The hydrogen storage performance and catalytic mechanism of the MgH2-MoS2 composite
Luxing Wng ,Yiwnting Hu ,Jiyu Lin ,Hiyn Leng ,Chenghu Sun ,Chengzhng Wu,* ,Qin Li,,*,Fusheng Pn
a State Key Laboratory of Advanced Special Steel &Shanghai Key Laboratory of Advanced Ferrometallurgy &School of Materials Science and Engineering,Shanghai University,Shanghai 200444,China
b Centre for Translational Atomaterials,Faculty of Science,Engineering and Technology,Swinburne University of Technology,Hawthorn,Victoria 3122,Australia
c National Engineering Research Center for Magnesium Alloys,College of Materials Science and Engineering,Chongqing University,Chongqing 400044,China
Abstract In this work,we synthesized MoS2 catalyst via one-step hydrothermal method,and systematically investigated the catalytic effect of MoS2 on the hydrogen storage properties of MgH2.The MgH2-5MoS2 composite milled for 5 h starts to release hydrogen at 259 °C.Furthermore,it can desorb 4.0 wt.% hydrogen within 20 min at 280 °C,and absorb 4.5 wt.% hydrogen within 5 min at 200 °C.Mo and MoS2 coexisted in the ball milled sample,whereas only Mo was kept in the sample after dehydrogenation and rehydrogenation,which greatly weakens the Mg-H bonds and facilitates the dissociation of MgH2 on the surface of Mo (110).The comparative study show that the formed MgS has no catalytic effect for MgH2.We believed that the evolution and the catalytic mechanism of MoS2 will provide the theoretical guidance for the application of metal sulfide in hydrogen storage materials.
Keywords:Hydrogen storage;Magnesium hydride;MoS2;Evolution;Catalytic mechanism.
1.Introduction
The burning of traditional fossil fuels caused severe environmental damage with the global economic growth [1],and hydrogen with the merits of abundance and environmental friendliness was recognized as one of the most promising energy sources in the 21st century [2].However,the lack of effective storage technology impeded the widespread application of hydrogen energy.As a hydrogen storage material,magnesium hydrides (MgH2) with high gravimetric(7.6 wt.%),low cost,safety and resources abundance attracted extensive attention [3,4].
Whereas,the practical application of MgH2was still limited due to its sluggish kinetics and high desorption temperature (above 400 °C).In the past decades,plenty of methods were used to improve kinetics and reduce hydrogen desorption temperature of MgH2,such as nano-confinement [5],alloying [6],catalyst addition [7]and etc.Alloying Mg with appropriate transition metal elements results in a decrease of hydrogen desorption temperature comparing with MgH2due to a lattice transformation,but the reversible capacity is significantly reduced due to the introduction of non-hydrogen absorbing metal elements [8].For nanoconfined strategy,the load amount of magnesium hydride is still limited,which also leads to low hydrogen storage capacity,which is not conducive to its practical application [9].
In contrast,catalyst addition,on the one hand,could significantly improve the hydrogen storage properties of MgH2;on the other hand,could get a better retention of the hydrogen capacity of composites [10–15].The catalytic effect of some catalysts has been studied [16–19].For instance,the initial dehydrogenation temperature of MgH2-TiO2,MgH2-Fe2O3,MgH2-Nb2O5and MgH2-MnFe2O4composites were 220 °C,289 °C,195 °C and 240 °C,respectively.It is noting that the onset dehydrogenation temperature of MgH2-MoSe2@FeNi3composite significantly reduced to 194°C,which indicates the Mo-containing catalyst has excellent catalytic activity [20].The additive of Mo can provide activity site for the diffusion of hydrogen atom,which means the kinetics of hydrogen absorption/desorption can be markedly promoted [21,22].According to the literature [23],on the one hand,the length of Mg-H bond can be extended from 1.71 to 2.32when the MgH2is absorbed on the Mo (110) surface;on the other hand,the bond strength between Mg and H can be significantly reduced due to the influence of Mo.The lengthening of bond length and weakening of bond strength mean that it is easier to dissociate MgH2in the presence of Mo.Therefore,the Mo-containing catalyst shows excellent catalytic effect.
