Fabrication of branch-like Aph@LDH-MgO material through organic-inorganic hybrid conjugation for excellent anti-corrosion performance
Maryam Chafiq ,Adelkarim Chaouiki,* ,Rachid Salghi ,Young Gun Ko
a Materials Electrochemistry Laboratory,School of Materials Science and Engineering,Yeungnam University,Gyeongsan 38541,Republic of Korea
b Laboratory of Applied Chemistry and Environment,ENSA,University Ibn Zohr,PO Box 1136,Agadir,80000,Morocco
Abstract Layered double hydroxides (LDH) frameworks have shown significant enhancement in stability and reusability,and their tailorable architecture brings new insight into the development of the next generation of hybrid materials,which attracted considerable attention in many fields over the years.One of the factors contributing to the widespread applicability of layered double hydroxides is their adaptable composition,which can accommodate a wide spectrum of potential anionic guests.This exceptional property makes the LDH system simple to adjust for various applications.However,most LDH systems are synthesized in situ in an autoclave at high temperatures and pressures that severely restrict the industrial use of such coating systems.In this study,LDH was directly synthesized on a magnesium alloy that had undergone plasma electrolytic oxidation (PEO) treatment in the presence of ethylenediaminetetraacetic acid,thereby avoiding the use of hydrothermal autoclave conditions.This LDH system was compared with a hybrid architecture consisting of organic-inorganic self-assembly.An organic layer was fabricated on top of the LDH film using 4-Aminophenol (Aph) compound,resulting in a smart hierarchical structure that can provide a robust Aph@LDH film with excellent anti-corrosion performance.At the molecular level,the conjugation characteristics and adsorption mechanism of Aph molecule were studied using two levels of theory as follows.First,Localized orbit locator (LOL)-π isosurface,electrostatic potential (ESP) distribution,and average local ionization energy (ALIE) on the molecular surface were used to highlight localization region,reveal the favorable electrophilic and nucleophilic attacks,and clearly explore the type of interactions that occurred around interesting regions.Second,first-principles based on density functional theory (DFT) was applied to study the hybrid mechanism of Aph on LDH system and elucidate their mutual interactions.The experimental and computational analyses suggest that the high π-electron density and delocalization characteristics of the functional groups and benzene ring in the Aph molecule played a leading role in the synergistic effects arising from the combination of organic and inorganic coatings.This work provides a promising approach to design advanced hybrid materials with exceptional electrochemical performance.
Keywords: Magnesium alloy;Surface modification;LDH;Organic-inorganic hybrid materials;Inter-/intra-molecular interactions.
1.Introduction
Just as the well-organized and interconnected pores of a mesoporous material facilitate the efficient transport of molecules and ions,the porous structure of layered double hydroxides (LDHs) enables the efficient transport of ions and molecules,making them attractive materials for applications in physical [1,2],electrical [3,4],chemical [5,6],and optical [7,8]domain.Determining the origin of the outstanding properties of the 2D nanomaterials has been a challenging task for the research community.Moreover,significant efforts,have been made over the past decades to enhance the performance of advanced multifunctional materials,particularly layered double hydroxides (LDH),as a specific type of 2D material that has been widely used in the field of material science [9–12].The lamellar structures of LDH can introduce cross-linked morphological networks with a high anion-exchange capacity,thereby making them a flexible system with a vast choice of practical applications [13,14].In addition,LDH is considered a perfect physical barrier to prevent corrosion of metal substrates,owing to its strong interlayer coupling and chemical stability.Although the synthesis of layered double hydroxides (LDHs) is a well-established process,scaling up production for industrial applications remains a significant challenge.The need for large quantities of high-quality materials,coupled with the high cost and energy requirements of conventional synthesis methods,has prompted researchers to explore alternative synthesis routes,thereby hindering LDH from being the best solution and restricting its range of applicability despite its outstanding properties.To overcome the challenges of conventional synthesis methods for layered double hydroxides (LDHs),this study presents a novel approach for the in situ growth of LDH using ethylenediaminetetraacetic acid (EDTA) as a complexing agent.In addition to addressing the extreme process condition requirements,another key challenge for LDH in the field of material engineering is to utilize layered coating materials as a bastion to improve the performance of functional materials,and to broaden their application domain in the field of materials science and engineering.
Favorable biodegradation properties and mechanical behaviors of Mg and its alloys make them attractive choices in various applications worldwide as one of the most important groups of engineering materials[15–18].They are widely used in the automotive industry,pharmaceutical and chemical applications [19,20],and the electronics sector [21,22].However,Mg alloys are susceptible to corrosion in humid air,seawater,acids,and salts because of their lower standard potential (2.36 V) than that of a conventional hydrogen electrode,which significantly limits their general application [23].Recently,hybrid coatings with self-assembled organic molecules,prepared by a combination of plasma electrolytic oxidation(PEO) and in situ deposition of LDH architecture,have received attention among different anti-corrosion techniques because of their effectiveness,high chemical stability,excellent adhesion,and acceptable mechanical properties[24,25].However,achieving a high and durable inhibition along with the extraordinary performance of LDH is still an exciting challenge,considering that the inevitable defects in the lamellar structure of perpendicular nano-petals architecture with gaps,that make the coatings corrosion-resistant,for long term,are not easily achievable using the pristine lamellar structure.
Organic-inorganic materials have been proposed as multifunctional hybrid materials owing to their specific advantages in high value-added applications.This particularity originates from the fact that the combination of both inorganic and organic properties of these materials offers a creative way to include a wide range of materials.These hybrids cover a range from materials having a high proportion of inorganic compounds,such as ceramics,to those having a low proportion of inorganic compounds,such as polymers.In recent years,this class of materials has been extensively studied.Msun et al.[26]reported that the inclusion of benzotriazole and halloysite nanotubes in silicate-based electrolytes boosts corrosion resistance while reducing the porosity of the coating.Toorani et al.[27]suggested that the PEO/silane/epoxy coating system performs the best in the presence of an organic inhibitor,8-Hydroxyquinoline,and develops active corrosion prevention capabilities.Recently,a novel coating system on AZ31 Mg alloy was developed by modifying an LDH film with WO3nanoparticles [28].The system was applied to improve the anti-corrosion ability using a surface treatment mechanism in which the flake structure of the LDH-ALB composite is responsible for capturing corrosive ions during the corrosion process.To the best of our knowledge,only a few studies have been conducted on the enhancement of metal surface protection using organic compounds on the top of the LDH layer,thereby preventing possible contact between the corrosion media and inorganic layers.Furthermore,the literature suggests that pure LDH could only provide short-term protection for the metal substrate;even in the presence of an incorporated organic inhibitor,the porous structure of the layered coating material remains a weak point that affects long-term corrosion inhibition.The structural flaws in the LDH film,organized perpendicular to the coating surface,would allow the corrosive media to attack both the coating and the underlying substrate.Therefore,an LDH layer alone can not provide dependable and long-lasting corrosion protection.Herein,we report the fabrication of LDH nano-petals using the chelating agent EDTA on the PEO surface and tuning of their inner structure similar to the nature-inspired architectural design of aminophenol compounds to achieve excellent anti-corrosion properties.
