations of 0.01 mol L-1,0.05 mol L-1and 0.1 mol L-1.Before adding solution A,solution B containing corrosion inhibitor needs to be activated by heating and stirring at 40 °C for 20 min.
The resulting coated samples are named MgAl-CO32-LDH,MgAl-PDCA0.01LDH,MgAl-PDCA0.05LDH and MgAl-PDCA0.1LDH,respectively.Whereas,the LDH powders are ascribed as MA-C LDH powder,MA-P0.01LDH powder,MA-P0.05LDH powder and MA-P0.1LDH powder,respectively.The reagents used in this work,including 2,5-PDCA,are all analytically pure.
2.2.Electrochemical measurement
The potentiodynamic polarization (PDP) curves and electrochemical impedance spectroscopic (EIS) tests were used to measure the electrochemical performance and corrosion resistance of the LDH coatings.Gamry (Interface 1000,model 07130) was tested in a three-electrode system consisting of a rectangular platinum counter electrode,a saturated calomel electrode (SCE) as a reference electrode and a working electrode exposed to an area of 1 cm2.In order to stabilize the system,open circuit potential(OCP)was measured for at least 30 min with the working electrode soaked in 3.5% NaCl.EIS was tested with an AC amplitude of 10 mV in the frequency range from 100 kHz to 0.05 Hz.ZSimpWin 3.5 software was used to fit the EIS spectra and the goodness of fitting was assessed by the values ofχ2and it was accepted when≤5×10-3was observed[29].PDP was measured with a scan rate of 0.5 mV s-1and a potential scan range of±500 mV vs.OCP.All the electrochemical tests were carried out in 3.5%NaCl solution with neutral pH.The 3.5% NaCl solution was considered as artificial simulated seawater and widely used for corrosion tests [31–36].
2.3.Coating peeling and characterization
To determine the effective intercalation of the corrosion inhibitor,XRD (Ultima IV,Rigaku) was used to characterize MgAl-CO32-LDH powder and MgAl-PDCAx(x=0.01,0.05 and 0.1) LDH powders in a range of 5–90° MDI Jade 6 was used to analyze XRD data.The chemical properties of LDH powders were identified by FTIR (Bruker,TENSOR-27) with a wavenumber range of 400–4000 cm-1.Besides,the surface morphology of LDH coated samples with different corrosion inhibitor concentrations and different reaction times were also characterized by scanning electron microscopy (SEM) (Hitachi S4800).Furthermore,the stripped coating was prepared by repeatedly bending the samples.LDH coating triggered a brittle fracture and was peeled off from the substrate due to the superplasticity of AZ31 Mg alloy and the brittleness of metal hydroxide,as shown in Fig.2.The stripped coating after grinding into powder was also characterized by the XRD (Ultima IV,Rigaku).The cross-sectional morphology of the stripped coating was also characterized by SEM,and the elemental analysis was measured by energy dispersive x-ray spectroscopy (EDS) attached to the SEM facility.In order to ensure the accuracy of the coating thickness,the cross-section of the substrate after peeling was characterized by SEM and EDS.Image-pro plus 6.0 was used to measure the length in the SEM images.In order to explore the coating formation process,the surface morphology under different reaction times was characterized by SEM.The bonding type of C and O elements on the coating surface is characterized by XPS(Thermo,K-alpha).Avantage was used to split and fit XPS data.

Fig.2.Schematic illustration for the peeling of the coating.
3.Results and discussion
3.1.Characterization of LDH powder
The powder LDH samples were characterized by XRD and FTIR to determine the successful synthesis of LDH as well as the intercalation between the spacing layers.Fig.3(a)shows the XRD results of MA-C LDH powder and MA-Px(x=0.01,0.05 and 0.1) LDH powders.It can be seen from the figure that there are LDH characteristic peaks of (003),(006) and (009) in all four types of LDH which confirms the successful synthesis of LDH [44].Table 1 shows the base layer spacing calculated from the 2θof the (003) peak using Bragg’s law (2dsinθ=nλ).The results show that there is almost no change in the layer spacing with the addition of 2,5-PDCA,which means that the 2,5-PDCA molecules could not intercalate into the interlayer [30].
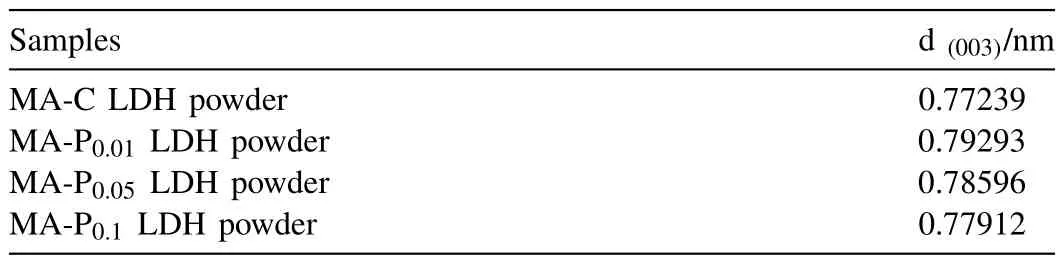
Table 1Layer spacing of LDH powders.