Recent years,the catalytic effect of transition metal sulfides on MgH2has been increasingly reported [24–31].In 2013,Jia et al.[25]observed that MgH2-16.7 wt.%MoS2can desorb 0.57 wt.% hydrogen,while the as milled MgH2only can desorb 0.1 wt.% hydrogen within 10 min at 300 °C.They considered that the formed Mo and MgS via the reaction of MgH2and MoS2could weaken the Mg-H bonds and offer active site for hydrogen diffusion.In 2015,MgH2catalyzed by the catalyst of WS2was reported,which exhibited the excellent catalytic effect on MgH2[26].The results revealed that the formed W and MgS in the reaction of MgH2and WS2during ball milling played a catalytic role in improving the kinetic properties of MgH2.The FeS2catalyst was added to MgH2in 2018 by Zhang et al.[28],which could release 1.24 wt.% hydrogen within 1400 s at 300 °C,while the pure MgH2could only release 0.18 wt.%.They proposed that the in situ formed Fe could significantly decrease the apparent activation energy of MgH2-FeS2composite,and the MgS could provide active sites and hydrogen diffusion channels.NiS was also added to MgH2to exhibit preferable catalytic effect [32].However,except for the catalytic effect of Ni,they believed that the catalytic effect of the formed MgS was currently uncertain.To sum up,transition metal sulfides could significantly enhance the hydrogenation and dehydrogenation kinetic performance of MgH2.The in situ formed metal,such as W and Fe,could improve the hydrogen absorption/desorption performance.Especially,transition metal Mo could remarkably weaken Mg-H bonds.Nonetheless,current studies on the evolution and catalytic mechanism of the MoS2have not been systematically clarified.
Inspired by these encouraging facts,MoS2catalyst was synthesized by one-step hydrothermal method,and added to MgH2by ball milling to improve the hydrogen storage performance of MgH2.The synthesized MoS2played an important role in improving the kinetic properties of hydrogen absorption and desorption of MgH2.What’s more,the evolution and catalytic mechanism of MoS2in MgH2-MoS2composite was elucidated by XRD,XPS,TEM and comparative study,which could provide mechanism guidance for the application of metal sulfides in hydrogen storage materials in future.
2.Experimental section
2.1.Synthesis of MoS2,WS2,CuS and MgS catalysts
The MoS2catalyst was prepared by one-pot hydrothermal method.(NH4)2Mo2O7·4H2O and CH4N2S were purchased from Sinopharm.In a typical synthesis,0.4944 g(NH4)2Mo2O7·4H2O and 0.4796 g CH4N2S were dissolved in 75 mL distilled water with stirring at room temperature for 1 h to obtain a uniformly mixed solution.Next,the pH value of the solution was tuned to 2 by adding hydrochloric acid dropwise.The solution was transferred into a 100 mL Teflon-lined autoclave,and kept at 220 °C for 24 h in oven.After the autoclave natural cooling to room temperature,the black powder was isolated by centrifugation,and washed with distilled water and alcohol for three times,respectively.The obtained powder was dried in oven at 60 °C for 12 h to remove the residual distilled water and alcohol.
In order to clarify the catalytic mechanism of MoS2,other metal sulfides,such as WS2,CuS and MgS were used for comparative study.WS2,Mg powder,sulfur powder and Cu(NO3)2·3H2O were purchased from Sinopharm.CuS was synthesized by hydrothermal method using Cu(NO3)2·3H2O(0.1 mol) and CH4N2S (0.1 mol) as raw materials after keeping at 140 °C for 15 h.MgS was prepared by ball milling Mg powder and sulfur powder at a molar ratio of Mg to S of 1:1 for 35 h under Ar atmosphere,with a rational speed of 400 rpm and ball-to-powder ratio of 35:1,respectively.
2.2.Synthesis of MgH2/metal sulfides composites
Commercial MgH2(purity>98%) was purchased from Shanghai Mg Power Tech.Co..The as-prepared MoS2with different content of 3 wt.%,5 wt.% and 10 wt.% was ball milled with MgH2together under 1.0 MPa hydrogen pressure,with rational speed of 400 rpm and ball-to-powder of 35:1.The products are denoted as MgH2-3MoS2,MgH2-5MoS2and MgH2–10MoS2,respectively.In addition,for comparison,samples of MgH2catalyzed by different sulfides,such as MgH2-10WS2,MgH2-10CuS and MgH2-10MgS were prepared as the same process for comparison.A QM-3SP2 planetary mill produced in Nanjing was used for all samples preparation by ball milling.
2.3.Characterization
Microstructure and phase composition of the samples were characterized by powder X-ray diffraction (XRD,D8 Advance,Bruker),scanning electron microscopy (SEM,FEI nova 450) and transmission electron microscopy (TEM,JEM-2100F).Acceleration voltage of TEM is 200 kV.X-ray photoelectron spectroscopy (XPS,Thermo Scientific K-Alpha+)was used in the surface analysis of the samples.Fourier transform infrared spectroscopy (FT-IR,Tensor 27) was used to observe the shift of chemical bonds in the sample with a wave number range of 400–2000 cm-1.
