Effect of wire-arc directed energy deposition on the microstructural formation and age-hardening response of the Mg-9Al-1Zn (AZ91) alloy
Glori Grf ,Petr Spoerk-Erdely,1,∗ ,Emd Mwd ,Mihel Burtsher ,Dniel Kiener ,Helmut Clemens ,Thoms Klein
aDepartment of Materials Science,Montanuniversität Leoben,Franz Josef-Straße 18,8700 Leoben,Austria
b Institute of Materials Physics,Helmholtz-Zentrum Hereon,Max-Planck-Straße 1,21502 Geesthacht,Germany
c LKR Light Metals Technologies Ranshofen,Austrian Institute of Technology,Lamprechtshausener Straße 61,5282 Ranshofen,Austria
Abstract In recent years,wire-arc directed energy deposition(waDED),which is also commonly known as wire-arc additive manufacturing(WAAM),has emerged as a promising new fabrication technique for magnesium alloys.The major reason for this is the possibility of producing parts with a complex geometry as well as a fine-grained microstructure.While the process has been shown to be applicable for Mg-Al-Zn alloys,there is still a lack of knowledge in terms of the influence of the WAAM process on the age-hardening response.Consequently,this study deals with the aging response of a WAAM AZ91 alloy.In order to fully understand the mechanisms during aging,first,the as-built condition was analyzed by means of high-energy X-ray diffraction (HEXRD) and scanning electron microscopy.These investigations revealed a finegrained,equiaxed microstructure with adjacent areas of alternating Al content.Subsequently,the difference between single-and double-step aging as well as conventional and direct aging was studied on the as-built WAAM AZ91 alloy for the first time.The aging response during the various heat treatments was monitored via in situ HEXRD experiments.Corroborating electron microscopy and hardness studies were conducted.The results showed that the application of a double-step aging heat treatment at 325 °C with pre-aging at 250 °C slightly improves the mechanical properties when compared to the single-step heat treatment at 325 °C.However,the hardness decreases considerably after the pre-aging step.Thus,aging at lower temperatures is preferable within the investigated temperature range of 250-325 °C.Moreover,no significant difference between the conventionally aged and directly aged samples was found.Lastly,the specimens showed enhanced precipitation kinetics during aging as compared to cast samples.This could be attributed to a higher amount of nucleation sites and the particular temperature profile of the solution heat treatment.
Keywords: Wire-arc directed energy deposition;Additive manufacturing;High-energy X-ray diffraction;Synchrotron;Mg-Al-Zn alloys;Age-hardening.
1.Introduction
As the lightest structural metallic materials with high specific strengths,Mg alloys demonstrate a great weight-saving potential for automotive and aircraft applications [1].Enhancing the percentage of Mg alloys used in the automotive and aircraft industry would thus help to reduce fuel consumption and the concomitant environmentally detrimental emissions.As the strength of pure Mg is poor,alloying elements are added.In the most widely used system,i.e.the Mg-Al-Zn system,Al is alloyed to enhance the strength via solid solution hardening,whereas additions of Zn,e.g.in the age-hardening AZ91 alloy(Mg-9Al-1Zn in m.%),reduce the solubility range and thus increase the amount ofβ-Mg17Al12precipitates [2].In general,Mg alloys can easily be processed,e.g.via casting,welding and machining [1,3].Their main disadvantage,however,is their poor formability at room temperature,owing to the hexagonal crystal structure [1,3].Consequently,the majority of Mg products in the automotive industry is fabricated by casting,even though wrought Mg alloys exhibit higher strengths [1].
A promising new approach to processing is additive manufacturing (AM).With this method,higher strengths can be achieved than via casting.In fact,the obtained strength level becomes even comparable to that of wrought material [4,5].Since additionally complex geometries with minimal waste of material can be produced and there is a possibility to automate the fabrication technique,research and industry have shown great interest in this approach recently [6,7].
AM can be classified based on the type of supplied material,i.e.into powder-based or wire-based methods [6,7].One common powder-based method is laser powder bed fusion(LPBF).Wei et al.[4] demonstrated that dense AZ91 samples with a fine-grained microstructure can be generated with this method.While a higher complexity in geometry[6]as well as slightly higher strengths [4,8] can be achieved with LBPF in contrast to wire-based methods,there are certain challenges when working with Mg powder.First,the combination of the low optical absorptivity and high thermal conductivity of Mg alloys [9] leads to a significantly different behavior of the already molten material and the powder [10].Phenomena such as balling and overheating of the melt can be the consequences [10].Furthermore,Mg has a low boiling point[9],which results in a reduction of the relative density due to evaporation [11].This can,however,be overcome with an increased atmosphere pressure inside the chamber[12].Moreover,Mg powder is highly reactive,i.e.it easily oxidizes and is even flammable and explosive in air.Hence,it imposes certain safety issues.