Fig.3.(a) XRD patterns of MA-C and MA-Px (x=0.01,0.05 and 0.1) LDH powders.(b) FTIR patterns of MA-C and MA-Px (x=0.01,0.05 and 0.1)LDH powders.
Fig.3(b)shows the FTIR spectra of MA-C LDH,MAPx(x=0.01,0.05 and 0.1) LDH powders and 2,5-PDCA powder.The vibration at 447 cm-1signifies the connection between Mg and hydroxide [34],similarly at 654 cm-1resembles the connection peak between Al and hydroxide [34],while the peak appears at 1382 cm-1is related to nitrate or carbonate [40],and 1612 cm-1is the peak position of interlayer binding water [40].3470 cm-1denotes the -OH peak position [40].It can be seen from the figure that with the increase of the concentration of PDCA added,a number of stretching vibrations appear at the position less than 1000 cm-1,which comes from the binding peak of 2,5-PDCA and Mg as 2,5-PDCA can form a weak complex with Mg[17].
Fig.4 shows the SEM micrographs of MA-C LDH and MA-P0.05LDH powders.The hexagonal LDH typical crystal grains can be observed in both of the figures.The MA-C LDH powder grain size range is 70–250 nm,most of the grain size between 100 and 200 nm.MA-P0.05LDH powder has obvious agglomeration and can be observed grain size of about 60 nm,but also the existence of grain size of 350 nm.
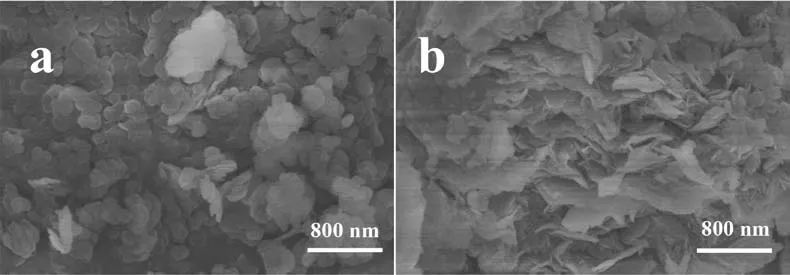
Fig.4.SEM micrographs of (a) MA-C LDH powder and (b) MA-P0.05 LDH powder.
The difference is that the LDH powder added with 2,5-PDCA agglomerates into a large mass,while the MA-C LDH powder is more dispersed.
This shows that 2,5-PDCA molecules are likely to be physically adsorbed on the surface of LDH through hydrogen bonds.In addition,2,5-PDCA molecules can form complexes with Mg.According to the hydrogen bond theory and chemical bond theory in the hard agglomeration mechanism of powders,the hydrogen bonds and chemical bonds between molecules are likely to cause hard agglomeration of nanopowders [45,46].Combined with the XRD and FTIR results of LDH powders,it can be seen that 2,5-PDCA is not inserted between the layers.Instead,it adsorbs on the surface of LDH and makes LDH powder easier to agglomerate.
3.2.Characterization of the stripped coating
The XRD results of the stripped coating are shown in Fig.5.It can be seen from the figure that there are obvious Mg(OH)2diffraction peaks in the XRD patterns of stripped coating.Meanwhile,there are weak diffraction peaks that correspond to the characteristic peaks of (003),(006) and (009)crystal planes of LDH crystals.It is proved that the stripped coating contains a large amount of Mg(OH)2crystal grains and a small amount of LDH crystal grains.In other studies,the Mg(OH)2peak is common in the XRD results of the hydrothermally synthesized LDH coating [34,35,41,47].It proves that Mg(OH)2is ubiquitous in LDH coatings prepared by the hydrothermal method.
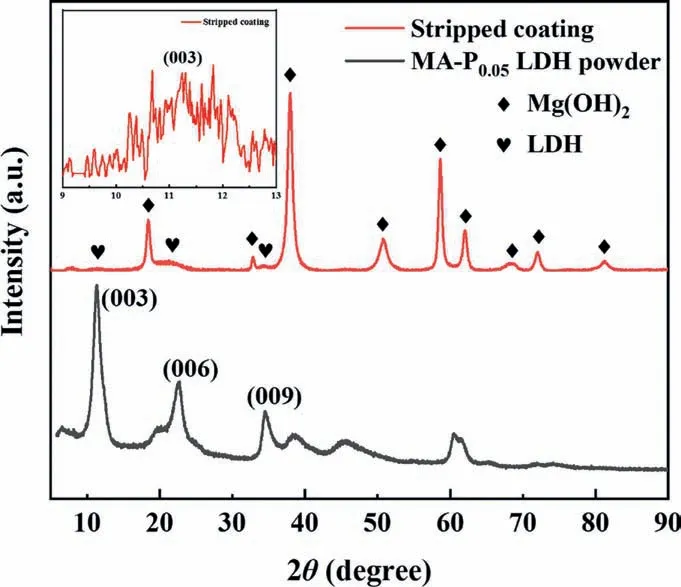
Fig.5.XRD patterns of MgAl-PDCA0.05 LDH stripped coating and MA-P0.05 LDH powder.