Hydrogen storage properties were measured using a homemade HPSA-auto apparatus,in which the modified Benedict-Webb-Rubin (MBWR) EOS was applied to calculate the hydrogen storage capacity.In order to improve the accuracy of the system,two high-accuracy pressure sensors (0.002% FS)and three thermocouples of Pt 100(0.01°C)was used to monitor the temperature and pressure.In the absorption and desorption measurements,an initial pressure of hydrogen absorption and desorption was performed at 3 MPa and 0.0004 MPa,respectively.All samples were handled in a glove box and the water vapour and oxygen levels were below 1.0 ppm to ensure that the samples will not be oxidized.
3.Results and discussion
3.1.Microstructure of MoS2 catalyst
The XRD pattern of as-prepared MoS2is shown in Fig.1.The characteristic peaks at 14.1°,33.9°,39.5 and 59.1°,are corresponding to the plane of (002),(101),(103) and (110) of MoS2,respectively,indicating the MoS2was successfully synthesized using the hydrothermal method.Based on Scherrer equation,the average grain size of MoS2was estimated to be 10.2 nm.Moreover,the morphology of the as-prepared MoS2was observed by SEM,and the images at different magnification are shown in Fig.2.It can be seen that a large number of MoS2are staggered and stacked together,and the average size of the catalyst is around 0.5 μm (Fig.2a).Moreover,the SEM image of MoS2at higher magnification shows that the as-prepared product was consisting of numerous nanosheets(Fig.2b),which could facilitate adequate contact with MgH2during ball milling and provide many active sites.The small grain size and nanosheets were beneficial to improve the hydrogen storage properties of MgH2.
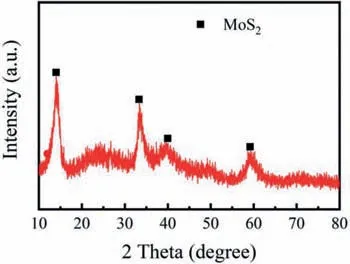
Fig.1.XRD pattern of as-prepared MoS2.

Fig.2.SEM images of MoS2 at different magnifications: (a) 50,000 magnifications;(b) 150,000 magnifications.
3.2.Influence of MoS2 content on hydrogen storage performance
The ball milling time for all the preparation of MgH2-MoS2composite with different MoS2content is 5 h.XRD patterns of ball milled MgH2,MgH2-3MoS2,MgH2-5MoS2and MgH2-10MoS2are shown in Fig.3.It can be seen that all the peaks were belong to MgH2,while the peaks of the catalyst can’t be found,which may be attributed to the poor crystallinity of MoS2catalyst prepared by hydrothermal method.The morphology of ball milled MgH2,and MgH2-3MoS2,MgH2-5MoS2and MgH2-10MoS2was observed by SEM (Fig.S1).All samples exhibit irregular shapes due to the uneven force during ball milling.The particle size of pristine and ball milled MgH2was 5.0 and 3.5 μm,respectively.However,with increasing the MoS2content from 3 to 10 wt.%,the particle size of MgH2-MoS2composites reduced to 2.9 μm,2.4 μm and 2.3 μm,respectively,which means that the MgH2-MoS2composites with different addition amount of MoS2has no obvious difference on particle size.
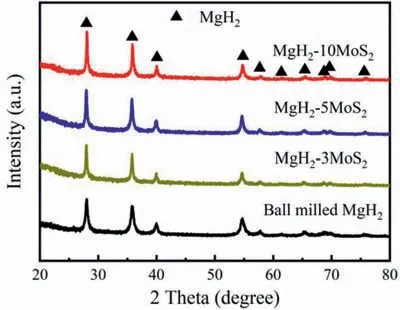
Fig.3.XRD patterns of ball milled MgH2,MgH2-3MoS2,MgH2-5MoS2 and MgH2-10MoS2.
The dehydrogenation performance of MgH2with different MoS2content was investigated by temperature programmed desorption (TPD) with a heating rate of 5 °C min-1.As shown in Fig.4,the initial hydrogen desorption temperature of the milled MgH2was 325°C,while the onset dehydrogenation temperature of the MoS2catalyzed MgH2was reduced to 264 °C for MgH2-3MoS2,259 °C for MgH2-5MoS2and 247 °C for MgH2-10MoS2,respectively.Obviously,the addition of MoS2catalyst could significantly reduce the onset hydrogen release temperature.
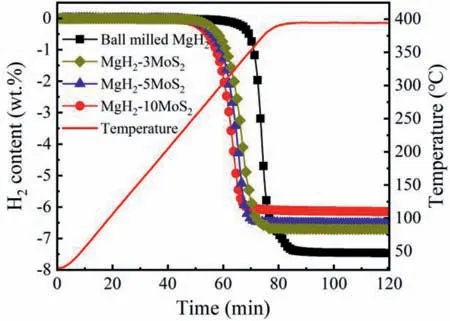
Fig.4.TPD curves of ball milled MgH2,MgH2-3MoS2,MgH2-5MoS2 and MgH2-10MoS2 with a heating rate of 5 C min-1.
