Realizing Cd and Ag codoping in p-type Mg3Sb2 toward high thermoelectric performance
Shijun Xio ,Kunling Peng ,Zizhen Zhou ,Hun Wng ,Sikng Zheng ,Xu Lu ,Gung Hn,Guoyu Wng,Xioyun Zhou,b,d,*
aCollege of Physics,Chongqing University,Chongqing 401331,China
b National Engineering Research Center for Magnesium Alloys,Chongqing University,Chongqing 400044,China
c Chongqing Institute of Green and Intelligent Technology,Chinese Academy of Sciences,Chongqing 400714,China
d Analytical and Testing Center,Chongqing University,Chongqing 401331,China
Abstract Mg3Sb2 has attracted intensive attention as a typical Zintl-type thermoelectric material.Despite the exceptional thermoelectric performance in n-type Mg3Sb2,the dimensionless figure of merit (zT) of p-type Mg3Sb2 remains lower than 1,which is mainly attributed to its inferior electrical properties.Herein,we synergistically optimize the thermoelectric properties of p-type Mg3Sb2 materials via codoping of Cd and Ag,which were synthesized by high-energy ball milling combined with hot pressing.It is found that Cd doping not only increases the carrier mobility of p-type Mg3Sb2,but also diminishes its thermal conductivity (κtot),with Mg2.85Cd0.5Sb2 achieving a low κtot value of~0.67 W m-1 K-1 at room temperature.Further Ag doping elevates the carrier concentration,so that the power factor is optimized over the entire temperature range.Eventually,a peak zT of~0.75 at 773 K and an excellent average zT of~0.41 over 300 -773 K are obtained in Mg2.82Ag0.03Cd0.5Sb2,which are~240% and~490% higher than those of pristine Mg3.4Sb2,respectively.This study provides an effective pathway to synergistically improve the thermoelectric performance of p-type Mg3Sb2 by codoping Cd and Ag,which is beneficial to the future applications of Mg3Sb2-based thermoelectric materials.
Keywords: Thermoelectric;p-type Mg3Sb2;Cd and Ag codoping;Lattice thermal conductivity;Carrier concentration.
1.Introduction
Thermoelectric materials can realize the mutual conversion between heat and electricity,and can be utilized to generate electricity from various heat sources,such as environmental heat and industrial waste heat,providing a green and convenient way for sustainable energy supply [1–3].There have been continuous and intense interests in improving the energy conversion efficiency of thermoelectric materials,which is mainly determined by the dimensionless figure of merit(zT),defined aszT=S2σT/κtot.In this formula,Sis the Seebeck coefficient,σthe electrical conductivity,Tthe absolute temperature,andκtotthe total thermal conductivity that is composed of the lattice (κL) and electronic (κe) thermal conductivity [4].Ideally,a material with exceptional thermoelectric performance would be with a combination of largeS,highσ,and lowκtot.However,these parameters are coupled to each other,so it is challenging to synergically regulate them to achieve exceptionalzTvalues.In the past decade,researchers have devoted themselves to the exploration of new thermoelectric materials together with performance optimization of existing thermoelectrics.For example,optimization of carrier concentration,adjustment of the Fermi level[5,6],and band convergence [7–9]can effectively improve the power factor (S2σ) of materials.Alternatively,the introduction of high-density grain boundaries [10,11],nanoparticles [12,13],and point defects [14–18]into materials can efficiently scatter phonons with various wavelengths,thus sharply reducing their lattice thermal conductivity.Additionally,the reduction of phonon group velocity plays a vital role in achieving diminishedκL[19,20].
Zintl compounds are a group of thermoelectric materials with the “electron crystal -phonon glass” features.In these compounds,the anionic groups connected by covalent bonds provide transport channels for carriers,providing properties resembling a “electron crystal”.Furthermore,the cationic and anionic groups bonded by ionic bonds facilitate the scattering of phonons,giving rise to “phonon-glass” properties [21–23].Among them,Mg3Sb2is a typical Zintl-type thermoelectric material,in which the covalent bonding regions are separated from the ionic regions: Mg1 is located in the cationic layer,Mg2 and Sb are covalently bonded to form [Mg2Sb2]2-anion framework,as illustrated in Fig.S1(a).Such structural characteristics play an important role in securing good thermoelectric properties for Mg3Sb2,such as low lattice thermal conductivity.Mg3Sb2normally exhibits p-type conducting behavior,while it can be converted to n type by introducing excessive Mg [24,25].As for n-type Mg3Sb2with multivalley conduction bands,Te-doped Mg3Sb1.5Bi0.5materials possess excellentzTvalues [26,27],e.g.a peakzTvalue of~1.65 at 725 K in Mg3Sb1.48Bi0.48Te0.04[27].Furthermore,Nb doping [28],Mn doping [29,30]and reducing excess Mg[31]were performed to synergistically optimize the electrical and thermal transport properties of Mg3.2Sb1.5Bi0.49Te0.01.For example,a peakzT~1.85 at 723 K and an averagezT~1.25 (from 300 K to 723 K) were achieved in Mg3.15Mn0.05Sb1.5Bi0.49Te0.01[30],by combining the intrinsic low thermal conductivity and doping-optimized electrical transport properties,demonstrating the great potential of n-type Mg3Sb2-based materials in high-performance thermoelectric devices.