2.Materials and experiments
2.1.Synthesis of the inorganic layer
The following reagent-grade chemicals were used to prepare the electrolytes: potassium hydroxide (KOH,≥90%),tripotassium phosphate (K3PO4,≥99%),ethylenediaminetetraacetic acid (EDTA,C10H16N2O8,≥ 99%),Aluminum nitrate nonahydrate (Al(NO3)3.9H2O,≥ 99%),Magnesium nitrate hexahydrate (Mg(NO3)2.6H2O,≥99%),(4-Aminophenol (C6H7NO,≥98%),sodium hydroxide (NaOH,≥99%),and sodium chloride (NaCl,≥99%).Deionized water was used throughout the experiments.All chemicals and reagents used in this work were of high-purity grade,purchased from Merck,and used directly without any further purification.A commercial-graded AZ31 Mg alloy plate (purchased from Posco steel,South Korea) with a chemical composition of 2.89 wt.% Al,0.96 wt.% Zn,0.31 wt.% Mn,0.15 wt.%Fe,0.12 wt.%Si,and a balance fraction of Mg was used as substrates.Prior to PEO,samples were ground to dimensions of 30 mm × 20 mm × 4 mm using SiC abrasive paper up to 1200 grit,washed in acetone using an ultrasonicator,and thoroughly rinsed with deionized water.An electrolyte containing KOH(6 g/L)and K3PO4(6 g/L)was used to fabricate the PEO coating.The electrolyte was kept in a glass container equipped with a magnetic stirrer and water-cooling system to maintain the system temperature at approximately 298 K in order to stabilize the electrochemical reactions throughout the process.The present sample was set as the anode while the stainless steel net was used as the cathode.Then,a series of PEO treatments were performed under an AC current at a constant frequency of 60 Hz and a current density of 100 mA/cm2for 10 min.
2.2.Synthesis of Aph@LDH composite
The surface-treated Mg substrates were vertically immersed in an aqueous solution containing Al(NO3)3,Mg(NO3)2with an Mg2+/Al3+ratio of 2:1,and varying molar concentration of EDTA (0.01,0.05 and 0.1 M) to deposit LDH film on the surface of the MgO coating.The pH of the prepared solutions was adjusted to 10 ± 0.1 using a NaOH solution.Subsequently,hydrothermal reaction was performed under electromagnetic stirring at 353 K for 3 h.After the LDH deposition process,the PEO-LDH coated samples were immersed in water solution for 12 hours to facilitate dip chemical coating (DCC) with aminophenol compound,thereby producing self-assembled organic-inorganic material.Finally,after the immersion treatment,the as-prepared samples were washed with deionized water and ethanol and then air-dried at room temperature.The final product was labeled Aph@LDH-MgO,and its synthesis steps are shown in Fig.1.

Fig.1.Schematic description of the fabrication process of the Aph@LDH-MgO composite.
2.3.Microstructural and compositional analysis
Chemical composition results were obtained by characterizing the as-prepared oxide and hybrid layers.Throughout the processing time of the PEO treatment,sequential realtime images were captured,providing visual documentation of the formation and evolution of micro-discharges on the treated surface.A scanning electron microscope (SEM,Hitachi,S-4800) equipped with energy-dispersive X-ray spectroscopy (EDS,Hriba EMAX) and an analytical transmission electron microscope (TEM,Philips,CM 200) with an acceleration voltage of 200 kV were used to investigate the surface morphologies of the coating surfaces.For TEM observation,samples were detached from the substrate and ground to fine particles.Fourier transform infrared spectroscopy (FTIR,Perkin Elmer,Spectrum 100) was used to determine the surface functional groups of the organic-inorganic materials in the range of 4000–500 cm-1.An X-ray diffraction (XRD,Rigaku,D-MAX 2500) with a step size of 0.05° and scan range of 20–90° was used to examine the crystal structures of the as-prepared organic and inorganic coatings.The chemical composition of the organic-inorganic materials was characterized in detail using X-ray photoelectron spectroscopy (XPS;VG Microtech,ESCA 2000).All samples were coated with platinum prior to SEM observations to avoid charging effect by the electrons.
2.4.Quantum chemical theory simulations
To comprehend the nature of the interactions between the organic compound and the inorganic layer,theoretical calculations were carried out using density functional theory (DFT).The interfacial mechanism and adsorption behaviors of the aminophenol molecules and LDH surface were examined.The Gaussian 16 W package was used to evaluate the molecular electronic properties [29].The molecular structures were geometrically optimized using the B3LYP functional with the 6-311G+(d,p) basis set under water conditions by integrating the polarizable continuum model (PCM) model [30].Furthermore,electronic structure analyses were performed on the optimized systems based on the electrostatic potential (ESP),electron localization function (ELF),average local ionization energy (ALIE),localized orbital locator (LOL),and density of states (DOS) to understand the charge transfer ability and quantitatively discuss the delocalization path ofπelectrons.