The isothermal hydrogen absorption and desorption properties of MgH2with different MoS2content was further investigated at different temperatures,and the results are shown in Fig.5.It can be seen that,the addition of MoS2obviously accelerated the hydrogen desorption rate.At 300 °C,the MgH2-3MoS2can desorb 2.7 wt.% within 7 min,while 3.8 wt.%hydrogen was desorbed for MgH2-5MoS2and 5.2 wt.% hydrogen for MgH2-10MoS2at same condition (Fig.5a).When the temperature drops to 280 °C,a hydrogen capacity of 3 wt.%,4 wt.% and 5.1 wt.% within 20 min was obtained for the content of MoS2in the samples were 3,5 and 10,respectively (Fig.5b).The MgH2-5MoS2can absorb 5.6 wt.%and 4.5 wt.% hydrogen in 5 min at 250 °C and 200 °C,respectively as shown in Fig.5c and d,while MgH2-10MoS2only absorb 4.8 wt.% and 4.0 wt.% hydrogen within the same conditions.That illustrated the increase of MoS2content can substantially improve the hydrogen desorption kinetics of the composites.It is worthy of noting that,in addition to the influence of catalyst amount on hydrogen storage performance,the particle size may also affect the hydrogen absorption and desorption performance of MgH2.Above particle size distribution shows that the particle size for all MgH2-MoS2composites is around 2.9~2.3 μm,indicating that the particle size of the samples after ball milled has been small enough to have no obvious influence on the performance.Therefore,the above performance difference was mainly attributed to different MoS2content.It is worth noting that MgH2-5MoS2shows faster kinetics and maintains a high hydrogen storage capacity.Thus,MgH2-5MoS2was selected for further study.
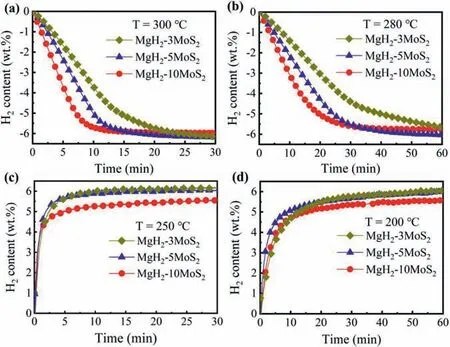
Fig.5.Hydrogen desorption curves of MgH2-3MoS2,MgH2-5MoS2 and MgH2-10MoS2 composites at 300 °C (a) and 280 °C (b),and hydrogen absorption curves at 250 °C (c) and 200 °C (d).The initial hydrogen pressure is 3 MPa for hydrogenation and 0.0004 MPa for dehydrogenation.
3.3.Influence of milling time on the hydrogen storage performance
MgH2-5MoS2composite was prepared for various milling time of 2 h,5 h,10 h,20 h,respectively.The XRD patterns of the MgH2-5MoS2composite with different milling time are shown in Fig.6,the peaks of all samples mainly correspond to MgH2.However,the peaks of MoS2were not found in the XRD patterns for the samples of various milling time.With the milling time increased,the diffraction peaks of MgH2became wider and weaker,indicating the crystallite size gradually became smaller.The morphology and corresponding particle size diagram of as-milled MgH2-5MoS2for different milling time was further observed by SEM (Fig.S2).Some larger particles can clearly observe in the sample of MgH2-5MoS2milled for 2 h.When the milling time increased but no more than 10 h,the average particle size becomes smaller without obvious particle aggregation.However,the agglomeration of particles can be observed for the sample of MgH2-5MoS2milled for 20 h,indicating the particle size may increase with the extension of ball milling time.The increase in particle size possibly resulted from the cold-welding of particles during the long milling process [33,34].It can be seen that the particle size for MgH2-5MoS2-2 h,MgH2-5MoS2-5 h,MgH2–5MoS2-10 h and MgH2-5MoS2-20 h is 3.3,2.4,2.1 and 2.2 μm,respectively.obviously,the relationship between particle size and ball milling time is non-linear.The nonlinear relationship between particle size and milling time indicates that over-long milling time is disadvantageous to the hydrogen storage properties.

Fig.6.XRD patterns of MgH2-5MoS2 composite for various milling times.