As a consequence,wire-based methods seem to be the more promising manufacturing technique at the moment,especially since the higher deposition rates are more suitable for industrial processes [6].To date many investigations on wirearc directed energy deposition (waDED),which is also commonly known as wire-arc additive manufacturing (WAAM),have shown that the process is viable to produce dense parts with regard to the Mg-Al alloy series [5,8,13–23].As mentioned above,the tensile strengths of LPBF samples are slightly higher than those of WAAM parts [4,8].However,a substantially greater ductility can be realized with WAAM as opposed not only to LPBF but also cast and wrought material [5,8,14,17,19–22].Alongside mechanical properties,another crucial characteristic of Mg alloys,which can determine their implementation,is their corrosion performance [1].For WAAM alloys of the Mg-Al series,this material quality has therefore also been in the focus of recent studies.Han et al.[16] and Fang et al.[14] reported an enhanced corrosion resistance of as-built WAAM AZ91D and AZ31 alloys,respectively,in comparison to the corresponding cast as well as rolled alloys.According to Zhang et al.[23] a solution heat treatment of the as-built AZ91 alloy can further improve the corrosion resistance.This was attributed to the dissolution of second phases and the elimination of non-basal orientations during the solution heat treatment.In contrast,another investigation by Li et al.[18] revealed an increased but stable corrosion behavior of the as-built AZ31 alloy in comparison to the cast material.Due to a finer grain size,intercrystalline corrosion is preferred over micro-galvanic corrosion of the cast alloy [18].
While the microstructure,the mechanical properties and the corrosion resistance of Mg-Al alloys fabricated via WAAM have already been studied,there is still a lack of knowledge in terms of the influence of the WAAM process itself on the age-hardening response.Guo et al.[24] investigated the influence of the T4,T5 and T6 heat treatment on the microstructure,mechanical properties and anisotropy of the AZ80M alloy fabricated by WAAM.This study revealed that both the T5 and T6 treatment lead to an increased strength.However,only the ductility after the T6 treatment improved,while the ductility after the T5 treatment was significantly reduced.Gneiger et al.[5] also found an improvement of strength and ductility after the T6 heat treatment of a WAAM AEX11 alloy.These studies,however,only focused on the final conditions.As the influence of WAAM on the age-hardening response could be different than for conventional fabrication techniques,there is still a knowledge gap concerning the microstructural changes in the course of the aging heat treatment.This shall be dealt with in the current study.
Age-hardening of Mg-Al alloys with a higher Al content of around 6-9 m.% is a convenient way of further enhancing the strength [25–28].This can be achieved with a solution heat treatment and a subsequent aging step,in which the body-centered cubicβ-Mg17Al12phase (space group I3m,a=10.6 ˚A [29]) can be precipitated [30].As opposed to other Mg alloying systems,no precipitation sequence,e.g.including the formation of Guinier-Preston Zones (GPZ) or other metastable phases,is observed in the Mg-Al system and the stableβ-Mg17Al12phase is formed directly [30].Furthermore,the precipitation can occur continuously inside the grain or in a discontinuous fashion at the grain boundaries [31,32].The latter is a cellular reaction,in which coarse lamellar precipitates are formed.In this case,the transformed microstructure consists of alternating near equilibriumα-Mg andβ.By contrast,during continuous precipitation,fine laths are formed inside the grain.Which one of the two precipitation types occurs,depends on the temperature range [31,32].In the case of Mg-Al alloys with Al contents ranging between 3.6-18.8 at.%,at higher annealing temperatures close to the solution temperature only continuous precipitation occurs,while at intermediate annealing temperatures exclusively discontinuous precipitation appears [31,32].In between these temperature ranges,both precipitation types are observed[31,32].Duly et al.[31] further revealed that at higher Al contents a transition from discontinuous to continuous precipitation with decreasing annealing temperatures takes place as well.
As WAAM is a process far from thermodynamic equilibrium,in which each material layer is provided with a complex thermal history,a detailed understanding of the precipitation processes during WAAM and an ensuing age-hardening step is crucial for the optimization of material properties.Consequently,the texture and formation of precipitates in as-built AZ91 samples have been characterized by means of highenergy X-ray diffraction (HEXRD) as well as scanning electron microscopy (SEM).Subsequently,the as-built samples have been exposed to different single-step and double-step aging heat treatments (also referred to as “two-step aging”in literature),which were performed both conventionally and directly.The evolution of precipitates has been recorded in the course of the heat treatments viainsituHEXRD experiments.Additionally,electron microscopy,i.e.SEM and transmission electron microscopy (TEM),has been performed to study the microstructural features of selected heat-treated conditions.Corroborating hardness tests have been conducted as well.Improved precipitation kinetics were found during aging of WAAM material as compared to cast material.The insights gathered in this study significantly contribute to the understanding of the microstructural evolution of magnesium alloys of the AZ alloy family during WAAM processing.This is a prerequisite for the adaption of alloy and technology on a wider scale.
2.Experimental
2.1. Material and processing
The chemical composition of the as-built specimen was determined by means of optical emission spectroscopy using a SPECTROMAXx 6 from SPECTRO Analytical Instruments GmbH and equals Mg-8.57Al-0.58Zn-0.2Mn in m.%.Values given were calculated by averaging eight individual measurements.
The specimen was fabricated using the parameters given in Table 1 with a Cold Metal Transfer welding characteristic.The adjustment of this parameter set was conducted based on a preliminary parameter study similarly as reported in Ref.[33].WAAM was conducted continuously with a constant layer-by-layer z-displacement and no interlayer waiting period was introduced.The specimen with dimensions of 100 mm × 30 mm × 60 mm with a wall thickness of ∼5 mm was manufactured as shown in Fig.1.No evidence of cracks was observed on the specimen surface.