In addition,first-principles of DFT simulations were carried out using the CASTEP algorithm [31],to investigate the adsorption mechanism of the organic layer on the LDH surface.All calculations were performed using the generalized gradient approximation in the Perdew-Burke-Ernzerhof exchange-correlation function (GGA-PBE) [32]and ultrasoft pseudopotentials to characterize the Aph-LDH interactions with molecules that adsorb on the lamellar framework .For the bulk lattice optimization,the Brillouin zone analysis was carried out using a Monkhorst-Pack grid with a smearing parameter of 0.02 Ry;the relaxations were performed using the Broyden-Fletcher-Goldfarb-Shanno (BFGS) algorithm with a force convergence cut-off of 0.01 eV/per atom,and the electronic wave functions were expanded using a plane-wave cutoff energy of 480 eV [33].To evaluate the interfacial mechanism of Aph on the LDH layer quantitatively,the adsorption energy (Eads) was calculated as follows:
whereELDH+Aph(eV),EAph(eV),andELDH(eV) denote the interaction energy of the aminophenol compound together with LDH,free energies of aminophenol,and LDH,respectively.
2.5.Insights into the electrochemical behavior
Anticorrosion measurements were performed in 3.5 wt.%sodium chloride (NaCl) solution to evaluate the corrosion properties of the oxide layer by an electrochemical impedance spectroscopy (EIS) test using an interface 600 potentiostat(Gamry Instruments).A conventional three-electrode cell was used in which the specimens (PEO and Aph@LDH hybrid coatings prepared on the Mg substrate) with an exposed area of 1 cm2served as the working electrode,a Pt plate as the counter electrode,and an Ag/AgCl electrode as the reference electrode.The long-term electrochemical behavior of the asprepared materials was investigated following immersion periods of 30 min,7,14,and 21 days in the 3.5 wt% NaCl solution.Subsequently,EIS measurements were performed in the frequency range of 0.1 to 106Hz with an amplitude of 10 mV rms sinusoidal perturbation vs.OCP.Prior to EIS measurement,the working electrode was immersed in the solution for 30 min to obtain a relatively stable open-circuit potential(OCP).All tests were performed at room temperature without agitation.Electrochemical measurement were repeated at least 3 times to ensure the reproducibility of the experimental results.
3.Results and discussion
3.1.Structure and morphology characterization of hybrid Aph@LDH-MgO material
Fig.S1 illustrates the changes in the responding voltages during PEO process together with couples of inset images indicating the characteristics of micro-discharges for 30,50,180,350,410,and 600 s.Fig.S1 demonstrates three distinct regions based on the increasing tendency of the responding voltage with respect to the coating time.Each stage is characterized by its unique slope,representing significant shifts in the behavior of the PEO process based on the development of the oxide layer and appearance of plasma sparks.During stage I,the surface of the Mg alloy undergoes instantaneous oxidation,resulting in the formation of a thin barrier layer.This oxidation process occurs concurrently with oxygen evolution reactions due to an increase in electrical resistance which resulted from the formation of the thin passive film formed initially on substrate.Notably,the voltage exhibited a steady increase over time at a consistent rate during this period and the absence of plasma discharges was observed.However,intense plasma discharges started to form on the oxide surface when the voltage reached a critical value(at~30s) that would be referred to as the breakdown voltage and they showed a tendency to grow in size as the responding voltage increased steadily during PEO process (region II).The occurrence of plasma discharges,as depicted in the inset of Fig.S1,accompanies this remarkable and pronounced change.The dielectric breakdown rapidly triggers an avalanche of high-energy electrons,leading to the emergence of a multitude of plasma discharges throughout the surface of the anode [34–36].Thus,a notable transition occurs in the growth mechanism,shifting from conventional chemical reactions that adhere to the linear relationship of Ohm’s law to plasma-driven electrochemical processes.This shift signifies a fundamental change in the underlying mechanisms driving the growth dynamics during this stage [37,38].Entering stage III,a gradual augmentation in both the lifetime and size of the micro-sparks was observed uniformly across the entire substrate surface.Concurrently,the voltage-time curves exhibited a comparatively lower slope in this region as compared to that in region II.Despite the substantial growth of the adhesive interfacial layer caused by intense discharges,the emergence of micro-pores and oxide nodules resembling volcanic craters is an inevitable consequence.These formations serve to counterbalance the thermal stress resulting from local heating effects.They actually lead to a deterioration of electrochemical stability under corrosive environment [39–41].Nevertheless,in the present work,the porous inorganic layer formed by PEO process and its post-treatment is expected to provide preferential sites for LDH nucleation.
To confirm and optimize the formation of LDH on the MgO surface under ambient pressure,concentrations of the chelating agent EDTA were varied from 0.01–0.1 M.As shown in Fig.2,the EDTA concentration has a significant impact on both the microstructure and surface topography.The pristine MgO surface(Fig.2a) exhibits several cracks and microporous defects with multilevel layouts,which are attribute to the powerful spark discharge that occurs throughout the PEO process [42,43].In contrast,formation of LDH on the MgO film at a concentration of 0.01 M EDTA significantly improves the surface morphology by reducing the pore size of the PEO surface (Fig.2b).However,it can not control the uniform precipitation to obtain a layered morphology over the entire film surface.Therefore,the formation of the LDH layer at a low chelating agent concentration is not sufficiently favored.For samples that were immersed in the solution with 0.05 M of EDTA(Fig.2c),the micropore defects on the MgO film are seen to be more effectively sealed than those on the inorganic surface.However,the sealing performance may be imperfect under these conditions.Furthermore,the architecture of the LDH-covered surface is significantly different when the synthesis concentration of EDTA is 0.1 M,as shown in Fig.2d.The LDH layer is seen completely formed at 0.1 M EDTA.The results are supported by the EDS spectra of the pristine PEO sample (Fig.2e) and the LDH-MgO surface(Fig.2f),indicating successful fabrication of the LDH coating.The surface area of the LDH structure could be considerably improved by changing from a 2D to a 3D morphology,leading to the formation of a unique flower-shaped network.The evolution of the 3D floral structure was strongly dependent on the concentration of EDTA;samples examined at different EDTA concentrations during the same reaction produced different morphological architectures,going from initial porous(0.01 M),to an effective sealed surface in the presence of 0.05 M of the chelating agent,and finally,to self-assembled and interlaced petal-like structures after the addition of 0.1 M.Fig.2g illustrates a schematic of the key steps that lead to the nucleation and growth of the flower-like architecture and indicates their dependence on the EDTA concentration.Notably,severe spark response and the melting of magnesium oxide and magnesium phosphate resulted in the formation of a relatively compact barrier film decorated by sizeable pores on the PEO surface,whereas LDH could generate a uniform surface assisted by the precipitation behavior in the pores formed by spark discharge during the PEO process[44–46].Moreover,the post-growth architectures exhibited a flower-like structure that agglomerates and stretches primarily from the micro-defects of the inorganic layer through the layer-by-layer behavior of the layered coating material [47].This could be because of the uneven disintegration of the substrate during synthesis and the continuous etching of the material during LDH formation,suggesting that the conforming structure morphology of the porous material can be modified owing to the organic synergy of the chelating agent with the inorganic component.In addition,porosity itself acts as a support,thereby accepting the nucleation of the nature-inspired network,hierarchically upgraded into the given defects.The estimation of surface porosity for both the PEO layer and LDH-based coating is depicted in Figs.S2 and S3,respectively.According to Fig.S2,it can be observed that intensive plasma discharges lead to a high level of porosity,which can be attributed to the accumulation of fine micro-discharges in the low-intensity region during the initial stage of PEO treatment.In contrast,the formation of the LDH layer at a low concentration of EDTA significantly reduces the porosity level(Fig.S3).This reduction indicates that the interlaced petallike structures not only possess a strong tendency to fill cracks and block pores in the PEO coating but also facilitate robust inter-and intra-molecular interactions in relation to electron transfer behavior of Aph compound.These results demonstrate that the porous MgO surface was successfully coated with LDH under ambient pressure.