The dehydrogenation properties of MgH2-5MoS2with different milling time were investigated by TPD.As shown in Fig.7,the onset temperature of hydrogen desorption was increased in an order of MgH2-5MoS2-5 h (259 °C),MgH2-5MoS2-10 h(264°C),MgH2-5MoS2-2 h(283°C)and MgH2-5MoS2-20 h (301 °C).It was worth noting that the MgH2-5MoS2-5 h had the lowest onset temperature of dehydrogenation for all samples.With the extension of ball milling time to 5 h,the initial dehydrogenation temperature further decreased to 259 °C.The long ball milling time of MgH2leads to the reduction of its grain size and the introduction of various defects,which allows the hydrogen atoms in MgH2to enable a faster diffusion process [33].But the sample milled for 20 h,the dehydrogenation temperature rises to 301 °C.The performance deterioration could be attributed to the serious agglomeration of particles,which was caused by long time ball milling.Furthermore,the cycle performance of MgH2-5MoS2composite was tested at 300 °C,as shown in Fig.8.It illustrated that the hydrogen storage capacity of the MgH2-5MoS2system stayed stable even after 10 cycles,indicating that MoS2was quite an effective and stable catalyst precursor for MgH2.In addition,elemental mapping of the Mg,Mo and S were observed for ball milled,dehydrogenated and rehydrogenated MgH2-5MoS2(Fig.S3).It can be seen that Mo and S elements were distributed evenly on the Mg/MgH2matrix,which was beneficial to reduce the reaction energy barrier during the hydrogenation and dehydrogenation [31].All the phenomenon mentioned above the ball milling time of 5 h without obvious aggregation delivered the excellent performance of hydrogen desorption.Therefore,we chose the milling time of 5 h for further study.
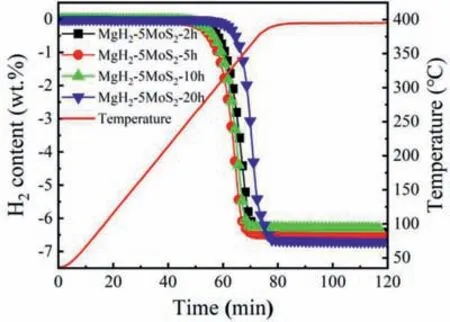
Fig.7.TPD curves of MgH2-5MoS2 composite for various milling times with a heating rate of 5 °C min-1.
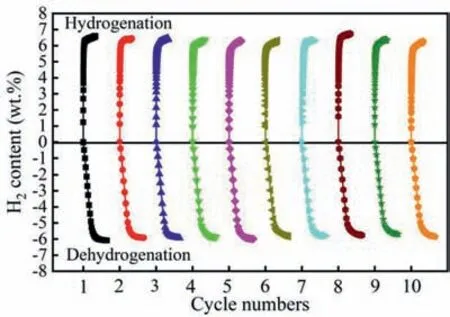
Fig.8.Cyclic curves of MgH2-5MoS2 composite at 300 °C.The initial hydrogen pressure is 3 MPa for hydrogenation and 0.0004 MPa for dehydrogenation.
The PCT measurements of hydrogen absorption and desorption was used to investigate the thermodynamics of MgH2-5MoS2.As shown in Fig.9,the absorption plateau pressure was measured to be 1.75 bar,2.90 bar and 4.75 bar and the desorption plateau pressure was shown as 1.41 bar,2.34 bar,and 4.04 bar at 300 °C,320 °C and 340 °C,respectively.The absorption and desorption enthalpy (ΔH) of MgH2-5MoS2was calculated by the Van’t Hoff equation,which was -73.25 ± 0.74 kJ mol-1and -76.82 ± 4.2 kJ mol-1,respectively.It can be seen that there is no essential change on the theoretical values [35],indicating that MoS2had no positive effect on the thermodynamic properties of MgH2.
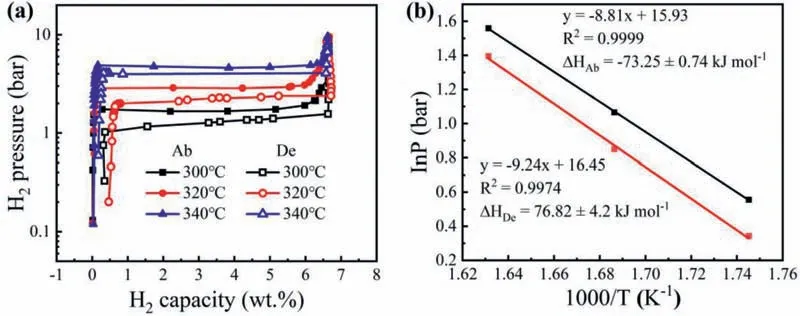
Fig.9.Hydrogen absorption/desorption PCT curves (a) at 300 °C,320 °C,340 °C and Van’t Hoff plots (b) for the MgH2-5MoS2 composite.