In contrast,thezTof undoped p-type Mg3Sb2remains below 1 due to the poor electrical properties [32],which is attributed to its low carrier concentration and mobility together with the low valence band degeneracy atΓpoint (Fig.S1(b)).In order to enhance the electrical properties of p-type Mg3Sb2,the main approach focuses on doping at Mg or Sb sites to regulate the carrier concentration.Specifically,the doping elements at the Mg site mainly include Li [33],Na [34],Ag[35],Na/Zn[36],Li/Zn[37]and Li/Cd[38],while the doping elements that occupy the Sb position are dominantly Pb [39],Bi [40]and Sn [41],among which the alkali metal doping is the most effective in regulating the carrier concentration.It is also of interest to note that compared with single-element doping,double-element codoping strategy can better regulate the thermoelectric properties of p-type Mg3Sb2.Although thezTof p-type Mg3Sb2has been improved by doping,its electrical and thermal transport properties should be further optimized to compete with its n-type counterpart.Considering that both p-and n-type semiconductors are needed to fabricate thermoelectric devices for practical applications,it is imperative to enhance thezTof p-type Mg3Sb2to accelerate the commercialization process of Mg3Sb2-based thermoelectric devices.
Herein,we perform codoping of Cd and Ag at Mg sites of p-type Mg3Sb2toward synergistic optimization of thermal and electrical transport properties.The introduction of Cd at the Mg position exerts positive influence on enhancing phonon scattering,which reduces the lattice thermal conductivity obviously,e.g.0.56 W m-1K-1for Mg2.85Cd0.5Sb2(at 773 K).Simultaneously,Ag doping at the Mg site of Mg2.85Cd0.5Sb2effectively enhances the hole concentration,and Cd and Ag codoping increases the density of states effective mass of Mg3Sb2,leading to effectively optimized electrical properties.Eventually,Mg2.82Ag0.03Cd0.5Sb2achieves a peakzTof~0.75 at 773 K and an averagezTof~0.41 (from 300 K to 773 K),which are~240% and~490% higher than those of Mg3.4Sb2,respectively.
2.Experimental and theoretical methods
2.1.Sample synthesis
Polycrystalline Mg3.4-xCdxSb2(x=0,0.1,0.3,0.5) and Mg2.85-yAgyCd0.5Sb2(y=0,0.01,0.03,0.05) samples were prepared by high-energy ball milling and hot pressing,as illustrated in Fig.1.Firstly,high-purity Mg (powder,99.5%),Sb (spheres,99.999%),Cd (powder,99.9%) and Ag (powder,99.9%) were weighed in a stoichiometric ratio and loaded into stainless-steel ball-milling jars in an Ar-filled glove box.Next,the raw materials were mechanically alloyed for 10 h utilizing a high-energy ball mill(SPEX 8000 Mixer/Mill).The obtained milled powders were loaded into a 10 mm diameter graphite die and then consolidated into dense pellets by hot pressing at~873 K for 20 min under a uniaxial pressure of~70 MPa.High relative densities (>95% of the theoretical density) are obtained for all samples (Table S1).
2.2.Characterization and measurements
The powder X-ray diffraction (XRD) patterns of all samples were collected on a PANalytical X’pert diffractometer using Cu Kαradiation.The microstructure of the samples was investigated through scanning electron microscopy(SEM,Thermo Scientific Quattro S)and transmission electron microscopy (TEM,Thermo Scientific Talos F200S),which were operated at 20 and 200 kV,respectively.The TEM samples were prepared from the Cd,Ag-codoped bulk pellets using mechanical polishing and ion milling (LEICA EM RES102).The chemical compositions of the Mg3.4Sb2,Mg2.85Cd0.5Sb2and Mg2.82Ag0.03Cd0.5Sb2samples (Table S2)were characterized by electron probe microanalysis (EPMA,JXA-8530F Plus Hyper Probe).The electrical transport properties were measured on bar-shaped samples with dimensions of~9 mm × 3 mm × 3 mm using an ULVAC ZEM-3 instrument from 300 K to 773 K,and the thermal diffusivity (D)was acquired from wafer-shaped samples (with a diameter of 10 mm and a thickness of 1 mm)utilizing a Netzsch LFA 457 instrument over the same temperature range.The electronic thermal conductivity (κe=LσT) can be estimated from the Wiedemann-Franz relationship with the Lorenz number (L)being calculated based on the single parabolic band (SPB)model [42].The temperature-dependent Hall carrier concentration (nH) was measured by a homemade Hall effect measurement apparatus under a magnetic field of ±1 T,and the carrier mobility (μH) was calculated using the formulaμH=σe-1nH-1.The uncertainties in the measurement ofS,σ,κandzTare 5%,5%,10% and 15%,respectively.
2.3.Theoretical calculations
The electronic properties of Mg3Sb2were investigated within the framework of the density functional theory[43,44],which was coded in the Vienna Ab-initio Simulation Package[45].The exchange-correlation functional was in the form of Perdew-Burke-Ernzerhof with generalized gradient approximation [46].The GW0approximation was considered for accurately predicting the band gap [47–50].The system was fully relaxed until that the magnitude of the forces acting on all the atoms is less than 0.002 eV-1.The phonon dispersion relations were computed based on the density functional perturbation theory [51].A 3 × 3 × 2 supercell withΓpoint was adopted to calculate the second-order interatomic force constants.