Fig.2.SEM images of PEO-treated Mg samples before (a) and after 3 h treatment at different EDTA concentrations: 0.01 M (b);0.05 M (c);and 0.1 M (d).EDS spectra of (e) PEO and (f) optimized LDH system.(g) Schematic illustration of LDH nucleation on the porous PEO surface.
Considering the crystallographic LDH arrangement is perpendicular to the coating surfaces,the weak microcrystalline structure of the LDH morphology may allow the corrosive anions to easily enter into the material substrate.This issue raises a concern to correct the porous morphology of the lamellar structure and identify the impact of corrected morphology on the long-term stability of the coating system in corrosive environments.The self-assembled hybrid Aph@LDH-MgO coating,which can offer stronger protection and suggest a suitable answer to our concerns,is investigated.As shown in Fig.3,the SEM images compare the microstructures and the corresponding EDS results obtained from naturemimic architectures consisting of floret-like 3-dimensional ultrathin nano-petals of LDH(Fig.3a)and the branch-like structure of the Aph@LDH-MgO coating (Fig.3h).Pristine MgO provides a solid framework of different-sized pores located on a relatively smooth surface,as shown in Fig.3a,thereby providing favorable conditions and long-range 3D spacing for the nucleation,growth,and agglomeration of the LDH layer.After the in situ growth of the LDH layer based on the floretlike network,the nano-petals were tightly and uniformly integrated into the PEO surface (Fig.3a).The successful loading of the LDH-MgO surface morphology was also confirmed by elemental mapping analysis,which shows that Mg,Al,C,and O are uniformly distributed throughout the selected area,as shown in Fig.3c-g.Upon complete precipitation of the LDH and its etching at the MgO surface,the duplex structure film serves as a compact template and transforms into a hybrid hollow structure assembly of uniform nanosheets over the entire surface.Subsequently,the obtained LDH was fabricated using a DCC in Aph solution to form an organic layer on top of the defects,where Aph self-assembly tends to deposit on the LDH morphological defects with a tree branch-like morphology and creates an intermediate flat cover distributed over the entire LDH layer with high coverage and unique sealing design characteristics.The as-formed micro-branch architecture,in turn,promotes long-term protection of the Mg alloy,making the final material a robust candidate capable of providing excellent protection by perfect reformation of the topography achieved by the network structure of the Aph molecules in synergy with the LDH architecture,as shown in Fig.3h.Elemental mapping of the self-assembled material (Fig.3j-n)confirms the uniform distribution of C,O,and N;the density of C is higher than that of other elements,implying the formation of an organic layer on the coating surface.The particular morphological evolution resulting from the unique combination of the two nature-inspired structures in the presence of the chelating agent could provide an alternative solution to the limiting conditions of autoclaves to facilitate and generalize the use of LDH systems and correct the inevitable defects of the LDH morphology as well.Besides,the key role played by Aph@LDH composite was also revealed by the comparison of the average thickness of the PEO coating before and after hydrothermal and DCC treatments.Fig.S4 illustrates the cross-sectional images of the PEO sample,while Fig.S5 showcases the cross-sectional images of the Aph@LDH-MgO sample.In a similar fashion to the surface morphology observed in Fig.2a,the cross-section of the PEO sample (Fig.S4) exhibited numerous micro-pores with varying sizes prior to any post-treatment.However,through in situ treatments involving the presence of EDTA and Aph,the micro-pores in Aph@LDH-MgO were effectively sealed.This sealing process occurred uniformly on the oxide layer during the subsequent post-treatment procedures,facilitating the formation of the hybrid coating (Fig.S5).Furthermore,the absence of a distinct boundary between the Ap@LDH coating and outer porous layer,as depicted in Fig.S5,indicates a robust adhesion between the Aph@LDH-based coating and the inorganic layer in the Aph@LDH-MgO sample.The presence of Mg,O,Al,and C in the sealed porous structure was confirmed through EDS elemental mappings,as depicted in Fig.S5.This observation indicates the formation of a highly compact film.The low detection of N using EDS in the Ap@LDH-MgO sample can be attributed to the challenge of detecting N as a low-Z element in the hybrid composite coating.Moreover,Figs.S4 and S5 reveal that the average thicknesses of the PEO and Ap@LDH-MgO coatings were calculated to be 12.73 ±1.2 μm and 14 ± 0.8 μm,respectively.This suggests that the hierarchical structures formed during LDH and DCC treatments significantly influenced the compactness and uniformity of the inorganic coating.The porous structure of PEO film provides favorable sites for the development of a 3D floral structure,which effectively repairs defects within the coating.Additionally,the LDH coating acts as an intermediate layer,enabling the successful intercalation of Aph compound,leading to the formation of uniform and compact hybrid coating.It is worth mentioning that the presence of a compact inner layer observed in Fig.S5 highlights the strong adhesion of the hybrid coating to the Mg substrate.This observation indicates that the Ap@LDH-MgO coating formed a tightly bonded interface with the underlying substrate,indicating a robust adhesion property.