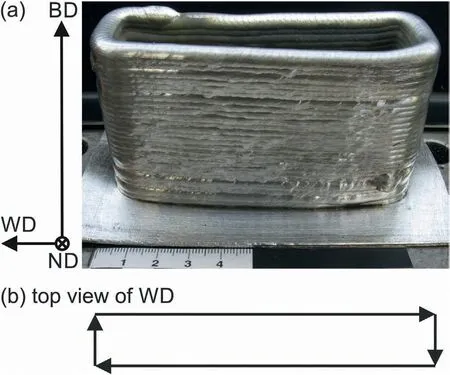
Fig.1.(a) As-built WAAM specimen.The building direction (BD),welding direction (WD) and normal direction (ND) are displayed in the coordinate system to the left,which is valid only for the front of the specimen.The WD and ND change depending on the position in the specimen,as can be seen in the top view in (b).
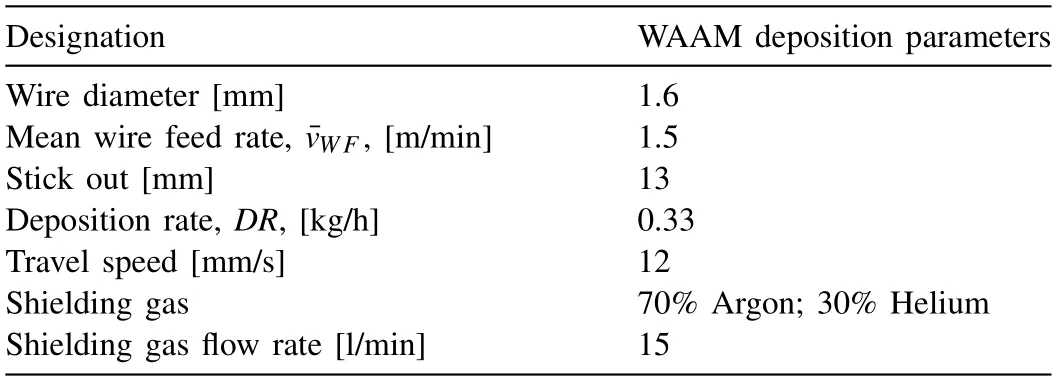
Table 1WAAM deposition parameters used for fabrication of the specimen shown in Fig.1.
2.2. High-energy X-ray diffraction
The HEXRD experiments were performed at beamline P07 and P07b run by the Helmholtz-Zentrum Hereon at PETRA III at the synchrotron facility Deutsches Elektronen-Synchrotron (DESY) in Hamburg,Germany.As the experiments were conducted in transmission geometry,the scattered signal was monitored with a centrically positioned PerkinElmer (PE) XRD 1621 flat panel detector.The detector has a total number of 2048 × 2048 pixels,with a size of 200 × 200 μm2each,and was situated at a distance of 1.3 m from the specimen.Moreover,the setup was calibrated using LaB6.The experiments performed at beamline P07,i.e.the texture measurement and the heat treatments,were probed with a mean photon energy of 73.9 keV and beam dimensions of 1 × 1 mm2.At beamline P07b a mean photon energy of 87.1 keV was utilized.
At beamline P07b an as-built sample was scanned along the building direction,i.e.along its height,to investigate the distribution of precipitates,which were formed during the WAAM process.For this,a step size of 0.1 mm was used and the beam dimensions were 0.1 mm parallel to the building direction and 0.3 mm perpendicular to it.
For the texture measurements at beamline P07,cylindrical as-built samples with a diameter of 5 mm were prepared with the cylinder axis parallel to the building direction.These samples were mounted on a rotary table,so they could be turned around their cylinder axis.During the experiment,they were rotated by a total angle of 180°,while individual frames were detected continuously over an angle range ofΔω=5°each.Azimuthal sections of the detector frames were then integrated using the software Fit2D [34].The pole figures,which are shown as upper hemisphere stereographic projections,were obtained from the orientation distribution functions (ODF) reconstructed with the Matlab MTEX toolbox[35].For the reconstruction the five lattice planesand (0002) ofα-Mg were used.
In order to study the age-hardening response after the WAAM process,aging experiments were conducted at beamline P07.For this,samples with a cylindrical cross-section and dimensions of 10 mm in length and 5 mm in diameter,were prepared from the as-built condition.Their cylinder axis was parallel to the welding direction and they were taken from the middle of the as-built specimen.A schematic representation of these aging heat treatments can be seen in Fig.2 and their respective aging temperatures,holding times and denotations are listed in Table 2.The aging heat treatments were conducted in two different ways– conventionally and directly.During conventional aging (CA),the specimens were quenched to room temperature after the solution heat treatment,followed by 9 weeks of natural aging with ensuing artificial aging,whereas during direct aging (DA),the specimens were quenched directly to the annealing temperature subsequent to the solution heat treatment.The solution heat treatment consisted of holding for 1 h at 410 °C.Subsequently,single-step annealing heat treatments at 250°C for 10 h and at 325 °C for 2.5 h were applied.Furthermore,doublestep heat treatments after the solution heat treatment were performed,in which the specimens were first held at 250 °C for 3 h and then directly heated to 325 °C at a rate of 2 K/s and held for 2.5 h.The heat treatments were conducted in a modified quenching dilatometer DIL 805A/D from TA Instruments under Ar atmosphere.A type S thermocouple was spot welded onto the specimens to trace the evolution of the temperature.In order to obtain diffraction patterns of good grain statistics,the detector frames were azimuthally integrated over an angle range of 360° with the software Fit2D [34].As the Al content in solution can be estimated with the lattice parameters ofα-Mg,Rietveld analysis was performed using the software Maud [36,37].Additionally,the phase fraction ofβwas evaluated.The refinement was performed with the batch mode of Maud and the value of the weighted profile R-factor Rwpdid not exceed 13.5.