3.Results and discussion
3.1.Reducing the lattice thermal conductivity by Cd doping
Since Mg is rather active and easy to volatilize during heating,we first realize the synthesis of single-phase Mg3Sb2phase (Fig.S2) by ball milling and sintering mixed Mg and Sb precursors with a higher Mg:Sb molar ratio (i.e.,3.4:2)than the stoichiometric ratio.Thereafter,we systematically investigate the effect of Cd doping at the Mg position and the concentration of Mg vacancies on the thermoelectric properties of p-type Mg3.4Sb2.Fig.2(a)shows the powder XRD patterns of Mg3.4-xCdxSb2(x=0,0.1,0.3,0.5),Mg2.85Cd0.5Sb2and Mg2.8Cd0.5Sb2samples synthesized by high-energy ball milling and hot pressing.All diffraction peaks can be indexed to hexagonal Mg3Sb2structure with the space groupPm1 (PDF 71-0404),except that a trace amount of secondary phase Sb is observed in the Mg2.8Cd0.5Sb2sample.A representative SEM image of the Mg2.85Cd0.5Sb2fractured surface is presented in Fig.2(b),revealing microstructures without obvious pore or micro-crack,which is consistent with the high relative density (95% of the theoretical density).Fig.2(c)-(e) display the energy dispersive X-ray spectroscopy (EDS) element maps,showing that all component elements (Mg,Sb and Cd) are evenly distributed in the sample.
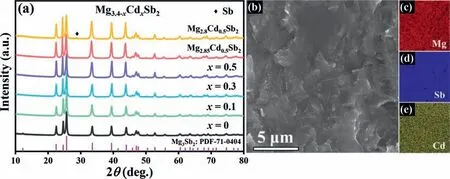
Fig.2.XRD and SEM characterization of Cd-doped Mg3.4Sb2:(a)XRD patterns for Mg3.4-xCdxSb2 (x=0,0.1,0.3,0.5),Mg2.85Cd0.5Sb2 and Mg2.8Cd0.5Sb2,(b) SEM image of the Mg2.85Cd0.5Sb2 sample,and (c -e) corresponding EDS maps of Mg,Sb and Cd,respectively.
In order to investigate the effect of Cd doping and Mg vacancies on the thermoelectric performance of p-type Mg3.4Sb2,the electrical (Fig.3) and thermal transport properties (Fig.4) of Mg3.4-xCdxSb2(x=0,0.1,0.3,0.5),Mg2.85Cd0.5Sb2and Mg2.8Cd0.5Sb2samples were measured.Fig.3(a) and (b) demonstrate the temperature-dependent electrical conductivity (σ) and Seebeck coefficient (S) for these samples,respectively.The increasingσand decreasingSwith elevated temperature indicate that these samples exhibit a typical nondegenerate semiconductor behavior (an indication of low carrier concentration).The positive Seebeck coefficient indicates that all samples are p-type.With the increasing Cd contents,the electrical conductivity of Mg3.4-xCdxSb2rises monotonously while the Seebeck coefficient predominantly shows the inverse trend.Hall effect measurement shows that the room-temperature Hall carrier concentration (nH) and mobility (μH) of the samples increase from~5.2 × 1016cm-3and 9.6 cm2V-1s-1for the Mg3.4Sb2to~6.5 × 1017cm-3and 27.8 cm2V-1s-1for the Mg2.9Cd0.5Sb2(Table S1),respectively.The increasednHis due to the decrease of activation energy of Mg3Sb2-based materialsviadoping Cd,which could shift impurity/defect levels toward valence band and/or narrow energy gap [52].The increase inμHis possibly related to two reasons: one is that the weakening of ionizing impurity scattering by doping Cd at the Mg site,similar to the cases of Mg3Sb2-xBix[53],Mg3-xAgxSb2[35],Mg3.2-xNbxSb1.5Bi0.49Te0.01[28]and Mg3.19-xLi0.01CdxSb2[38],and the other is that the weakening of the scattering effect of grain boundary on carriers [54–58].In addition,in order to further improve the power factor (S2σ),Mg vacancies were created by reducing the Mg content on the basis of Mg2.9Cd0.5Sb2,leading to the further increased hole concentration (Table S1).Specifically,as to the Mg2.85Cd0.5Sb2sample,the room-temperature carrier concentration is further increased to~9 × 1017cm-3,contributing to a power factor of 0.36 mW m-1K-2at 773 K.
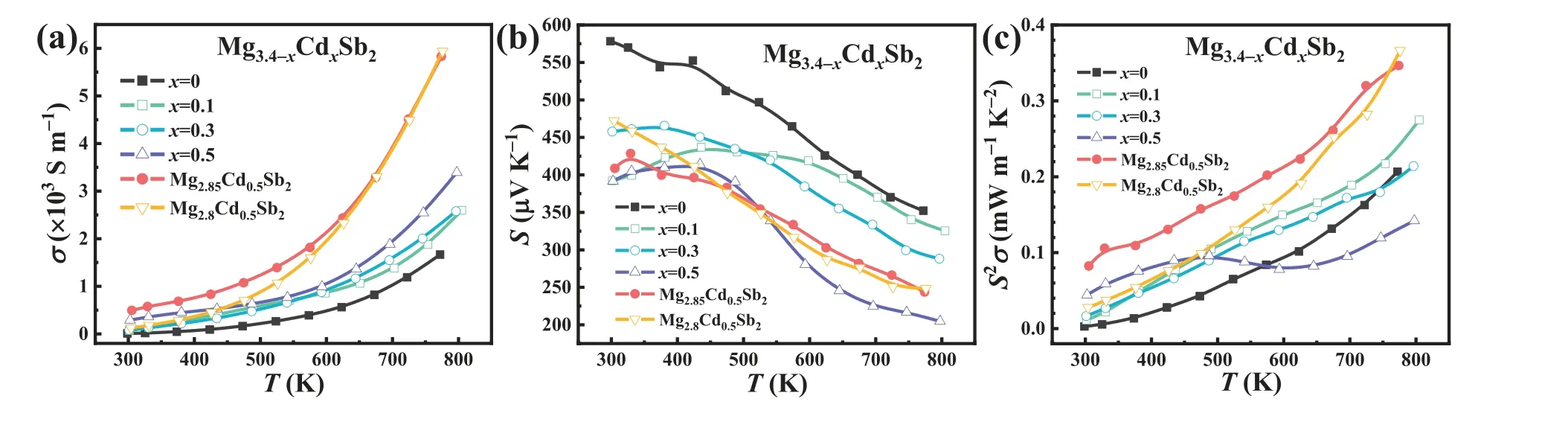
Fig.3.Temperature-dependent electrical transport properties of Mg3.4-xCdxSb2 (x=0,0.1,0.3,0.5),Mg2.85Cd0.5Sb2 and Mg2.8Cd0.5Sb2: (a) electrical conductivity,(b) Seebeck coefficient,and (c) power factor.