3.3.Morphological analysis
The SEM micrographs of MgAl-CO32-LDH and MgAl-PDCAx(x=0.01,0.05 and 0.1) LDH coating surface are shown in Fig.6.It can be seen from the figure that the surface of MgAl-CO32-LDH is flake-shaped vertical dendrite growth,which is loose and porous.The crystal size ranges from 100 to 400 nm,and the thickness is less than 50 nm.The surface of the coating added with 2,5-PDCA is no longer the morphology of the vertical growth of LDH grains but a denser planar film.The formed surface film of 0.01 mol L-12,5-PDCA added LDH is uneven and there are a lot of defects and large holes.The LDH surface film layer added with 0.05 mol L-12,5-PDCA is dense and uniform,and the LDH surface film layer added with 0.1 mol L-12,5-PDCA has blocky protrusions.The SEM results reveal that the compactness of the surface film is related to the concentration of 2,5-PDCA added.The morphology is the densest and most uniform with the addition of 0.05 mol L-12,5-PDCA.

Fig.6.SEM micrographs of AZ31 with (a,b) MgAl-CO32- LDH coating (c,d) MgAl-PDCA0.01 LDH coating (e,f) MgAl-PDCA0.05 LDH coating (g,h)MgAl-PDCA0.1 LDH coating,respectively.
Fig.7 shows the SEM images of the stripped MgAl-PDCA0.05LDH coating.It can be seen from Fig.7(a)that the total thickness of the stripped coating is 19.9 μm.There is no obvious bending deformation,which indicates that the stripped coating is a brittle fracture so that the original morphology of the composite coating is retained.The coating consists of three parts.The inner layer is about 18.5 μm.The EDS result in Fig.7(a1)shows that it contains a large amount of Mg,O and a small amount of Al.Combined with the XRD results of the stripped coating,it is shown that the layer is Mg(OH)2,and a small amount of Al is mainly derived from the 3% Al content present in AZ31.Fig.7(b)displays that the middle layer is loose and porous and the crystal grains are arranged vertically,which conforms to the characteristics of LDH crystal grains,and the thickness is 1.2 μm.The EDS result of this part is shown in Fig.7(b1).This layer contains an excessive amount of C,N,and O elements,as well as Mg and Al elements.The content of the Al is higher than that of the Mg(OH)2layer and the ratio of Mg to Al is close to 3:1,indicating that the layer is MgAl-LDH.The C element mainly comes from the 2,5-PDCA molecule because the experimental process completely occurs in a nitrogen atmosphere to avoid too much CO2dissolving in the solution.Since 2,5-PDCA is not inserted between layers,it verifies that the large amount of 2,5-PDCA is adsorbed on the LDH grains grownin-situ.In addition,Fig.7(c)demonstrates that the thickness of the outermost layer is about 0.2 μm.The EDS result in Fig.7(c1)shows that the element composition is similar to that of the LDH layer,but the content of Mg and Al elements is reduced,and the content of C element is increased,which indicates that the content of LDH grains decreases and the ratio of 2,5-PDCA molecules increases.At the same time,it can be observed that the elemental content of Al is very low,which indicates that most of the Mg exist in the form of complexes of 2,5-PDCA molecules rather than in the form of LDH.The results show that the layer is mainly composed of complexes of 2,5-PDCA molecule with Mg and a small amount of LDH crystal grains.

Fig.7.SEM micrographs and EDS analysis of the stripped coating of MgAl-PDCA0.05 LDH.
Fig.8 shows the EDS result of line scanning on the surface of the cross-section of the stripped coating.It can be seen from the figure that 2.3 μm and 3.7 μm on the abscissa are the boundary point of different layers.In the range of 0–2.3 μm,the ratio of Mg and O elements is very high,which is consistent with the EDS result of the spot scanning.This shows that the inner layer is Mg(OH)2.The middle layer is relatively loose in the range of 2.3–3.7 μm on the abscissa.The line scanning result of the Al element shows that the Al content at this layer first increases significantly,and then decreases.The significant increase in Al content indicates that the layer is MgAl-LDH.Due to the existence of voids in this layer,sufficient depth was not hit during the line scanning,and the content was not accurate enough,so the content of the Al dropped after that.There is a grain on the surface of the cross-section in the range of 2.3–2.8 μm on the abscissa.Its elemental content is more representative of the elemental content of the middle layer.The increase of C content indicates that there are a large number of 2,5-PDCA molecules in the middle layer,which is consistent with the EDS result of spot scanning.In the outermost layer on the abscissa of 3.7–3.9 μm,the content of Al and Mg elements decreases,while the content of the C element is still high,which is consistent with the EDS result of spot scanning.

Fig.8.EDS line scan result of stripped coating of MgAl-PDCA0.05 LDH.