Fig.2.Temperature profiles of the different heat treatments.Conventionally aged samples were quenched to room temperature after the solution heat treatment(sht) and subsequently naturally aged (n.a.) for 9 weeks before they were artificially aged.The solution heat treatment was conducted at 410 °C for 1 h.
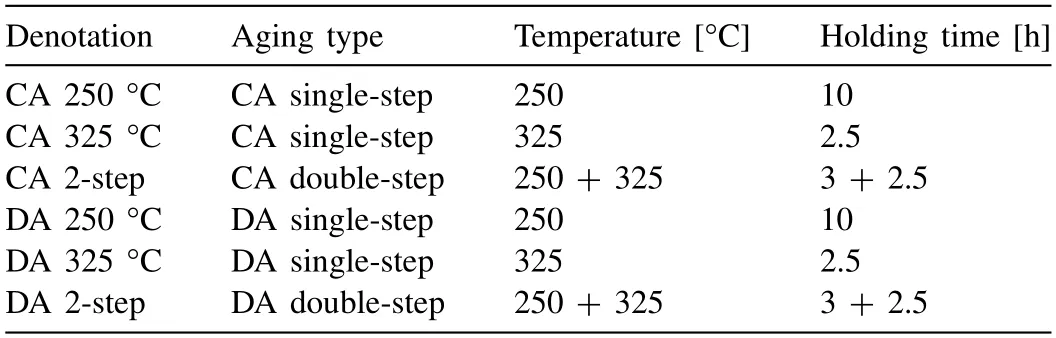
Table 2The denotations,aging temperatures and holding times for the different heat treatments.The single-and double-step heat treatments were performed either conventionally (CA) or directly (DA) (see text).Prior to each aging heat treatment,a solution heat treatment at 410 °C for 1 h was conducted.
2.3. Microstructural investigation
The microstructure of selected heat treatment conditions was investigated by means of SEM and TEM.Cylindrical asbuilt samples with a diameter of 5 mm were first heat-treated with the aid of a quenching dilatometer DIL 805 A from TA Instruments.As quenching gas N2was used and a type S thermocouple controlled the temperature.After the heat treatment,the specimens were ground with SiC-paper of grit sizes 500-1200 and polished with a diamond suspension of 3 μm and 1 μm grain size,respectively.Lastly,chemo-mechanical polishing with colloidal silica was applied.Most samples were examined in the unetched condition using a Clara Tescan scanning electron microscope in secondary electron (SE) mode and backscattered electron (BSE) mode at 10-15 kV.In order to reveal the grain boundaries and the welded layers,one sample in the as-built condition was etched for 10 s with the picric acid solution CRIDA– QT plus from CRIDA Chemie.The grain morphology of this sample was revealed with light optical microscopy using an Axio Imager.M1m from Zeiss.In order to investigate the distribution of the second phases within the layers,SEM in BSE mode was performed as stated above.
Furthermore,for the TEM investigation specimens with a thickness of 0.3 mm were cut from the heat-treated samples and ground to a thickness of 0.1 mm.Afterward,they were electrolytically prepared with the aid of a TenuPol-5 from Struers.The process was conducted at-20°C and the A3 electrolyte from Struers was used.After the preparation,the specimens were stored in isopropanol to avoid oxidation during the transport to the TEM.The microstructure was then investigated utilizing a JEM-2200FS from Jeol,which is equipped with a field emission gun (FEG) operated at 200 kV and is corrected by an in-column energy filter (Omega filter).To analyze the chemical composition of the nm-scaled dispersoids,an area scan was performed by means of electron dispersive X-ray spectroscopy (EDS) in scanning TEM mode using a spot size of 1 nm as well as an exposure time of 20 min.For this,an Oxford Ultim Max EDS detector running on the software AZtekLive was operated.
2.4. Hardness
The Vicker’s hardness of selected heat-treated conditions was determined with a Qness 60 A/A+EVO.The specimens were tested in welding direction.For each condition,the average value of three indents at random positions at a load of 10 kg was evaluated.
3.Results and discussion
3.1. As-built condition
Fig.3(a) shows a representative light optical micrograph of the as-built AZ91 alloy in welding direction.In order to reveal the grain boundaries,the sample has been etched.It can be seen that the entire sample consists of fine equiaxedα-Mg grains,which were observed not only in welding direction,but also in normal direction (not depicted).
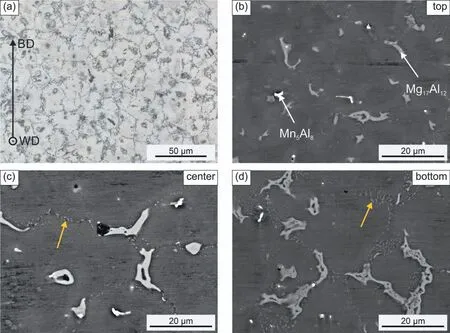
Fig.3.Micrographs of the as-built WAAM AZ91 sample: (a) light optical micrograph of an etched sample revealing the fine equiaxed grains.The coordinate system shows the position of the microstructure with respect to the specimen building direction (BD) and welding direction (WD);(b-d) SEM micrographs of unetched samples at a higher magnification at the top (b),at the center (c) and at the bottom of the specimen (d).The orange arrows mark positions of discontinuous precipitation.The images were taken in BSE mode.