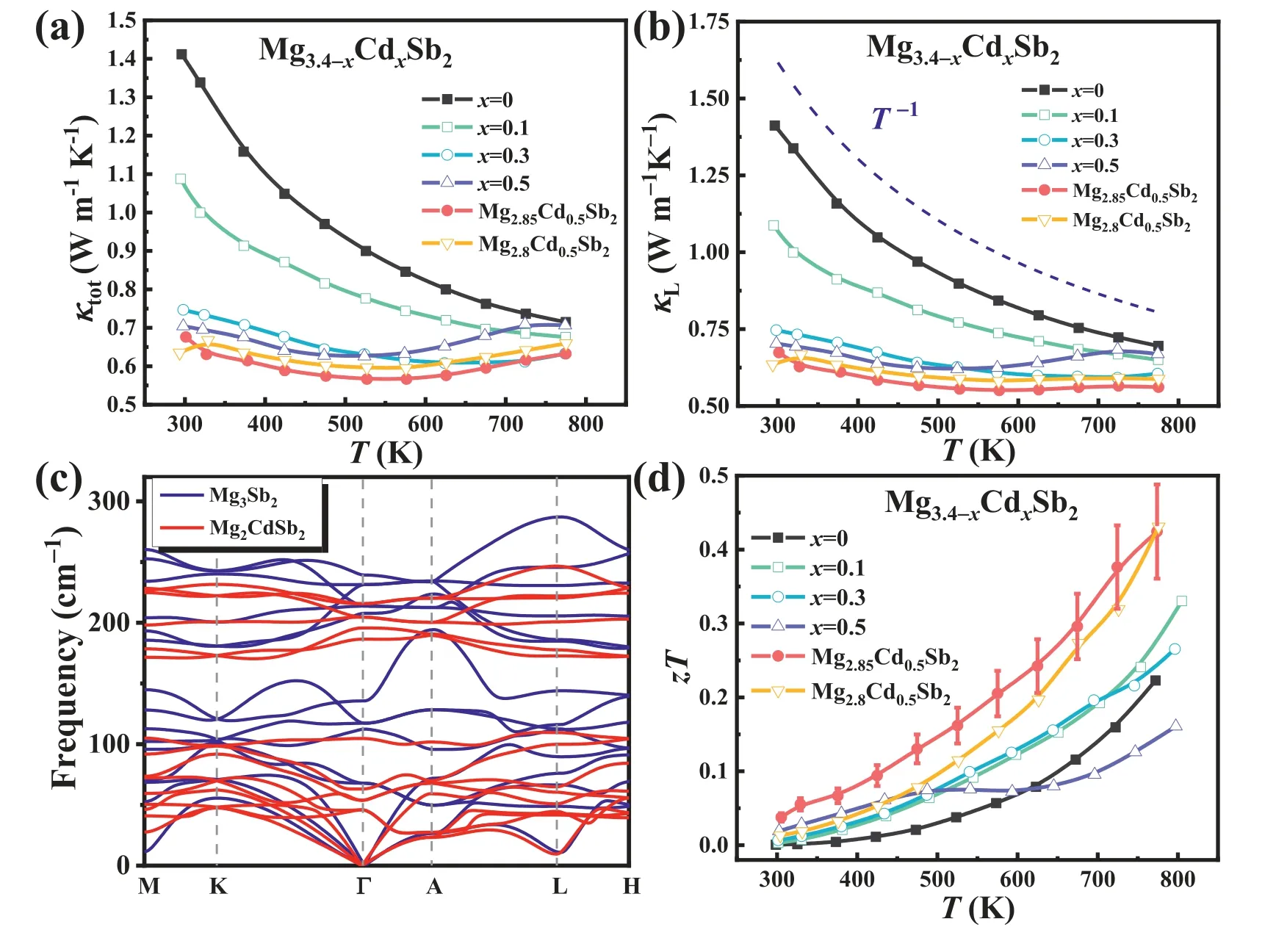
Fig.4.Temperature-dependent (a) κtot,(b) κL,and (d) zT values for Mg3.4-xCdxSb2 (x=0,0.1,0.3,0.5),Mg2.85Cd0.5Sb2,and Mg2.8Cd0.5Sb2.(c) Phonon dispersions of Mg3Sb2 (blue line) and Mg2CdSb2 (red line) (For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.).