Fig.3.SEM images and the corresponding EDS spectra of (a-g) PEO-LDH and (h-n) Aph@LDH-PEO composite.
The crystal structures of PEO,LDH,and the Aph@LDHMgO hybrid coatings prepared on the Mg substrate were further analyzed by X-ray diffraction.As shown in Fig.4a,Mg,MgO,and a small amount of Mg3(PO4)2(JCPDS #00-043-0225) are detected in the sample generated by the PEO process resulting from the reactions between the Mg2+cations from the substrate and the different ions produced by the electrolyte.Peaks related to metallic Mg (JCPDS #01-089-4894) were observed in the patterns due to X-ray penetration through the Mg substrate.Based on the peak intensities,the relative amount of MgO in the inorganic layer is the highest among all the peaks,indicating MgO (Reference No.03-065-0476) is the dominant oxide in the inorganic layer.In addition,five characteristic XRD peaks at 12.1°,21.7°,34.5°,58.5°,and 63.2° are characteristic of the (003),(006),(009),(110),and (113) diffraction planes of pure LDH phases,respectively,implying an effective cooperation between the organic layer and LDH surface [9,12,48–51].Because of the interactions between the Mg,Al,and hydroxyl groups,certain diffraction peaks of Mg(OH)2and Al(OH)3with low intensities were also observed.Results suggest that LDH layers were successfully deposited on the MgO surface.The crystallinity of the Aph@LDH-MgO particles was further investigated by the XRD patterns.The diffraction peak positions of the hybrid film match the spectrum of the LDH phases exactly,proving that the LDH samples remained intact following surface modification.The inclusion of the Aph compound on the LDH layer marginally affected the peak intensity by changing the X-ray adsorption ability.The high X-ray penetration depth allowed the identification of the chemical phase of the metallic Mg.Conversely,new peaks that are part of the reflection peaks of aminophenol emerged as a result of the interaction between (–OH),which is related to the LDH framework and the active sites of organic molecules.Therefore,the fabrication of the hybrid coating resulting from the combination of the LDH framework and organic layer was confirmed.To identify the bonding in the double-layer coatings,Fig.4b shows the FT-IR spectra of the self-assembled material,LDH structure,and PEO coating.As shown in Fig.4b,the O–H stretching of hydroxyl groups could be the origin of a distinctive broad band at 3400 cm-1[52,53].This is comparable to the Aph@LDH-MgO spectrum,in which the significant increase in the intensity of the hydroxyl bands confirms the presence of (–OH) groups resulting from both the hydroxide groups of LDH and aminophenol,which is indicative of the deposition of the organic self-assembled layer on the LDH structure.In the LDH system,the bands at 2920 cm-1and 2840 cm-1were assigned to the tensile vibration of the C–H group [54–57],which demonstrates role of EDTA as a chelating agent in the successful production of LDH at atmospheric pressure.This result was confirmed by the presence of peaks located at 1580,1350,and 1100 cm-1,attributed to the N–H[58,59],C–O [60,61],and C–N [62,63]bands,respectively,thereby indicating successful complexation by the chelating agent.Moreover,compared to the LDH coating,the significant increase in the intensity of the peaks corresponding to C–O and N–H indicates the evident adsorption of Aph,probably triggered by the formation of a self-assembly at the top of the lamellar structure.Furthermore,new characteristic peaks ranging from 960 to 740 cm-1are also seen in Fig.4b,which are attributed to the agglomeration of Aph molecules on the surface and on top of the LDH morphological structural defects,confirming the presence of organic self-assembly on the layered coating material [64,65].The surface composition and bonding states of the constituent elements in various coating layers were examined using XPS.As shown in Fig.4c,the PEO sample surface primarily consists of Mg,O,and P elements.In contrast,the peaks of the C,O,Al,and Mg elements can be seen in the XPS survey profiles of PEOLDH and Aph@LDH-MgO,which demonstrate the synthesis of LDH in both composites.According to the peak intensities,the spectra of C (2s),O (1s),and N (1s) became more intense with the addition of the organic layer,implying effective cooperation between the Aph self-assembly and the LDH surface.Moreover,the narrow-scan spectra of Mg 2p,O 1s,and C 1s are described for the hybrid coating simple in order to better comprehend the chemical states of the elements and the chemical interaction between the Aph self-assembly and LDH coating.The high-resolution Mg 2p spectrum can be deconvoluted into three sub-peaks assigned to MgO,Mg,and Mg(OH)2after curve fitting at 49.2,51.1,and 52.7,respectively,as shown in Fig.4d [66,67].Following curve fitting,it was discovered that the surface C atom exists in three different chemical states: the peak at 284.4 eV,which is associated with the energy of the C–C bonds in the aromatic ring of the aminophenol molecule,and two additional peaks at 285.3 and 288.6 eV,which are associated with the C–N and C–O bonds,respectively [68].The presence of a peak at 399.2 eV,which was attributed to C–N,also highlighted the nucleation and growth behavior of the organic layer,which manifested as a branch-like architecture.The structural details of the resulting Aph@LDH-MgO material were examined using TEM,as shown in Fig.5.From HR-TEM observations,micropetal frameworks are seen to be indicative of the floral-shaped layer of the LDH film covered by the organic layer,which agrees with the obtained SEM images,showing a magnified and clear view of the conformational features.The observed lattice presented in Fig.5c has spacings of 0.33 and 0.12 nm,which correspond to the (009) plane of the LDH network and the agglomerated organic chains on the lamellar surface,respectively.In addition,the interlayer distance in the agglomerated Aph molecules in the short-range well-ordered planar nanostructure is 0.62 nm,which constitutes a constructive unit of corrective self-assembly of the LDH morphology micro defects as illustrated in Fig.5d.These findings strongly highlight the forte-interaction between the different constituents of the duplex structured film,confirming the successful fabrication of the new Aph@LDH-MgO material.