Unetched samples have been investigated using SEM to disclose the nature of second phases (see Fig.3(b-d)).Small shrinkage cavities (black areas) can sporadically be observed in the microstructure.Additionally,Al-rich and Mn-,Al-rich segregations are located at the grain boundaries,which can be assigned to theβ-Mg17Al12phase and the Mn5Al8phase,respectively,as the HEXRD investigation revealed.It should be noted that the latter phase is known to undergo several transformations upon cooling to room temperature under equilibrium conditions [5,38].However,during additive manufacturing,the cooling rates are too high to promote the transformation of the Mn5Al8phase [38].Thus,no additional MnxAlyphases were detected employing HEXRD.This is also consistent with recent findings by Zhang et al.[23],who reported the presence ofβ-Mg17Al12and Mn5Al8in the as-built sample of a WAAM AZ91 alloy.Concerning the formation of precipitates along the grain boundaries,significant differences can be seen at different positions within the specimen along the building direction.In the top layer of the specimen,which is displayed in Fig.3(b),apart from the coarse segregations already discussed,the grain boundaries are almost free of second phases.Only a few fine precipitates are visible.In contrast to this,the grain boundaries in the center of the specimen are already decorated with sub-micrometer scaledβ-Mg17Al12phase,as illustrated in Fig.3(c).In Fig.3(d),at the specimen bottom,areas of discontinuous precipitation are clearly visible along the grain boundary seam and their boundaries started to migrate to the center of the grain.This can be explained as follows: Due to the high cooling rates during the WAAM process,the matrix is supersaturated in Al.Discontinuous precipitation is then initiated owing to the heat input during the deposition of subsequent layers and the elevated temperatures present during the manufacturing process [39].Consequently,discontinuous precipitation is already further advanced at the bottom of the specimen,owing to repeated and longer heat exposure.Guo et al.[15] reported on similar features of the as-built microstructure of an AZ80 alloy after the WAAM process.