The temperature-dependent total thermal conductivity and lattice thermal conductivity of Mg3.4-xCdxSb2(x=0,0.1,0.3,0.5),Mg2.85Cd0.5Sb2and Mg2.8Cd0.5Sb2samples are presented in Fig.4(a) and (b),respectively.We can observe that Cd doping on Mg site effectively reduces theκtotof Mg3Sb2-based materials.Specifically,theκtotat room temperature dramatically decreases from 1.41 W m-1K-1for the pristine Mg3.4Sb2to 0.67 W m-1K-1for the Mg2.85Cd0.5Sb2sample(Fig.4(a)),which is attributed to the drastically decreasedκL(Fig.4(b)).Further,it is noted that for the Mg3.4Sb2sample,κLshow a nearlyT-1dependence,indicating the dominance of phonon-phonon Umklapp scattering.However,the temperature-dependentκLof Mg3.4-xCdxSb2gradually deviates from the dependency ofT-1with increasing the Cd content,which is likely related to bipolar effect at elevated temperature.Additionally,by creating extra Mg vacancies (i.e.,the synthesis of Mg2.85Cd0.5Sb2and Mg2.8Cd0.5Sb2),the bipolar effect at the high temperature region is effectively suppressed (Fig.4(b)).
Room-temperature longitudinal (vl),transverse (vt) and mean (vs) sound velocities of Mg3.4-xCdxSb2decrease with increasing the Cd concentration (Table S1),which indicates a beneficial effect of Cd doping for reducing theκL(κL=,whereCvis the heat capacity,vis the phonon velocity,lis the phonon mean free path,andτis the phonon relaxation time).To understand the origin of the reduced phonon group velocity,we calculated the phonon dispersions of Mg3Sb2and Mg2CdSb2along the high symmetry lines (Fig.4(c)).Compared with Mg3Sb2,the cutoff frequency of phonon spectrum for Mg2CdSb2is surpassed,and the sound velocity decreases significantly around the center of Brillouin zone for transverse acoustic TA1,TA2and longitudinal acoustic LA (Fig.S3(a)–(c)),which is consistent with the experimental results.In addition,the phonon group velocity also sharply decreases for some optical mode,especially the frequency from 100 cm-1to 200 cm-1as shown in Fig 4(c) and S3(d),which also significantly contribute to the lattice thermal conductivity.To summarize,the decreasedκLafter doping Cd is mainly attributed to two factors: the decreased phonon velocityviaintroducing heavier element Cd,and the reduced phonon relaxation time by enhanced point defect phonon scattering.Eventually,the highestzTof~0.43 is achieved in Mg2.85Cd0.5Sb2at 773 K,which is~95% higher than that of the undoped Mg3.4Sb2(~0.22) (Fig.4(d)).
3.2.Electrical performance optimization by Ag doping
To obtain the best thermoelectric properties,the optimal carrier concentration is usually on the order of 1019-1020cm-3for most materials,that is,a heavy doping (or degenerate) state [59].We notice that replacing Mg2+by Cd2+does not significantly increase the hole carrier concentration of p-type Mg3.4Sb2.Considering that Ag has one less valence electron than Mg,Ag doping would be expected to effectively increase the hole concentration of Mg3Sb2-based materials.XRD patterns of Mg2.85-yAgyCd0.5Sb2(y=0,0.01,0.03,0.05) sintered samples are exhibited in Fig.5(a),in which the main diffraction peaks are consistent with the ones of Mg3Sb2phase,although there is a weak diffraction peak corresponding to Sb for the samples withy=0.03,0.05.TEMEDS analysis(Fig.5(b)-(f))shows the existence and uniform distribution of Mg,Sb and Cd in the Mg2.82Ag0.03Cd0.5Sb2sample together with the local enrichment of Ag.A selected area electron diffraction (SAED) pattern collected from the Mg2.82Ag0.03Cd0.5Sb2sample is displayed in the inset of Fig.5(g),and is indexed as the one taken along the [20]zone axis of Mg3Sb2.The corresponding high-resolution TEM(HRTEM) image shown in Fig.5(g) reveals a set of crystal planes with lattice spacings of 7.2and 3.9,which can be identified as the (0001) and(010)crystal planes,respectively.
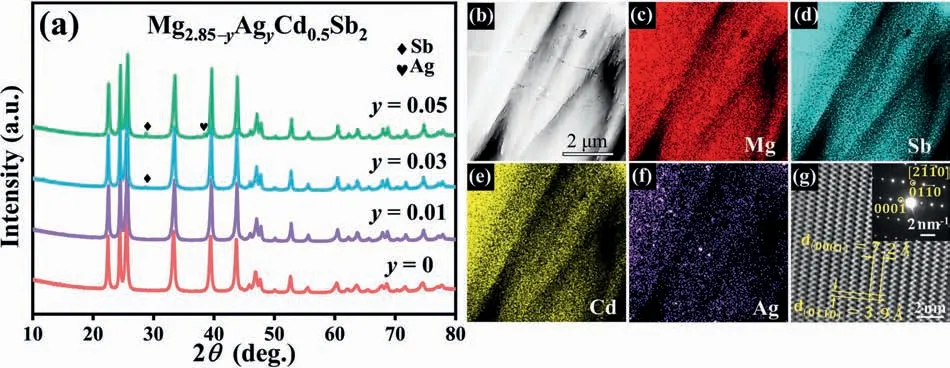
Fig.5.(a) XRD patterns of Mg2.85-yAgyCd0.5Sb2 (y=0,0.01,0.03,0.05);(b) The high-angle annular dark field-scanning TEM (HAADF-STEM) image of the Mg2.82Ag0.03Cd0.5Sb2 sample,(c)-(f) EDS elemental mappings for Mg,Sb,Cd,and Ag,respectively,and (g) SAED pattern along the [20]zone axis(the inset) and the corresponding HRTEM image.