3.2.Electronic characteristics and the interfacial effect of Aph on MgAl-LDH
According to FT-IR analysis,the functional groups (OH and NH2) of Aph were observed on the surface of MgOLDH,suggesting that the binding strength can be easily improved by classical electrostatic interactions.Various quantitative analyses of the molecular surfaces were combined to ensure the credibility of the results.Localized orbit locators based onπorbitals (LOL-π)) can vividly reflect the conjugate properties and preferential electron delocalization path.As shown in Fig.6a,the LOL-π-constructed isosurface map is primarily distributed at the O and N atoms,and the benzene ring of the Aph molecule as well,implying the strong delocalization characteristic ofπelectrons.It is remarkable that the red or orange regions in the LOL-πplane colorfilled map (Fig.6d) are related to the favorable delocalization path.Additionally,theπelectron density isosurface of the Aph molecule indicates that the same regions are rich in electrons,as shown in Fig.6b.Likewise,in Fig.6e,theπelectron density clearly shows the above areas as a colorfilled map.A novel real-space function named the interaction region indicator (IRI) was used to graphically reveal the interaction regions in a chemical system.Fig.6c and f present the isosurface map and plane color-filled map of IRI-π(variant of IRI) of the Aph molecule,in which the region and type of the interaction can be easily identified by different colors.IRI-πanalysis not only differentiates the type ofπinteractions but also exhibitsπ-interaction strength [69].The carbon-oxygen bond,carbon-nitrogen bond,and benzene ring in the Aph molecule exhibit apparentπ-conjugation characteristics.IRI-πnicely reveals the intramolecular and intermolecular interactions.In Fig.6f,the orange and green areas (IRI<1.0) indicate regions where notable chemical bond interactions and weak interactions occur,and the partial steric effect and attractive dispersion effect can be clearly visualized.Consequently,the highπ-electron density and delocalization characteristics of the functional groups and benzene ring in the Aph molecule allowed it to induce electrostatic interactions with the LDH andπ-πinteractions among the Aph molecules in the Aph@LDH-MgO composite.Moreover,the orbitalweighted dual descriptor (OWDD) was evaluated from Fukui functions;this has been established as an accurate method to predict the reactive sites for electrophilic and nucleophilic reactions.The OWDD can simultaneously reveal both types of reactions with (quasi-) degenerate frontier molecular orbitals.The region with a prominently negative (positive)Δfwcorresponds to the site with notable nucleophilicity (electrophilicity).The benzene ring,hydroxyl,and amino groups exhibit a high degree of electrophilicity,and the benzene ring of the Aph molecule also exhibits some nucleophilicity (Fig.6g).These results indicate that the high chemical reactivity of the Aph molecule facilitates charge transfer and overlap between the reactive sites and LDH-MgO layer.This is confirmed by the energy positions of the HOMO and LUMO orbitals,and the contribution of different fragments of the Aph molecule to the total and partial density of states (T/PDOS),as shown in Fig.6h.Additionally,Fig.6i shows that the occupied frontier molecular orbitals are more contributed by p orbitals,indicating that the conjugate characteristics of the Aph molecule dominate its electron charge transfer behavior.
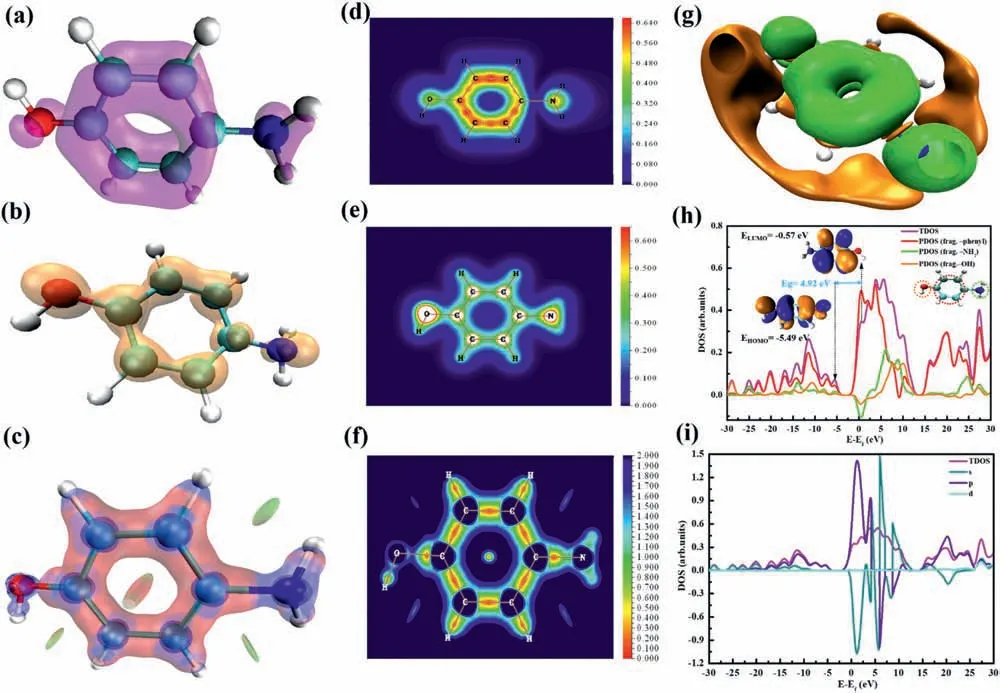
Fig.6.(a) LOL isosurface of π electrons and (d) color-filled map (XY plane,Z=1 ) of Aph molecule with isovalue of 0.29.(b) π electron density isosurface and (e) color-filled map of Aph molecule with isovalue of 0.02.(c) IRI-π isosurface and (f) color-filled map (XY plane,Z=0 ) of Aph molecule with isovalue of 2.37.(g) Orbital-weighted dual descriptor isosurfaces (isovalue=± 0.0003) of Aph molecule.Green/orange (positive/negative parts) represent the electron acceptor and electron donor regions,respectively.(h) Energetic position of molecular orbitals and the contribution of different fragments (PDOS)of Aph molecule on the TDOS.(i) PDOS for s,p,d atomic orbitals of Aph molcule.