Fig.9(a)is the cross-section of the substrate after peeling.The SEM cross-section exhibits that there is still a coating with a thickness of 11.5 μm remaining on the substrate after peeling.The EDS result in Fig.9(b)confirms that the coating consists of Mg(OH)2.Combined with the cross-section of the stripped coating,it can be seen that the total thickness of the Mg(OH)2layer is 30 μm.Fig.10 shows the schematic illustration of the thickness of the three-layer composite coating.Fig.9(c-d)is the SEM morphology of the substrate surface after peeling.From the figure,it can be observed that the Mg(OH)2crystal grains are "rice-like" compactly distributed.The reason for this morphology is that AZ31 reacts under high temperature and high-pressure conditions in an autoclave.After the reaction,the pH value is reached in the range of 12–13,which does not drop but rises compared to the pH value of 10 before the reaction.It also shows that a large amount of Mg from the substrate reacts with water to generate OH-ions as in Eq.(1).

Fig.9.Cross-sectional (a) SEM micrographs and (b) EDS spectrum of the substrate after peeling,(c-d) SEM images of the surface after peeling off the coating.

Fig.10.Schematic illustration of the thickness of the three-layer composite coating.
From the literature,it has been assumed that both the porous and dense layers are LDH layers as reported by Vahdat et al.[41]used Ce3+as trivalent and Zeng et al.[33]added MoO42-inorganic anion in the preparation of LDH.But,the results show that the Mg(OH)2layer is very common to formulate in the preparation of LDHs,which is also consistent with the obtained XRD result of stripped coating.
3.4.Characterization of the coating formation process
C1s and O1s high-resolution XPS spectra of MgAl-CO32-and MgAl-PDCA0.05LDH coatings are shown in Fig.11.C1s spectrum of MgAl-CO32-LDH coating is deconvoluted into two peaks appearing at 284.87 and 288.79 eV.The peak at 284.87 eV is attributed to C–C/C–H groups and the second peak at 288.79 eV is attributed to carbonate species(Fig.11(a))[47].Furthermore,the O1s spectrum of MgAl-CO32-LDH coating is deconvoluted into two peaks representing the carbonate ion and Mg(OH)2at 532.74 eV and 531.20 eV,respectively(Fig.11(c))[34].C1s spectrum of MgAl-PDCA0.05LDH coating is deconvoluted into four peaks appearing at 284.85 eV,287.13 eV,289.28 eV and 291.49 eV,respectively.The peak at 284.85 eV is attributed to C–C/C–H groups [47].The second peak at 287.13 eV is assigned to C–N group [34].The third peak at 289.28 eV is attributed to O=C-O from the carboxylic acid group of 2,5-PDCA[47].Whereas,the last peak at 291.49 eV is attributed to theπ→π*conjugate peak from the pyridine ring of 2,5-PDCA(Fig.11(b)).In addition,the O1s spectrum of MgAl-PDCA0.05LDH coating is deconvoluted into two peaks appearing at 531.45 eV and 533.77 eV.The peak at 531.45 eV is attributed to Mg(OH)2.The peak at 533.77 eV is attributed to O-(C=O)-C of organic molecules,which is derived from the carboxyl group on the 2,5-PDCA(Fig.11(d))[48].This indicates that the outermost layer of MgAl-PDCA0.05LDH coating contains a large number of 2,5-PDCA molecules and some LDH grains.
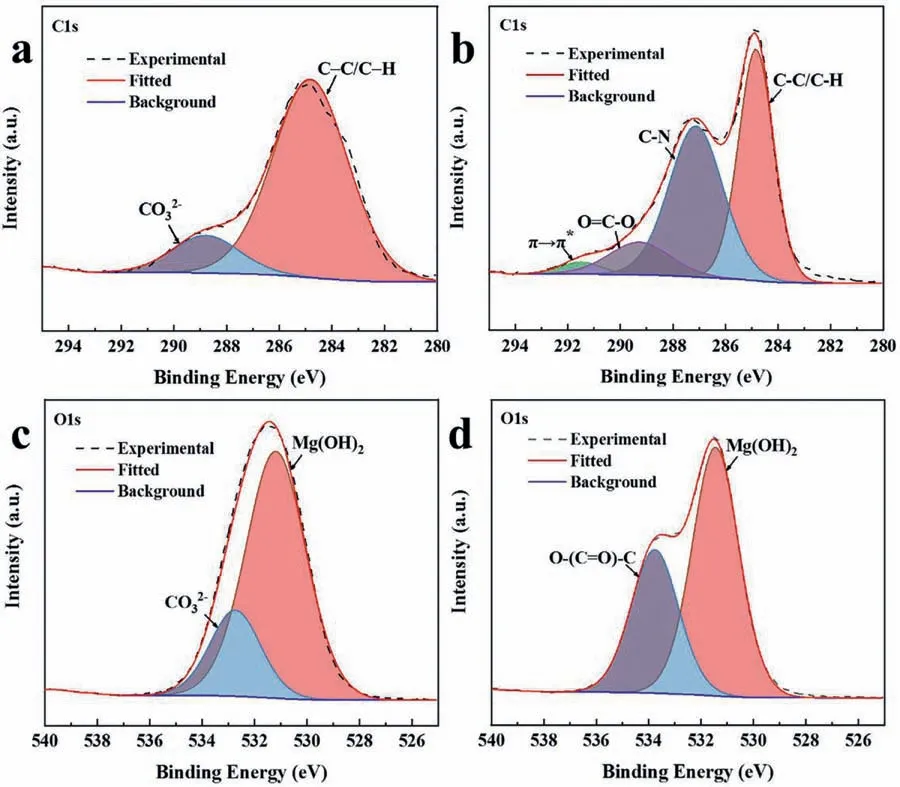
Fig.11.XPS (a) C1s and (c) O1s high-resolution spectra of MgAl-CO32-,(b) C1s and (d) O1s spectra of MgAl-PDCA0.05 LDH coatings on AZ31.