Figs.6 and 7 show the electrical transport properties of the Mg3.4Sb2and Mg2.85-yAgyCd0.5Sb2(y=0,0.01,0.03,0.05) samples.It is clear that the electrical conductivity values of the Ag-doped samples are much larger than those of Mg3.4Sb2and Mg2.85Cd0.5Sb2(Fig.6(a)),which is primarily attributed to the increasednHover the measurement temperature range,for example,2.62 × 1019cm-3for Mg2.8Ag0.05Cd0.5Sb2as compared to 9.03 × 1017cm-3for Mg2.85Cd0.5Sb2at room temperature (Fig.6b,Table S1).Notably,the electrical conductivity of the Mg2.82Ag0.03Cd0.5Sb2and Mg2.8Ag0.05Cd0.5Sb2samples reveals three different temperature-dependent variation trends,and Hall effect measurement was performed to understand the mechanism.(i)σfirst increases slowly when the temperature rises from 300 K to~450 K,which is primarily attributed to the slowly increasednHdue to the ionized impurities (Fig.6(b)).With increasing the temperature from 300 K to~450 K,theμHincreases first,exhibiting the temperature-activated behavior that is related to resistive grain boundaries [54,55],and then decreases due to the increased lattice vibration scattering,as shown in Fig.6(c).(ii) Over 450 -623 K,σelevates sharply with the increase of temperature.This could result from the dissolution of Sb secondary phase into the matrices [60]and in turn the generation of more Mg vacancies,which dramatically increases the carrier concentration (Fig.6(b)).Although the carrier mobility is declined gradually (Fig.6(c)),its impact on theσis overwhelmed by that of the sharply increasednH.(iii)σdecreases with increasing the temperature from 623 K to 773 K,which is due to the diminished carrier mobility resulting from the strong lattice vibration scattering (the carrier concentration does not change significantly within this temperature range).It should be noted that the carrier mobility of all samples coincides with theμH≈T–1.5relation over 473–773 K,indicating that acoustic phonon scattering dominates the charge carrier scattering.
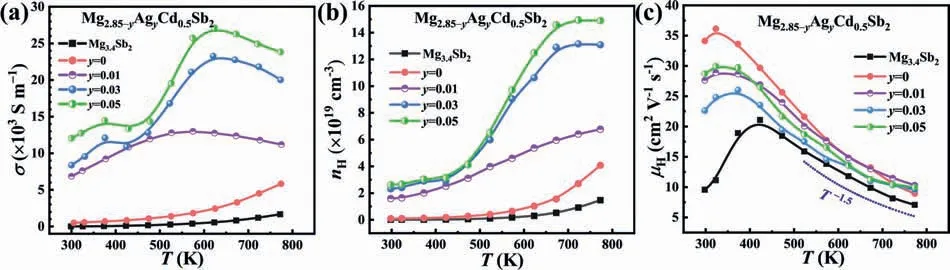
Fig.6.Temperature-dependent (a) electrical conductivity,(b) Hall carrier concentration,and (c) carrier mobility for Mg3.4Sb2 and Mg2.85-yAgyCd0.5Sb2(y=0,0.01,0.03,0.05).
In addition,the Seebeck coefficient (Fig.7(a)) of the samples gradually decreases with the increase of Ag content.For the Mg2.82Ag0.03Cd0.5Sb2and Mg2.8Ag0.05Cd0.5Sb2samples,the decreasedSwith increasing temperature from 450 K to 623 K is because of the significant increase in the carrier concentration.Due to much-improvedσand still decentS,Ag doping significantly increases the power factor of p-type Mg2.85Cd0.5Sb2,for example,0.7 mW m-1K-2at 773 K for Mg2.82Ag0.03Cd0.5Sb2as compared to 0.36 mW m-1K-2for Mg2.85Cd0.5Sb2(Fig.7(b)).The Pisarenko relationship at 300 K (Fig.7(c)) indicates that the density of states (DOS)effective mass of Cd,Ag-codoped samples (Table S1) rises remarkably compared to that of Mg3.4Sb2.According to the relationshipS∝md*T(3n)-2/3[4],a sharp increase in carrier concentration leads to the decreased Seebeck coefficient for the Cd and Ag codoped samples,although the DOS effective mass increases.Additionally,the temperature-dependent electronic quality factor (BE),which can describe the electronic contribution to the thermoelectric quality factorB=BET/κL(Bdetermines the maximumzTfor a thermoelectric material)[61–63],was calculated based on theSandσmeasured in the high temperature range.It is evident that Ag doping can effectively improve theBEof p-type Mg3Sb2(Fig.7(d)).It should be noted that any variation inBEwith temperature or doping indicates some extra effects in electrical transport,such as band convergence,additional electron scattering,and bipolar conduction [61].