To gain deeper insights into the electrostatic potential(ESP) distribution,the ESP statistical distribution on the entire molecular surface of Aph was examined.Fig.7a shows a large molecular surface area with a small ESP,which ranges from -36 to 36 kcal/mol.In this area,the negative part is primarily due to the contribution of the benzene ring and lonepair electrons to the ESP (effect of the abundantπ-electron clouds),whereas the positive part mainly originates from the positively charged C–H hydrogens.Nevertheless,as seen in the inset,this area occupies 51.6% of the total area.The blue area with smaller ESP values denotes the occupation of N and O atoms.ESP colored van der Waals (vdW) surface along with the surface extrema was further examined(Fig.7b).Blue,white,and red correspond to the ESP distribution from -34 to 55 kcal/mol,while the yellow and cyan spheres correspond to the ESP maxima and minima on the vdW surface.The blue area with smaller ESP values corresponds to the occupation of N and O atoms.Furthermore,the average local ionization energy (ALIE) was evaluated,and the relevant preferred electrophilic reaction sites were confirmed (Fig.7c).In the ALIE map of the molecular surface,the blue area is relatively small,which reflects electrophilic attack,while the cyan spheres denote the surface minimum of the ALIE.The figure shows that the benzene ring and oxygen and nitrogen atoms occupy more blue areas,indicating that oxygen and nitrogen atoms can easily form hydrogen bonds with the hydroxyl groups of LDH-MgO layers.Fig.7d shows a shaded surface map displaying the projection effect of the electron localization function (ELF) to further reveal the different features of the Aph molecule.The benzene ring and lone-pair regions show high degrees of electron localization in the Aph structure,which is consistent with the results of the LOL-πand IRI-πfunctions.
An investigation of the adsorption configuration of the Aph molecule on the MgAl-LDH layers is beneficial to acquire deeper insights into the interfacial mechanism and bonding behavior.DFT-based first-principles calculations were carried out,and the optimal adsorption configurations are shown in Fig.8.The Aph molecule is located on the LDH surface,and the most stable configuration corresponds to the interaction of the Aph molecule in a nearly parallel pattern(Fig.8a).The adsorption of the Aph molecule generates many hydrogen bonds with the hydroxyl groups of the LDH surface,particularly with the oxygen and nitrogen atoms.The O—H and N—H bond distances are 1.01 and 1.12,respectively,indicating that the hydrogen bond is strong.Moreover,the adsorption energy (Eads) was evaluated,where a low Eadsindicates that the interaction between the Aph molecule and LDH surface is energetically more robust and the structure of the system is more stable [70,71].Simultaneously,the superstructure models for the 3Aph and 6Aph molecules were investigated to evaluate the effect of Aph-Aph interactions on the stability of the system (in terms of Eads).As expected,the adsorption of the 6Aph molecules (Fig.8c) on the LDH layers is more stable than that of the 3Aph molecules (Fig.8b).The interaction between the Aph molecules makes the largest contribution to the adsorption energy,and therefore,to the stability of the system.Consequently,the robust interaction between Aph molecules and the charge transfer between the adsorbed Aph molecules and LDH layers plays a crucial role in the assembly process for the formation of the Aph@LDH composite.The electron density difference (EDD) of a single Aph molecule on the surface of the LDH was analyzed (Fig.8d).The results show a charge distribution at the interface of the Aph@LDH composite,i.e.,electron-accumulated (green isosurface) and electron-deficient (blue isosurface) regions are present for the hydrogen bonds formed with the hydroxyl groups of the LDH.Thus,charge transfer occurs between reactive sites and the LDH surface.The density of states (DOS)of the LDH and Aph@LDH systems was also analyzed,and the results are shown in Fig.8e,f.The Fermi energy level was set to 0 eV (dashed line).The easiest transferable electronic structure originates from states close to the Fermi level.Fig.8e shows that the p orbital from the LDH layers contributes to the total DOS.Interestingly,the Aph@LDH system (Fig.8f) shows a continuous and increased DOS around the Fermi region,compared to the pure LDH.These results confirm that the Aph molecule possess good electron transfer ability,and consequently,the Aph@LDH hybrid material presents excellent corrosion resistance,which is consistent with the electrochemical results.

Fig.8.The final optimized geometries indicating the most stable hydrogen-bonded arrangements of aminophenol (Aph) molecules and their corresponding adsorption energies as calculated by the DFT.(a) 1 Aph molecule,(b) super-structure for three molecules,(c) super-structure for six molecules,along with the distances between the H atom of OH in the layers and heteroatoms in Aph.(d) The electron density difference distribution for the adsorption of Aph from the side view.The calculated PDOS of (e) pristine LDH and (f) Aph@LDH composite using first principles DFT.
3.3.Electrochemical Properties
For application in a corrosive environment,nature-inspired architectures using the LDH and organic layers should not only offer good anti-corrosion performance but also possess a strong and especially,durable anti-corrosion capability [72].In this work,the anti-corrosion potential of the surface coatings was evaluated through EIS measurements with a tripleelectrode system;the working electrodes were immersed in a 3.5 wt% NaCl solution for the MgO and Aph@LDH-MgO films.Fig.9 shows the electrochemical results for the different fabricated coatings exposed to the aggressive solution for various immersion times (30 min-21 days).The EIS data in Fig.9 show the Nyquist and Bode plots of MgO (Fig.9a,c,e),and Aph@LDH-MgO (Fig.9b,d,f),and how the capacitance and the protective film evolve with time when each layer is fabricated.In the Nyquist plots for the pure PEO coating,the impedance arc radius considerably decreases with increasing immersion time,indicating a deterioration in the anticorrosion capability of the coating over time [73–75].This is,as expected,because of the permeability of the porous layer of the coating to the corrosive solution.Based on Fig.9a,the anticorrosive properties of the MgO material are considerably improved after fabrication with a duplex structure resulting from the layered coating structure in combination with the self-assembled layer [76–78].This improvement can be inferred in terms of the largest capacitance loop and impedance amplitude.Thus,the highest Rcvalues of the Aph@LDHMgO specimen strongly support the excellent performance of the final material.To enable a more quantitative analysis of the electrochemical properties and specific structural features of the coatings,the EIS spectrum is interpreted using the proposed equivalent circuit (EC) models,as shown in Fig.S6.In these circuit models,Rs is the electrolyte resistance;Ro and CPEo are the resistance and constant phase element of the outer layer;Ri and CPEi represent the resistance and constant phase element of the inner compact layer.Additionally,the impedance response of the PEO coating was characterized by capacitive properties (CPECP),which appear at intermediate frequencies,and L describes the corrosion behavior at low frequencies during the dissolution of the AZ31 Mg alloy.Based on the fitted results,it is of importance to note that a significant difference in Ri and Ro values was found between the PEO treated samples without and with additional post-treatment.The results indicate that the values of Ri and Ro exhibited significant improvement after the posttreatment of LDH and DCC for different immersion periods.For example,the values of Ri and Ro of Aph@LDH-MgO composite coating for 30 min and after 21 days of immersion are (Ri(30min)=1.0 × 105Ωcm2),(Ro(30min)=7.2 × 103Ωcm2),(Ri(21day)=495.4Ωcm2),and (Ro(21day)=962.9Ωcm2),which are higher than that in the samples treated only by PEO;(Ri(30min)=1.9 × 103Ωcm2),(Ro(30min)=16.83Ωcm2),(Ri(21day)=116.2Ωcm2),and (Ro(21day)=439.1Ωcm2).The present result values can be assigned to the interfacial effect and high ion-exchange ability of LDH interlayer with the presence of Aph compound which can be intercalated and assembled through different inter-molecular interactions,hence,increasing the barrier effect of the inner layer.Interestingly,the value of Ro exhibited significant improvement after the post-treatment by in situ treatment to form Aph@LDH composite even after long-term immersion as compared to that in PEO coating,demonstrating that the deposition of Aph molecules on the surface of an LDH film,which has a branch-like structure,provided significant enhancement in the protective properties of the PEO coating.To further validate the outstanding corrosion protection offered by the Aph@LDH-MgO hybrid material in its prepared state,we compiled and compared a range of hybrid organic and inorganic components that were fabricated on Mg alloy.The purpose was to compare the present results with the previously reported electrochemical properties of each material under specific conditions,as summarized in Table 1 [79–89].