3.4.Evolution of MoS2 and the catalytic mechanism
Above results show that the addition of MoS2significantly improved the hydrogen absorption and desorption kinetics,and decreased the hydrogen desorption temperature.According to the reported literature[25],MoS2could be reacted with two equivalents of MgH2during hydrogen absorption and desorption measurement,resulting in the formation of MgS and the release of two equivalents of H2(see Eq.(1)).
According to the equation,two equivalents of MgH2can react with an equal amount MoS2to form two equal amounts MgS and Mo,and release two equivalents of hydrogen molecules.When the addition amount of MoS2is 5 wt.%,the capacity of MgH2-5MoS2composite is about 6.5 wt.%by calculation.Therefore,except for the influence of catalyst weight,the total hydrogen release content of MoS2catalyzed MgH2should be less than that of transition metal catalyzed MgH2(Fig.4).In addition,during the ball milling process,it is possible of that the MoS2has already reacted with MgH2,which means partial Mo has already been formed.In order to clarify the catalytic mechanism of MoS2,the evolution of MoS2need to be studied firstly.
The phase structure of ball milled,dehydrogenated and hydrogenated MgH2-5MoS2composites was checked by XRD,and the results are shown in Fig.10.XRD pattern of ball milled MgH2-5MoS2composite just shows the diffraction peaks of MgH2.There are no diffraction peaks of MgS and Mo phases in the ball milled composite.It is not certain whether Mo and MgS are not present in the ball mill sample or because nano-sized/amorphous particles produced by the ball milling process [14].For the sample of dehydrogenated MgH2-5MoS2composite,except for the peaks of Mg,the peaks of MgS phase were also detected,indicating that Mg has partially transformed to MgS during dehydrogenation process.Moreover,the MgS phase was kept in the rehydrogenated sample.It is worthy of noting that,owing to the possible low content or nano size,no Mo specie was detected in the XRD patterns of MgH2-5MoS2composite at the three states.
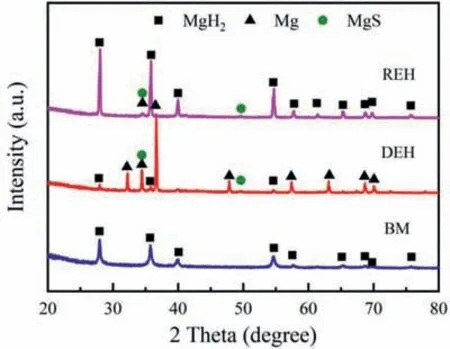
Fig.10.XRD patterns of ball milled (BM),dehydrogenated (DEH) and rehydrogenated (REH) MgH2-5MoS2 composite.
In order to gain the further insight into the evolution of MoS2,the XPS was employed to investigate the chemical states of Mo and S in pure MoS2and different states of MoS2catalyzed MgH2composites.Here,in order to obtain the stronger signals of Mo and S elements,the addition amount of 10 wt.% MoS2was selected for XPS study.The peaks at 228.8 and 232.0 eV on MoS2catalyst (Fig.11a)were assigned to Mo4+3d5/2and Mo4+3d3/2[36].Except the peaks of Mo4+,It is worth noting that the characteristic peaks of Mo appeared in the ball milled MgH2-10MoS2,indicating that part of Mo and other unreacted MoS2coexisted in the ball milled sample.According to the two peaks area of Mo and Mo4+,about 72% MoS2was transformed to Mo element after ball milling,i.e.,about 4.3 wt.% Mo is existed in the ball milled sample of MgH2-10MoS2.In comparison of the ball milled sample,it can be seen that the main peaks are from Mo after dehydrogenation and rehydrogenation,illustrating MgH2and MoS2are completely reacted to form Mo.In addition,two peaks at 228.2 and 231.5 eV assigned to Mo4+3d5/2and Mo4+3d3/2appear with relatively weaker intensities for dehydrogenated and rehydrogenated states of MgH2-10MoS2,indicating the slightly MoS2still existed.The peaks at 229.6 and 233.2 eV were located at Mo5+3d5/2and Mo5+3d3/2,and the peaks at 231.5 and 235.4 eV were assigned to Mo6+3d5/2and Mo6+3d3/2[36,37].The Mo5+and Mo6+could have stemmed from incomplete sulfurization of MoO3-xspecies in the hydrothermal process [36].The peaks at 226.1 eV allocated for S 2s of MoS2catalyst and different states MgH2-10MoS2[36].

Fig.11.XPS spectra of (a) Mo 3d and (b) S 2p for the pure MoS2,ball milled,dehydrogenated and rehydrogenated MgH2-10MoS2 composites.