Fig.12 shows the surface morphology of MgAl-PDCA0.05LDH coating with reaction times of 1,3,6 and 12 h,respectively.It can be seen from the figures thatin-situgrowth of LDH has been formed on the surface of the film 1 h after the start of the reaction.The size of the LDH grains is about 50 nm.In addition,some defects with the same orientation can be observed on the surface,which may originate from the scratched grooves produced during the pre-grinding of the substrate(Fig.12(a-b)).After 3 h of reaction,LDH on the surface has grown up with a grain size of 300–400 nm.Meanwhile,some planar block structures are deposited on the vertical LDH surface(Fig.12(c-d)).After 6 h of reaction,the vertical LDH was covered.Along with some holes,the surface film is composed of crystal grain connections.The crystal grain size is 100–200 nm(Fig.12(e-f)).After 12 h of reaction,the boundaries of the crystal grains became blurred compared with 6 h.Grains gradually grow into a whole,while there are still some holes with a size of about 50 nm(Fig.12(g-h)).The results showed that the LDH layer grownin-situwas covered by the deposits after the formation,and the small particles and the deposits were connected to form a unique surface dense layer.Combined with the SEM micrographs of the LDH powder in Fig.4,it can be inferred that the block structure is the hard agglomerated LDH nanopowder deposited on thein-situLDH.

Fig.12.SEM micrographs of MgAl-PDCA0.05 LDH coating prepared for (a,b) 1 h,(c,d) 3 h,(e,f) 6 h,(g,h) 12 h,respectively.
3.5.Electrochemical test
3.5.1.Potentiodynamic polarization
The potentiodynamic polarization curves are shown in Fig.13.The corrosion current density was determined by the extrapolation of the cathodic branch.A line was drawn in the cathodic branch between the corrosion potential (Ecorr) and-100 mV(vs.OCP) (Fig.13b).The corrosion current of the point at which the slope line intersects with the horizontal line drawn from theEcorrwas considered asicorr[49].The fitting results are shown in Table 2.Compared with MgAl-CO32-LDH coating,theEcorrof the 2,5-PDCA-contianed LDH coating was significantly increased,indicating that the coating with 2,5-PDCA could inhibit the dissolution of magnesium in the anodic reaction.The achieved corrosion current density (icorr) of the LDH coating added by 0.01,0.05 and 0.1 mol L-12,5-PDCA is two orders of magnitude less than that of the MgAl-CO32-LDH(1.63×10-7A cm-2)coating.Theicorrof MgAl-PDCA0.05LDH coating is the lowest among the three concentrations,which is 3.18 ×10-9A cm-2.From the corrosion efficiency and the polarization curves,it can be seen that when the concentration of 2,5-PDCA used is 0.01 mol L-1,the breakpoint potential appears in the anodic region of the polarization curve and the corrosion resistance of the film is poor.With the increase of the concentration,the corrosion efficiency reaches the maximum value (98.05%) at the concentration of 2,5-PDCA is 0.05 mol L-1.The corrosion inhibition efficiency decreases when the concentration continues to increase.The results show that the corrosion resistance of LDH coating is significantly improved after adding 0.05 mol L-12,5-PDCA.
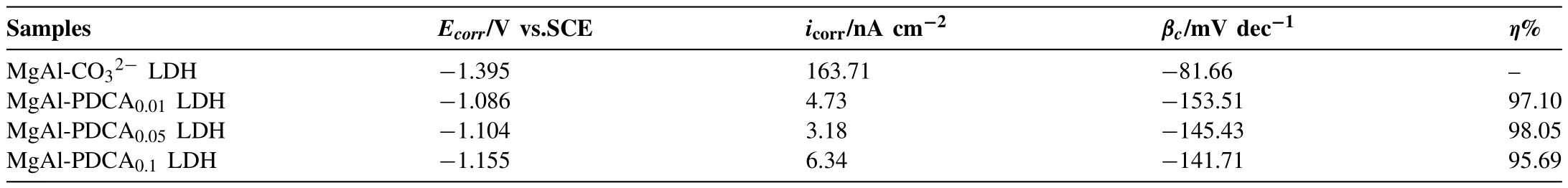
Table 2Fitting results of potentiodynamic polarization test.