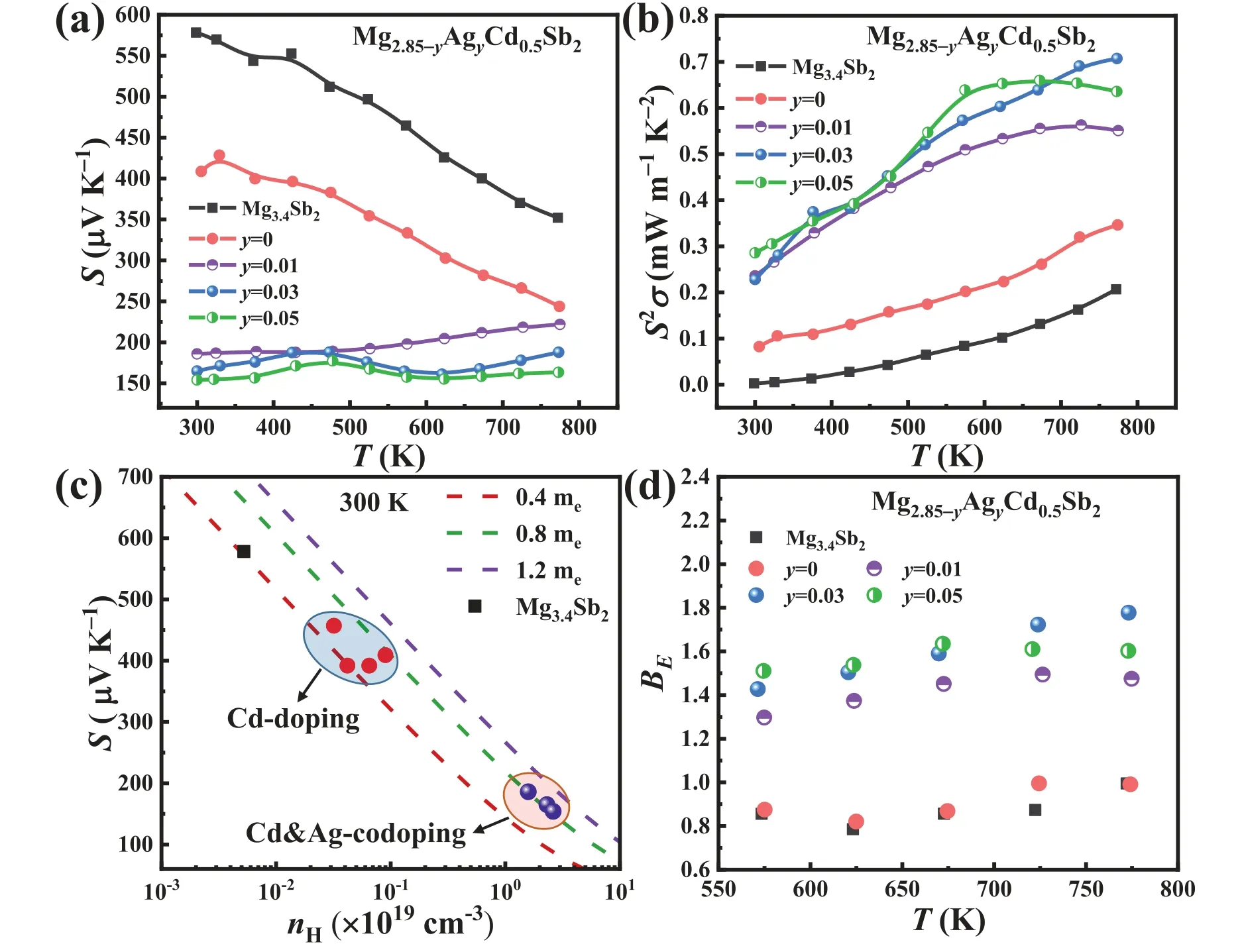
Fig.7.Temperature-dependent (a) seebeck coefficient and (b) power factor for Mg3.4Sb2 and Mg2.85-yAgyCd0.5Sb2 (y=0,0.01,0.03,0.05).(c) Pisarenko relationship for all samples at 300 K,in which both the calculated results (based on the single parabolic band model [62],dashed lines) and experimental data (spherical symbols) are included.(d) Temperature-dependent BE calculated based on a single parabolic band transport with acoustic phonon scattering model [61].
The total thermal conductivity of Cd,Ag-codoped samples,shown in Fig.8(a),is remarkably low,ranging between 0.65 and 0.8 W m-1K-1over 300–773 K.Due to the muchimproved electrical conductivity,the Mg2.85-yAgyCd0.5Sb2samples attain increasedκe,as shown in Fig.8(b),which leads to higherκtotin Mg2.8Ag0.05Cd0.5Sb2at 773 K than Mg3.4Sb2.Fig.8(c) presents the lattice thermal conductivity,indicating that Cd,Ag codoping can lead to the lowκLin the samples.Specifically,Mg2.8Ag0.05Cd0.5Sb2obtains the lowestκLof 0.43 W m-1K-1at 773 K,which is about 39%lower than that of Mg3.4Sb2(0.7 W m-1K-1).Eventually,due to the significantly reducedκLand enhancedS2σcontributed by Cd and Ag doping,respectively,an excellentzTvalue of 0.75 is obtained in the Mg2.82Ag0.03Cd0.5Sb2sample at 773 K,which is about 240% and 74% higher than that of Mg3.4Sb2(0.22) and Mg2.85Cd0.5Sb2(0.43),respectively(Fig.8(d)).