In Fig.4,the results of a vertical HEXRD scan from the top of the as-built specimen towards the bottom are shown.As can be seen in Fig.4(a),the volume fraction ofβis alternating.The peak-to-peak distances approximately equal the respective layer heights,i.e.the alternations can be explained by the layer-by-layer build-up of the specimen.Furthermore,the difference in theβvolume fraction between the minimum and the maximum of the investigated area equals 3.4 vol.%.By correlating the layer height to the volume fraction ofβ,it can be found that the fraction ofβis enhanced close to the fusion line of the layers.
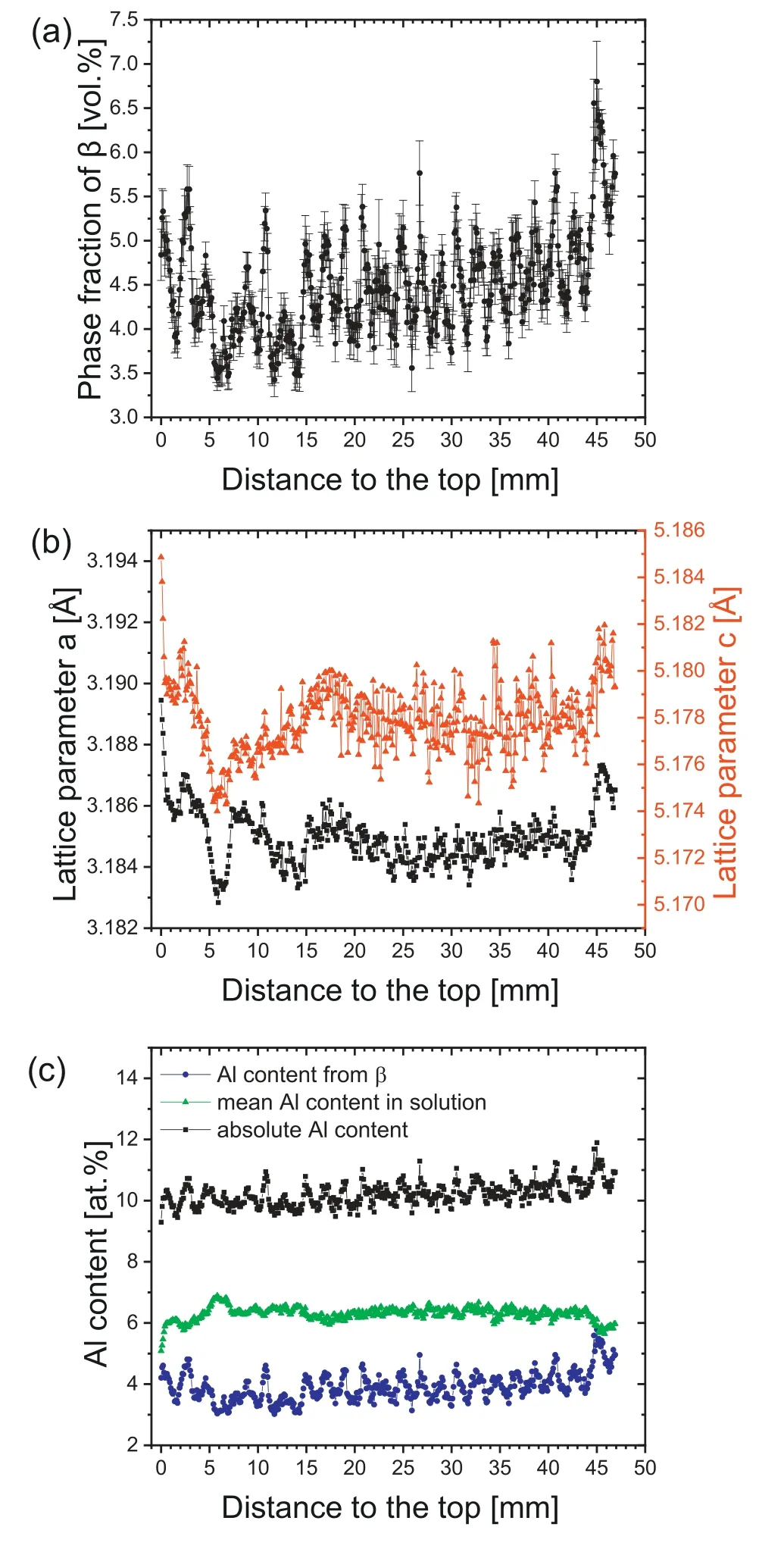
Fig.4.(a) Variation of the phase fraction of β along the building direction of the as-built sample;(b) variation of the lattice parameters a (squares) and c (triangles) of α-Mg with increasing distance to the top of the specimen;(c) mean Al content in solution estimated based on the lattice parameters of α-Mg and the contribution of Al from β-Mg17Al12,when the stoichiometric composition is assumed.Furthermore,the absolute Al content calculated from both contributions is illustrated as well (see text).
This circumstance has also been confirmed with SEM,as illustrated in Fig.5.The image shows an area spanning from the center of a layer to the center of the subsequent layer.Within each of the layers the amount ofβis lower,while in the interlayer region,i.e.the region close to the fusion line,the amount ofβis slightly enhanced.This area is bordered with the dashed lines in Fig.5.The HEXRD investigation suggests a variation inβof only 1-2 vol.% between the layer and interlayer regions.Since these differences are only small they might be harder to distinguish in Fig.5,however,they can be anticipated and confirm the results of the HEXRD study.
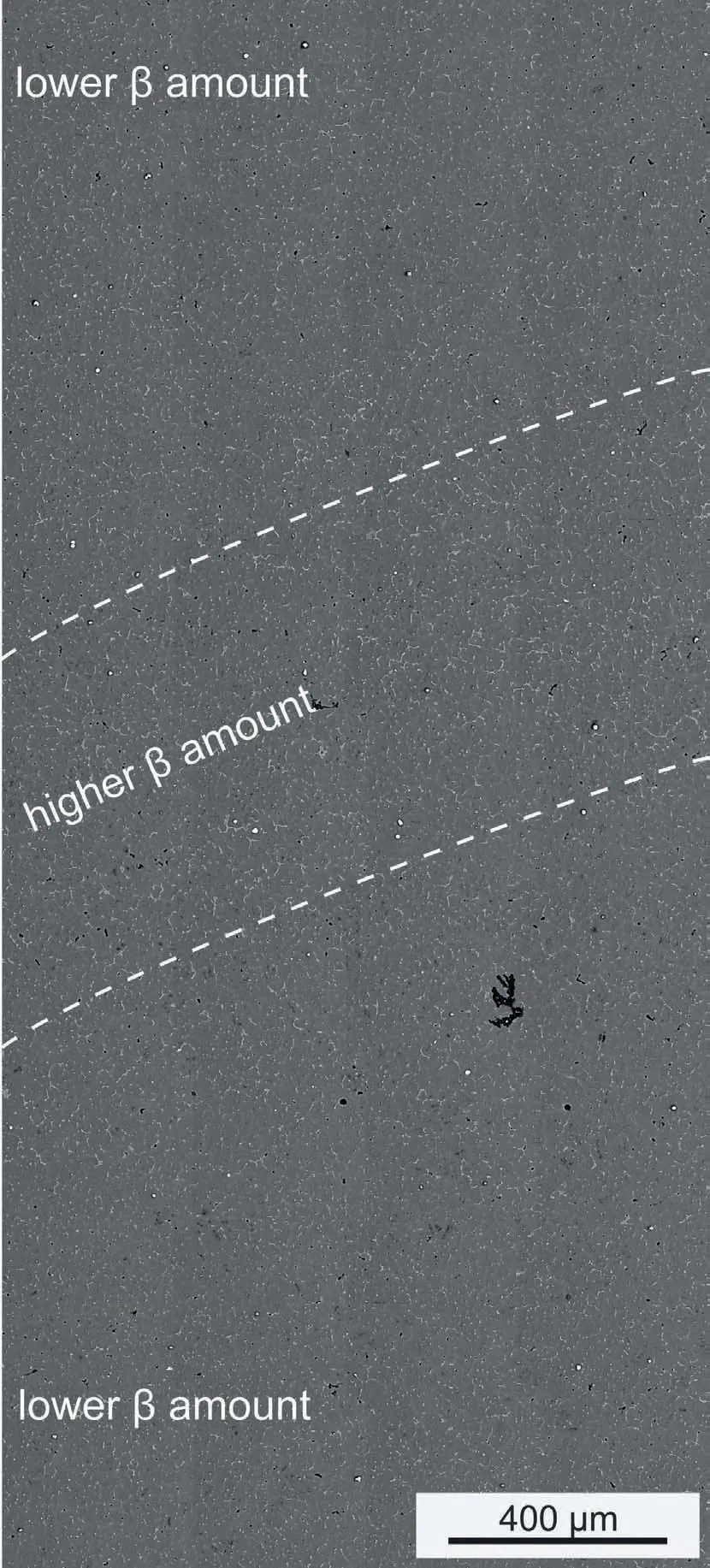
Fig.5.SEM BSE micrograph taken at the center of the as-built sample.The area between the two dashed lines marks an area of enhanced content of β which is located in the vicinity of the fusion line.In order to reveal this enhanced β content,the sample has been etched.The black areas are cavities which originate from etching.