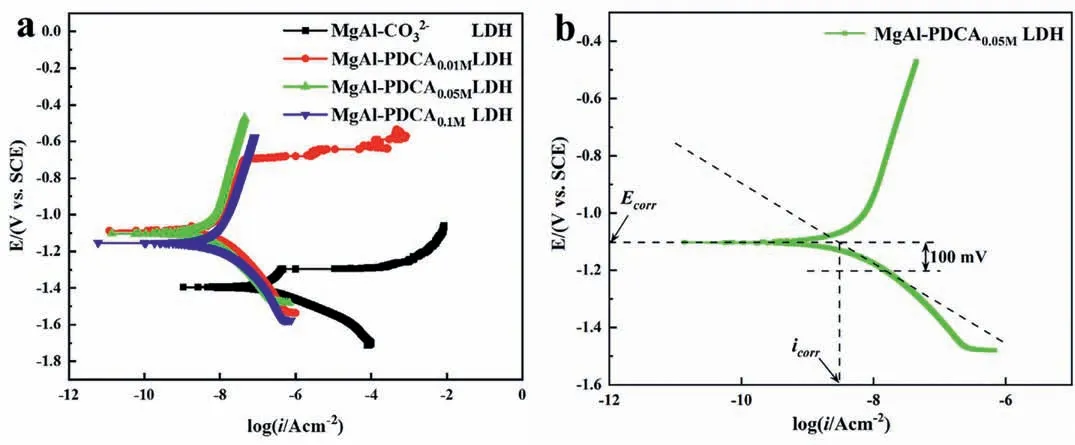
Fig.13.(a) Potentiodynamic polarization curves of AZ31 with MgAl-CO32- LDH and MgAl-PDCAx (x=0.01,0.05 and 0.1) LDH coating.(b) Illustration of determining icorr from Tafel plot.
3.5.2.EIS test
Fig.14 shows the EIS test results and equivalent circuit of MgAl-CO32-LDH and MgAl-PDCAx(x=0.01,0.05 and 0.1) LDH coatings.The low-frequency impedance modulus (|Z|0.05Hz) of MgAl-CO32-LDH and MgAl-PDCAx(x=0.01,0.05 and 0.1) LDH coatings are 2.55 × 103,2.50 × 105,5.81 × 105and 5.58 × 105Ωcm2,respectively.The fitting data is shown in Table 3.There is a back inductance part in the Nyquist curve of MgAl-CO32-LDH,which is related to the adsorption of corrosion products on the interface [50].Therefore,inductance elements are added to the equivalent circuit of the MgAl-CO32-LDH coating.The outermost layer of MgAl-PDCA0.05LDH and MgAl-PDCA0.1LDH coating is dense,which uses CDENand RDENto represent the outermost film.QMHand RMHcircuit components are used in the equivalent circuit to simulate Mg(OH)2layer.There are three-time constants in the Bode phase of the MgAl-PDCA0.01LDH coating.The time constant at high frequencies is attributed to the outermost layer,and the time constants at medium and low frequencies show the Mg(OH)2layer and the charge transfer process,respectively.The same equivalent circuit is used in the LDH coating of a similar structure on the aluminum alloy substrate[51].Since the LDH layer is very thin,loose and porous,the impedance of the LDH layer can be ignored compared to other layers.It can be seen from Table 3 that the MgAl-PDCA0.05LDH coating has the largest RCT(5.77 × 105Ωcm2),which is consistent with the results of the polarization curve.
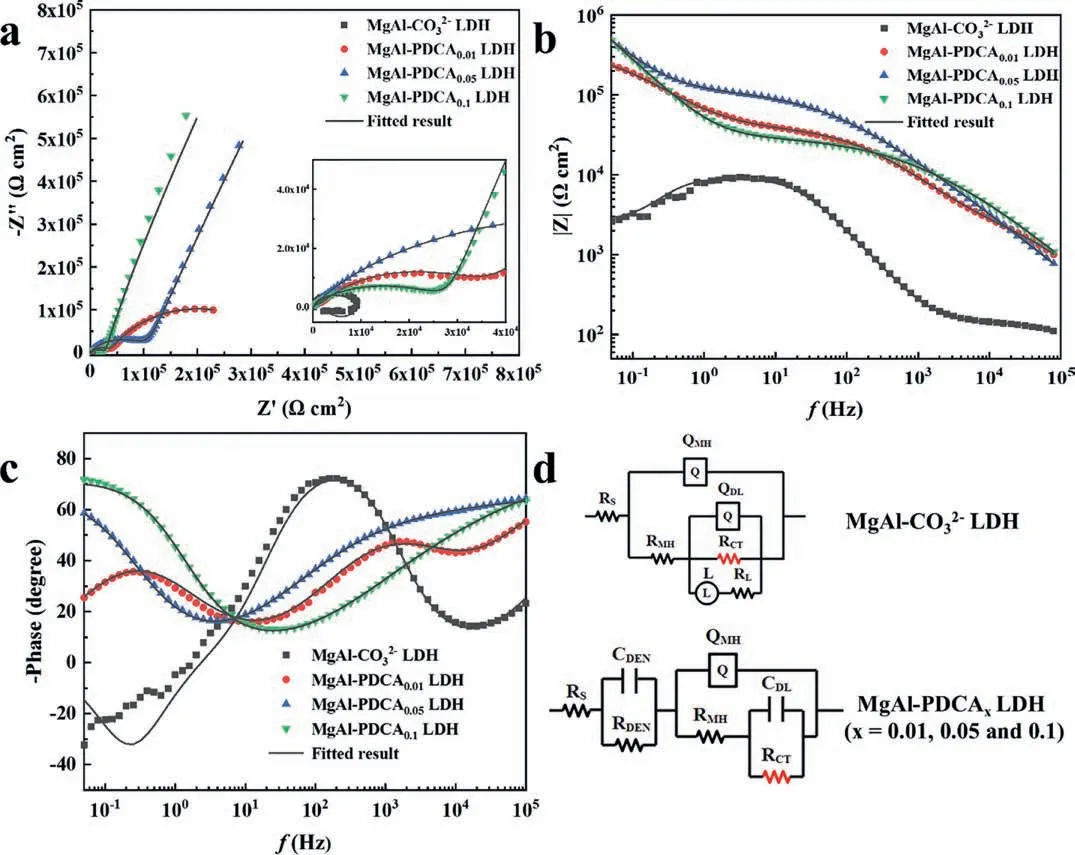
Fig.14.(a) Nyquist plot,(b) bode plot,(c) phase angle and (d) equivalent circuit of AZ31 with MgAl-CO32- LDH and MgAl-PDCAx (x=0.01,0.05 and 0.1) LDH coating.