As shown in Fig.11b,the peaks at 161.7 and 163.0 eV on MoS2catalyst were attributed to S2-2p3/2and S2-2p1/2[36].Two characteristic peaks of the 2p3/2-2p1/2(160.4/161.3 eV)spin orbit doublet of S2-were found for ball milled,dehydrogenated and rehydrogenated states MgH2-10MoS2,which corresponds to Mg-S bonds [38],indicating the MgS formed during ball milling process and kept after hydrogenation and dehydrogenation process.
Above XPS results show us the evolution of MoS2catalyst during ball milling and following hydrogenation and rehydrogenation,indicating that Mo and MoS2coexist in the ball milled MgH2-10MoS2.Moreover,Mo was kept in the dehydrogenated and rehydrogenated states samples.Huang et al.[23]thought that the bond length of Mg-H was significantly extended from 1.71 to 2.32when the MgH2molecule was absorbed on the Mo (110) surface.Due to the influence of Mo,the electrostatic interaction between Mg and H were remarkably reduced,resulting in the MgH2molecule dissociate more easily with the assistance of Mo.Moreover,in order to verify whether there is a change of Mg-H bond energy,FT-IR was used to investigate the wavenumber shift of Mg-H bond between MgH2and rehydrogenated MgH2-10MoS2composite.As can be seen from Fig.12,the band at 668 cm-1can be assigned to Mg-H bending vibration for both samples[39].It is noting that the Mg-H stretching vibration (987 cm-1) of rehydrogenated sample shifted towards lower wavenumbers comparing with pristine MgH2(1047 cm-1),which indicates the bond length of Mg-H bonds was extended due to the effect of Mo and is beneficial to weaken the Mg-H bond [23,39].In addition,the band from 649 to 439 cm-1can be assigned to Mg-S,implying the existence of MgS [40],which is consistence with the results of XPS (Fig.11).

Fig.12.FT-IR spectra of MgH2 and rehydrogenated MgH2-10MoS2 composite.
In comparison of the role of different Mo species on the hydrogen desorption properties of MgH2,TPD measurement was adopted to determine the performance variation of MgH2-5MoS2in ball milled and rehydrogenated states.As shown in Fig.13,the onset dehydrogenation temperature of ball milled MgH2is 325 °C,while the temperature decreases to 281 °C for ball milled MgH2-5MoS2and 259 °C for rehydrogenated MgH2-5MoS2,respectively.Obviously,the formation of Mo and MgS during ball milling and dehydrogenation process would be beneficial to the hydrogen release comparing with the originally added MoS2.Additionally,the hydrogen desorption capacity of ball milled MgH2,ball milled MgH2-5MoS2,rehydrogenated MgH2-5MoS2are 7.5 wt.%,7.1 wt.% and 6.5 wt.%,respectively,which is attributed to the influence of catalyst weight and the formation of MgS consumes equal amount of Mg according to the Eq.(1).
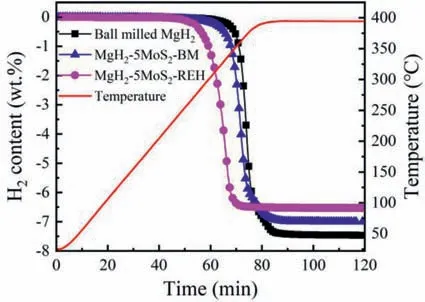
Fig.13.TPD curves of ball milled MgH2,ball milled and rehydrogenated MgH2-5MoS2 composites.
Above results indicated that the Mo and/or MgS plays an excellent catalytic effect on MgH2.The formed Mo can weaken Mg-H bonds and promote the dissociation of H2molecules on MgH2surfaces to significantly improve the kinetics of MgH2[23].However,the catalytic effect of MgS is still uncertain.Some authors speculated that the MgS offers active site for hydrogen diffusion to improve the hydrogen storage performance of MgH2[25].To further clarify the catalytic effect of MgS,several metallic sulfides,such as MgS,WS2,CuS,were used as comparative catalysts.Therefore,the composites of MgH2-10MgS,MgH2-10WS2and MgH2-10CuS were synthesized and the hydrogen desorption kinetics of all composites were tested at 300 °C.Obviously,MgH2-10MoS2and MgH2-10WS2exhibit the excellent hydrogen desorption kinetics,but MgH2-10CuS and MgH2-10MgS show the poor kinetics performance (Fig.14).MgH2-10MoS2and MgH2-10WS2can desorb 5.4 wt.% and 4.5 wt.% hydrogen at 300 °C within 10 min,respectively.However,MgH2-10CuS,ball milled MgH2and MgH2-10MgS can only desorb 0.4 wt.%,0.3 wt.% and 0.1 wt.% hydrogen at a same condition,respectively.