Fig.4(b) presents the lattice parameters ofα-Mg as evaluated by means of Rietveld analysis.According to Duly et al.[40] and Hardie et al.[41] the lattice parameters ofα-Mg increase linearly with decreasing Al content in solution.Hence,the Al content of the solid solution can be estimated from the lattice parameters.By tracing the overall evolution of the lattice parameters in building direction starting from the top of the specimen,they first show a steep decrease.With increasing distance from the top they increase again and finally a value at a roughly constant level is reached,which is enhanced again towards the bottom of the specimen.These observations are also in agreement with the overall evolution of the volume fraction ofβalong the building direction,as shown in Fig.4(a).Here,it can be seen that the amount ofβfirst decreases and then increases again with further distance to the top.From this,it can be deduced that the deposition of subsequent layers leads to the dissolution ofβin the previous layers,as long as the solution temperature is exceeded.Thus,the Al content in solution increases in these layers.Furthermore,towards the bottom of the specimen,at some point only temperatures below the solution temperature are reached.Consequently,βis precipitated again in the form of discontinuous precipitates as also shown in the SEM investigation above.
In order to determine the absolute Al level as a function of the distance from the top of the specimen,the Al content in solution as well as the Al content inβwas estimated.For the evaluation of the Al percentage inβ,the stoichiometricβ-Mg17Al12phase was assumed.Moreover,for the estimation of the Al percentage in solution,the variation of the lattice parameters with the Al content in solution for binary Mg-Al alloys was used [40,41].Since the volume fraction of Mn5Al8does not exceed 0.2 %,its contribution can be neglected.The results are displayed in Fig.4(c),in which the mean Al content calculated from both lattice parameters is shown.As the Al content of the alloy determined by optical emission spectroscopy equals 7.8 at.% (see Section 2.1),the absolute Al content is overestimated.The reason for this might be that the stoichiometric composition ofβand the presence of a binary alloy were assumed.Yet,while these assumptions do not fully reflect the present case,especially since other alloying elements are present as well,they still appear sufficient for a first estimation.From Fig.4(c) it can be seen that the Al content fromβis alternating,while the mean Al content in solution is nearly constant over a wide range.It appears that at the cooling rates imposed by the WAAM process a solid solution of around 6.3 at.% Al is adjusted and excess Al is precipitated in the form ofβ.Thus,on the basis of the abovemade assumptions it can be concluded that the higher amount ofβnear the fusion line is caused by an overall higher Al level.One plausible reason is the evaporation of Mg due to its lower boiling point and higher vapor pressure [42].This effect is frequently observed in laser-based methods,where Wei et al.[4],for example,found a higher Al content with increasing energy density in LPBF-produced AZ91 samples.Marya and Edwards [43] reported that the Al content in the fusion zone of AZ91 laser welds increased towards the top,i.e.Mg evaporates at the top of the melt pool.However,Mg loss also occurs in arc-based techniques [44,45].While the Mg distribution was uniform over several layers despite the loss of Mg in WAAM specimens produced by Yuan et al.[44],the Mg content was lower in the top and higher at the bottom of the layers in WAAM specimens fabricated by Zhao et al.[45].The uniform element distribution in the study of Yuan et al.[44] was explained by extensive fluid flow due to the Lorenz force and surface tension before solidification during WAAM.In the current study,Mg is also inhomogeneously distributed inside the layers similar to the investigation by Zhao et al.[45],i.e.a higher Al content is observed close to the fusion line.
Texture analysis was performed on as-built samples by means of HEXRD and the {100},{101} and {0001} pole figures estimated based on the ODF are illustrated in Fig.6.As different measurements along the building direction all show the same trend,the measurement displayed in Fig.6,which was performed in the center of the specimen,is representative for the entire specimen height.No preferential orientation is present in the specimens,i.e.no texture can be identified.This was also found in literature,where a random grain orientation was observed in the as-built WAAM microstructures of the AZ91 alloy [23] and the AZ31 alloy [14,18].The specimens all showed an equiaxed grain structure.