Fig.15 shows the EIS test results of MgAl-PDCA0.05LDH soaked in 3.5%NaCl for 7 and 14 days,respectively.The lowfrequency impedance modulus (|Z|0.05Hz) of MgAl-PDCA0.05LDH 7day and MgAl-PDCA0.05LDH 14day coatings are 2.90 × 105and 1.25 × 105Ωcm2,respectively.The fitting results are shown in Table 3.It has been shown that the Bode phase of the sample after 7 days of soaking is similar to that of the sample before soaking,indicating that the outermost dense layer has not been damaged after soaking for 7 days.The sample after 14 days of soaking is no longer sustained and the membrane layer has been destroyed.The RCTvalue before soaking and after soaking for 14 days is 5.77 × 105Ωcm2and 1.24 × 105Ωcm2,respectively,indicating a significant decrease in the corrosion resistance of the coating.
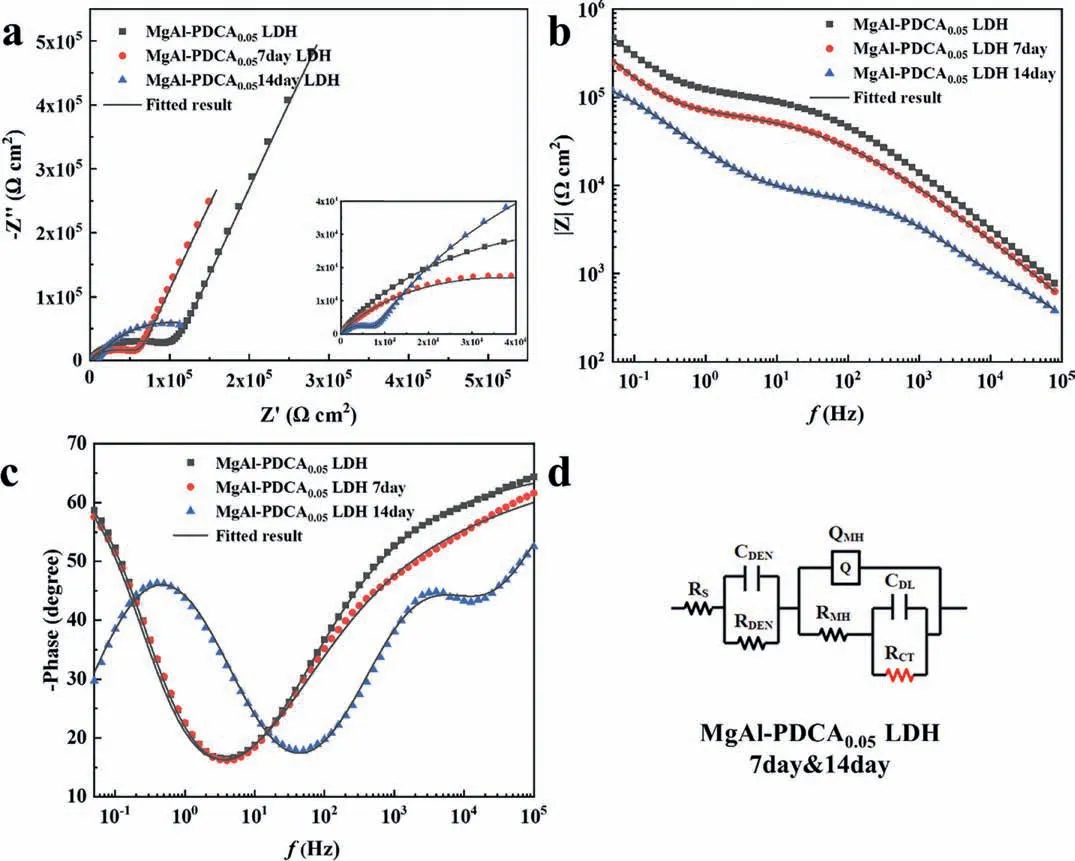
Fig.15.(a) Nyquist plot,(b) bode plot,(c) phase angle and (d) equivalent circuit of AZ31 with MgAl-PDCA0.05 LDH coating for different immersion times.