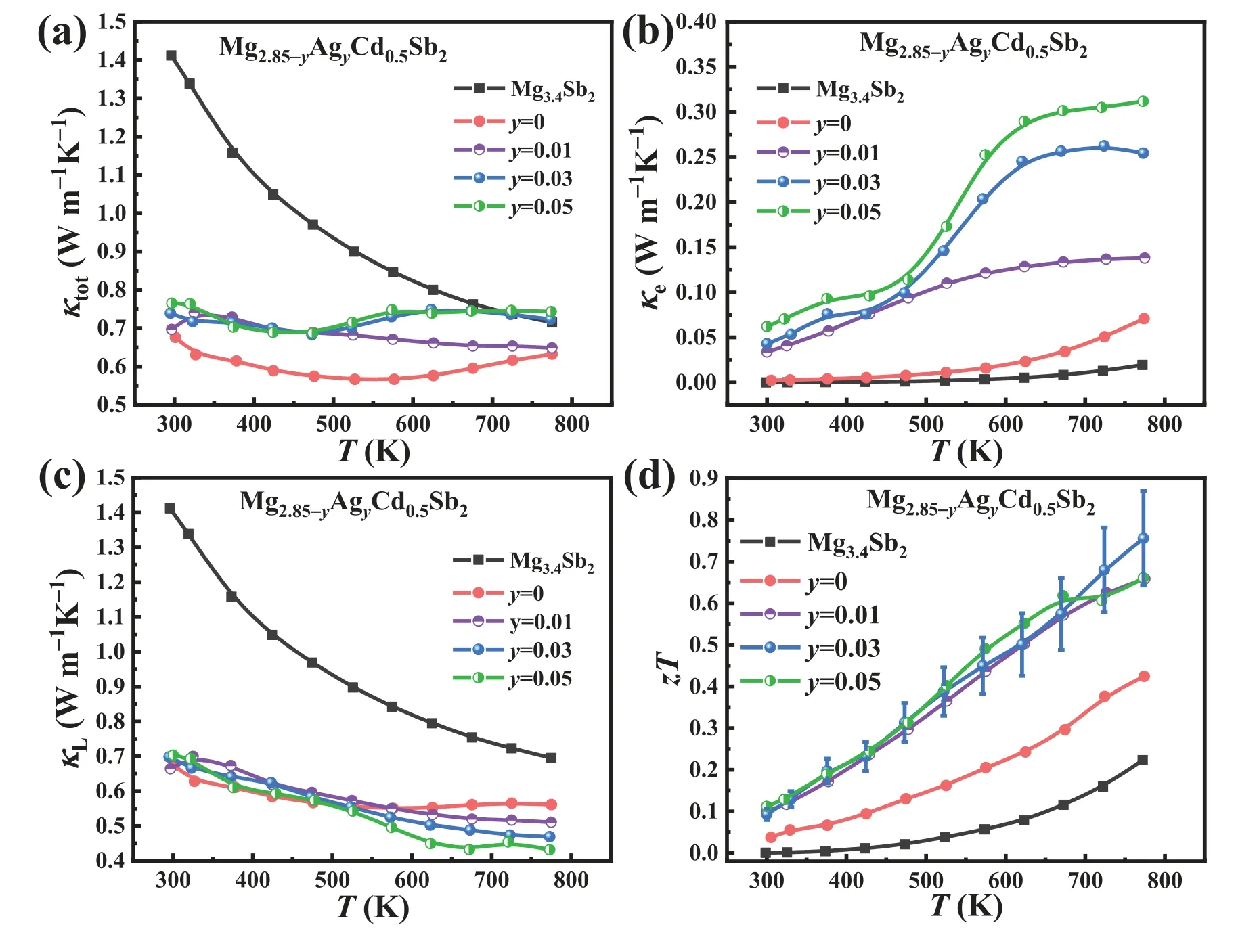
Fig.8.Temperature-dependent (a) κtot,(b) κe,(c) κL,and (d) zT values of Mg3.4Sb2 and Mg2.85-yAgyCd0.5Sb2 (y=0,0.01,0.03,0.05).
Fig.9(a) and S4 demonstrate the temperature-dependentzTof p-type Mg3Sb2-based materials reported in this study and previous literatures.As can be seen,thezTvalues of our Mg2.82Ag0.03Cd0.5Sb2compares very favorably to those of single element-doped (dashed lines) and codoped (solid lines) Mg3Sb2-based thermoelectrics previously reported.Notably,the Mg2.82Ag0.03Cd0.5Sb2sample also delivers an excellent averagezT(zTavg) of~0.41 over the temperature range from 300 K to 773 K,which is considerably higher than those of pristine p-type Mg3Sb2and most of other reported p-type Mg3Sb2-based materials(Fig.9(b)).This study demonstrates an effective Cd and Ag codoping strategy to synergistically optimize the thermal and electrical transport properties of Mg3Sb2,contributing to the high-performing p-type Mg3Sb2thermoelectrics.
4.Conclusions
In this work,Mg3.4-xCdxSb2(x=0,0.1,0.3,0.5) and Mg2.85-yAgyCd0.5Sb2(y=0,0.01,0.03,0.05) samples have been synthesized by ball milling and hot pressing.Cd doping not only increases the carrier concentration and mobility of p-type Mg3Sb2,but also introduces a large number of point defects and diminishes the phonon velocity,leading to a low room-temperature thermal conductivity value of 0.67 W m-1K-1in Mg2.85Cd0.05Sb2.Further,additional Ag doping effectively elevates the carrier concentration and in turn significantly improves the electrical conductivity and power factor,resulting in improvedzTover the whole temperature range.A notable example is the Mg2.82Ag0.03Cd0.5Sb2sample,which approaches a highzTvalue of 0.75 at 773 K and a highzTaveof~0.41 over the temperature range from 300 K to 773 K.These results indicate the effectiveness of Cd and Ag codoping in synergistically optimizing the electrical and thermal transport properties of p-type Mg3Sb2toward excellent peak and averagezT.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgments
The work is financially supported by the National Natural Science Foundation of China (Grant No.52071041,11874356,51802034).The work performed at the Chongqing Institute of Green and Intelligent Technology,Chinese Academy of Sciences (CAS) is supported by the Key Research Program of Frontier Sciences,CAS (Grant No.QYZDB-SSW-SLH016).We acknowledge Guiwen Wang from the Analytical and Testing Center of Chongqing University for her assistance in thermoelectric property measurement.
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2021.09.012.
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- “Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