Table 1The comparison of the electrochemical performance of hybrid organic-inorganic coating-based Mg alloy comprising various hybrid components reported in the literature.
By comparing the impedance responses observed during various electrochemical processes,it becomes evident that the Ap@LDH-MgO hybrid material,synthesized using the proposed strategy involving PEO and subsequent in situ treatment with EDTA to generate LDH,showcases significantly enhanced electrochemical stability.This method outperforms other approaches examined in terms of maintaining the material’s stability under long-term conditions.The current approach,which involves employing the hydrothermal method as a post-treatment and incorporating Aph as an electron donor,demonstrates exceptional efficacy in creating a protective coating for Mg-based alloys.This remarkable outcome can be attributed to the synergistic effects resulting from the combination of LDH layers and the network structure of Aph molecules.The presence of a branch-like organic layer contributes significantly to enhancing the chemical reliability of the coating through a variety of intermolecular and intramolecular interactions.This can also be attributed to the capture capacity of the LDH chloride ions and,simultaneously,to the role played by the organic layer in correcting the structural defects of the LDH morphology.This process is explained in Fig.10,which shows the mechanism of protection of the designed coatings in two different pathways.The first pathway illustrates the role played by the selfassembled Aph molecules,which constitute the first physical barrier against the corrosive ions on the surface of the LDH in a branch-like architecture.Although this nature-mimicking structure corrects the inevitable defects of the lamellar structure,the anticorrosive performance of the LDH,especially in the long term,remains to be clarified.In the second pathway,the lamellar structure plays a dual role:first,as a barrier,it can prevent water,oxygen,and chloride ions from penetrating the MgO coating,and acts as a physical blocker for the material to prevent external substances from coming into direct contact with the metal support;second,the spaces between the lamellae absorb Cl-ions by virtue of the ion exchangeability of the LDH phases between corrosive species and nitrate anions,which explains the improved anticorrosion properties.Thus,the triplex structure is influenced by a combination of PEO,the barrier effect of the LDH,the exchange of chloride ions,and the branch-like self-assembly of Aph,resulting in a smart combined system of organic-inorganic hybrid layers that enhance the protective effect of the coating in extreme environments for an exceptionally long period.

Fig.10.Proposed mechanism for the anticorrosion performance of the Aph@LDH-MgO hybrid composite.
4.Conclusions
In the end,we have reported the fabrication of Aph@LDHMgO materials with “floret”-“branch”-type nature-mimicking architectures possessing favorable anticorrosion properties.These materials serve as powerful functionalized coatings for Mg alloys.The LDH coating was successfully prepared using an EDTA chelating agent on the PEO surface under relatively moderate conditions.Thus,the layered coating materials could be fully utilized,without limiting conditions,and are promising for application in nanotechnology to improve functional materials and broaden their application domain.Moreover,by virtue of the anion-exchange potential of the lamellar LDH phases as well as the physical barrier effect of the layered architecture,the combination of the LDH structure and organic self-assembly features would reduce the risk of corrosion and enhancing the durability and performance of the Mg alloy.Consequently,the as-developed Aph@LDH-MgO material serves as a powerful barrier against Cl-ion shuttling,leading to the formation of a robust and durable highperformance system in aggressive environments.The new approach suggested in this paper can help fabricate novel materials with improved anti-corrosion performance under suitable experimental conditions to meet industrial requirements.It also provides novel opportunities for researchers to further develop various anti-corrosion systems by combining LDH structures with organic self-assembled frameworks as new material platforms for effective corrosion protection.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
CRediT authorship contribution statement
Maryam Chafiq:Conceptualization,Methodology,Investigation,Writing– original draft.Abdelkarim Chaouiki:Conceptualization,Investigation,Formal analysis,Writing–original draft,Writing– review &editing.Rachid Salghi:Visualization.Young Gun Ko:Supervision,Funding acquisition,Writing– review &editing.
Acknowledgements
This work was supported by the Fundamental-Core National Project of the National Research Foundation (NRF)funded by the Ministry of Science and ICT,Republic of Korea (2022R1F1A1072739).
Supplementary materials
Supplementary material associated with this article can be found,in the online version,at doi:10.1016/j.jma.2023.06.002.
 Journal of Magnesium and Alloys2023年7期
Journal of Magnesium and Alloys2023年7期
- Journal of Magnesium and Alloys的其它文章
- Recent progress in MgB2 superconducting joint technology
- “Smart” micro/nano container-based self-healing coatings on magnesium alloys: A review
- Recent advances using equal-channel angular pressing to improve the properties of biodegradable Mg–Zn alloys
- Twin evolution in cast Mg-Gd-Y alloys and its dependence on aging heat treatment
- Effects of Ce content on the modification of Mg2Si phase in Mg-5Al-2Si alloy
- Solute drag-controlled grain growth in magnesium investigated by quasi in-situ orientation mapping and level-set simulations