3.6.Coating growth mechanism
The coating growth mechanism is shown in Fig.16.At the beginning of the reaction,the solution contains Mg2+,Al3+,OH-,Na+and anions An-,the anions include 2,5-PDCA2-and NO3-.In the initial stage of the reaction,the Mg alloy substrate reacts with the H2O in the solution under the conditions of high temperature and high pressure in the kettle to form the Mg(OH)2layer on the substrate.This process will continue until the end of the reaction.With the growth of the Mg(OH)2layer,the diffusion of Mg on the substrate and OHin the solution is hindered,and the thickness gradually stabilizes in the subsequent reaction process.At the same time,in the solution,Mg2+,Al3+,OH-and anion An-(mainly NO3-) formed many hexagonal LDH crystal nuclei Eqs.(2),and ((3)).
In the next reaction process,the Al3+in the solution partially replaced the Mg2+on the surface of the Mg(OH)2layer and combined with the anion An-(mainly NO3-) in the solution to form the LDH crystal nuclei and grow on the magnesium alloy substrate.Since the Al3+and the anion An-required for the growth of the LDH layer come from the solution,the farther away from the liquid-solid interface,the higher the concentration,so the LDH grains formedin situon the substrate grow vertically.At the same time,the LDH crystal nucleus gradually grows up in the solution.Since 2,5-PDCA contains N and the double-bonded oxygen in the carboxyl group which can provide a lone pair of electrons,it formed hydrogen bonds with the H in the outer layer of LDH and physically adsorbed on the surface of LDH crystal grains.Therefore,2,5-PDCA molecules in the solution could be adsorbed in large quantities on the LDH crystal grains grownin situon the substrate and LDH crystal grains in the solution.
As the reaction progresses,LDH nanocrystals with 2,5-PDCA adsorbed in the solution are hard agglomerated and deposited on the vertical LDH crystal grains grownin situ.With the deposition of LDH grains in the solution,the LDH layer grownin situis gradually covered.Under the combined action of boundary fusion during grain growth and the hole-filling effect of the composite formed by 2,5-PDCA and Mg.The outermost layer gradually becomes dense.The dense layer hinders the diffusion of Cl-,so that the corrosion resistance of the LDH coating added with 2,5-PDCA is significantly improved compared to MgAl-CO32-LDH coating.
Finally,a three-layer composite coating is formed on the surface of the Mg alloy,as shown in Fig.17.The bottom layer is Mg(OH)2layer with a thickness of about 30 μm,and the middle layer is an LDH layer grownin situ,which grows vertically,with a thickness of about 1.2 μm.The outermost layer with a thickness of about 0.2 μm is a dense film composed of LDH agglomerates with complexes of 2,5-PDCA and Mg.

Fig.17.Schematics of three-layer composite coating on the surface of the magnesium alloy.
When the coating comes into contact with corrosive media containing Cl-,the outermost dense film first plays a key role in blocking the attack of Cl-,and if Cl-reaches the LDH layer,an ion-exchange reaction will take place,as a result,Cl-is entrapped between the layers.The inner brucite layer plays the final blocking role.This kind of three-layer composite coating provides the multiple physical barriers to protect the AZ31 magnesium alloy.
4.Conclusions
This work explored thein-situhydrothermal preparation of 2,5-PDCA-containing MgAl-LDHs anti-corrosion coating on AZ31 substrate.A mechanical method was found to be able to peel off the LDH coating from the substrate.The coated Mg samples were bended repeatedly until the coating peeled off from the substrate.With the help of SEM,EDS and XRD,it was found that the peeled coating showed a three-layer composite structure,in which,the inner layer consisted of Mg(OH)2and with the thickness of~20 μm,the intermediate layer which was vertically grown and mainly consisted of LDH and with the thickness of~1.7 μm,the outside layer which was very thin (~0.2 μm) but dense,and composed of LDH and complex of 2,5-PDCA-Mg.The corrosion resistance of the coating was evaluated by the electrochemical measurements in 3.5 wt.% NaCl solution,and the corrosion current density of sample coated with MgAl-PDCA0.05LDH was reduced by two orders of magnitude as compared to the MgAl-CO32-LDH coating (from 163.71 nA cm-2to 3.18 nA cm-2).The increase in corrosion protection was due to the construction of the three-layer composite structure which not only provided the multiple physical barriers,but also the 2,5-PDCA delivered an enhanced protection effect to the AZ31 magnesium alloy.The findings of this research provided valuable insights into the structure,growth process and corrosion protection mechanism of hydrothermally synthesized LDH coatings loaded with 2,5-PDCA.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.05.017.
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- “Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