Fig.14.Isothermal desorption of MgH2-10MoS2,MgH2-10WS2,MgH2-10CuS,MgH2-10MgS and ball milled MgH2 at 300 °C.
The peaks of MgS were detected after dehydrogenation in MgH2-10MoS2,MgH2-10WS2,MgH2-10CuS and MgH2-10MgS composites,indicating WS2and CuS react with MgH2during dehydrogenation process (Fig.S4).The formation of MgS means that the difference of hydrogen desorption behavior is mainly resulted from the formed metal.As other authors reported,Mo and W can weaken the Mg-H bond and accelerate the dissociation of MgH2[26,41],but the MgCu2generated by the reaction of MgH2and CuS can’t effectively dissociate MgH2[42,43].Particularly,the prepared MgS has no positive catalytic effect for MgH2.Above results indicate that the catalytic effect of MoS2is mainly in situ Mo rather than MgS.
Additionally,we chose the as-milled and rehydrogenated MgH2-10MoS2as the research object to study the microstructures and compositions via TEM and EDX,as shown in Fig.15.TEM images of ball milled MgH2-10MoS2composite at a different magnification are shown in Fig.15a and b.The lattice spacing of 0.251 nm is corresponding to (101) plane of MgH2and the lattice spacings of 0.223,0.288 nm and 0.263 nm are corresponding to (110),(100) and (200) planes of Mo,MoS2and MgS,respectively.The TEM results proved that the coexistence of Mo and MoS2in the ball milled sample.However,as shown in the Fig.15c and d,not MoS2but Mo was found in the rehydrogenated composite.These results further confirmed that partial MoS2reacted with MgH2during ball milling,but MoS2completely transformed to Mo and MgS during hydrogen absorption and desorption,which is in agreement with the results of XPS analysis.It is worth to note that TEM images were taken as soon as possible during the test for avoiding the hydrogen release due to the electronic irritation.In addition,the elemental mapping shows that the element of Mo in the rehydrogenated sample is more uniformly distributed on MgH2matrix (Fig.15f) than that in the ball milled sample (Fig.15e).The intimate contact between catalyst and MgH2could further improve the hydrogen storage performance.

Fig.15.TEM and HRTEM images of MgH2-10MoS2 composite at different states: ball milled (a,b) and rehydrogenation (c,d),and the EDX mapping of ball milled (e) and rehydrogenation (f) MgH2-10MoS2 composite.
Based on the above analysis,the evolution process of MoS2and its catalytic mechanism on improving the hydrogen storage performance of MgH2can be illustrated.For the ball milled process,MoS2partly reacts with MgH2to form Mo and MgS phases,indicating the Mo and MoS2coexist in the ball milled sample.The Mo was kept after dehydrogenation and hydrogenation,which can significantly weaken the Mg-H bonds and facilitate the dissociation of MgH2on the surface of Mo (110) to improve the kinetic properties of the system.However,the result of the comparative study indicates that the formed MgS may not be positive effect for MgH2.
4.Conclusion
In summary,we used the one-step hydrothermal method to synthesize the MoS2catalyst with excellent catalytic performance and illustrated the evolution and catalytic mechanism of MoS2.MgH2-5MoS2composite milled for 5 h showed the best hydrogen storage properties,which the onset hydrogen desorption temperature was reduced to 259°C.The composite can desorb 4.0 wt.% hydrogen within 20 min at 280 °C and absorb 4.5 wt.% hydrogen within 5 min at 200 °C,exhibiting excellent kinetics properties.
The evolution and catalytic mechanism of MoS2can be described as follows.Mo and MoS2coexisted in the ball milled sample due to the reaction between MgH2and MoS2.Mo was kept after dehydrogenation and rehydrogenation.On the one hand,the in situ formed Mo can significantly reduce the electrostatic interaction between Mg and H,which greatly facilitates MgH2molecule dissociation;on the other hand,the absorption of MgH2molecule on the surface of Mo (110) can remarkably extend the bond length of Mg-H,which also weakens the Mg-H bonds.The results of comparative study indicate that the formed MgS maybe no catalytic effect for MgH2.Overall,the evolution and catalytic mechanism of MoS2could provide the theoretical guidance for the application of metal sulfides in hydrogen storage materials in future.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the financial supports from Science and Technology Commission of Shanghai Municipality (No.19ZR1418400),the National Natural Science Foundation of China (No.51971126),Guangdong Innovation Research Team for Higher Education (2017KCXTD030)and the Science and Technology Committee of Shanghai(19010500400).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2022.06.001.
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- “Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