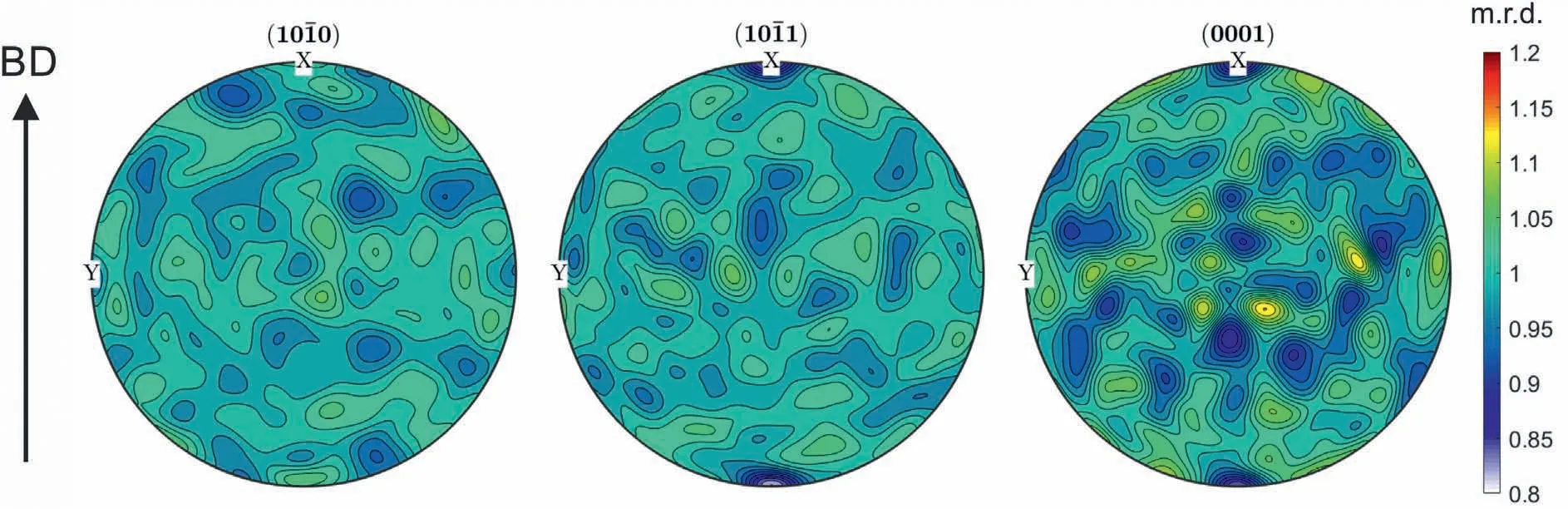
Fig.6.{100},{101} and {0001} pole figures of α-Mg in the as-built condition obtained from the reconstructed ODFs.The specimens were measured with HEXRD and the arrow indicates the direction of the building direction (BD).
In general,a microstructure with randomly oriented equiaxed grains is desired,since components with isotropic mechanical properties can be fabricated.Textured parts with columnar grains,by contrast,exhibit anisotropic properties and are only preferred for specific applications,such as directionally solidified turbine blades[46].Studies have shown that in Mg-Al alloys with a low Al content,e.g.the AZ31 alloy,a columnar and columnar dendritic solidification microstructure in as-built WAAM samples is often observed,which can transform to an equiaxed microstructure close to the top of the layer [19,21].However,completely equiaxed WAAM microstructures were produced as well [13,14,18,20].In Mg-Al alloys with a higher Al content,such as AZ61 [5,17,22] or AZ91 [8,16,23],the majority of studies on WAAM reports a microstructure consisting of equiaxed grains.The exception is an investigation by Guo et al.[15] on a WAAM AZ80 alloy,in which columnar grains were observed near the fusion line,and towards the top of the layer a transition to equiaxed grains was found.The dependence of the microstructural formation on the Al content can be well explained by the interdependence theory by StJohn et al.[47].Mg-Al alloys with a higher Al content exhibit a stronger tendency for the formation of equiaxed grains,since the higher solute content promotes constitutional supercooling.This provokes the formation of equiaxed grains in the area ahead of the solidification front,if potent nucleant particles are present in the melt.An enhancement of the solute content,in order to induce a columnar to equiaxed transition (CET) and grain refinement,is a widely used approach also in other alloying systems,e.g.Ti base alloys [48].Another important factor for the selection of grain morphology is the interplay between the solidification parameters,such as the temperature gradient,the solidification rate and the cooling rate [14,48].Those parameters can be influenced by the processing parameters and the manufacturing process itself [14,48].Hence,it can be concluded that in the current study the fine equiaxed microstructure is favored due to a high solute content and beneficial processing parameters.
3.2. Solution heat treatment
Results of theinsituHEXRD experiment during the solution heat treatment and a subsequent aging heat treatment at 250 °C are visible in Fig.7(a).A remarkable amount ofβis already present in the initial condition,i.e.the as-built condition (see also Section 3.1).In the course of the solution heat treatment,the initial amount ofβis reduced rapidly,but not dissolved completely within one hour.For cast alloys,the holding time is usually at least 24 h [25,27,28,49].However,as the grain size of the as-built condition is one order of magnitude smaller and such a long holding time is not feasible during the beamtime at the synchrotron,a reasonable holding time of 1 h was selected.Yet,aβphase fraction of around 1 vol.% remains after the solution heat treatment in the current study.This implies that the solution heat treatment did not fully homogenize the as-built WAAM microstructure.Nonetheless,the effect might be reduced with a longer holding time,which was,however,not subject of the current investigation.Moreover,as a result of theβdissolution,the Al amount in theα-Mg matrix increases,leading to a continuous decline in the lattice parameters of the matrix during the solution heat treatment [40,41].
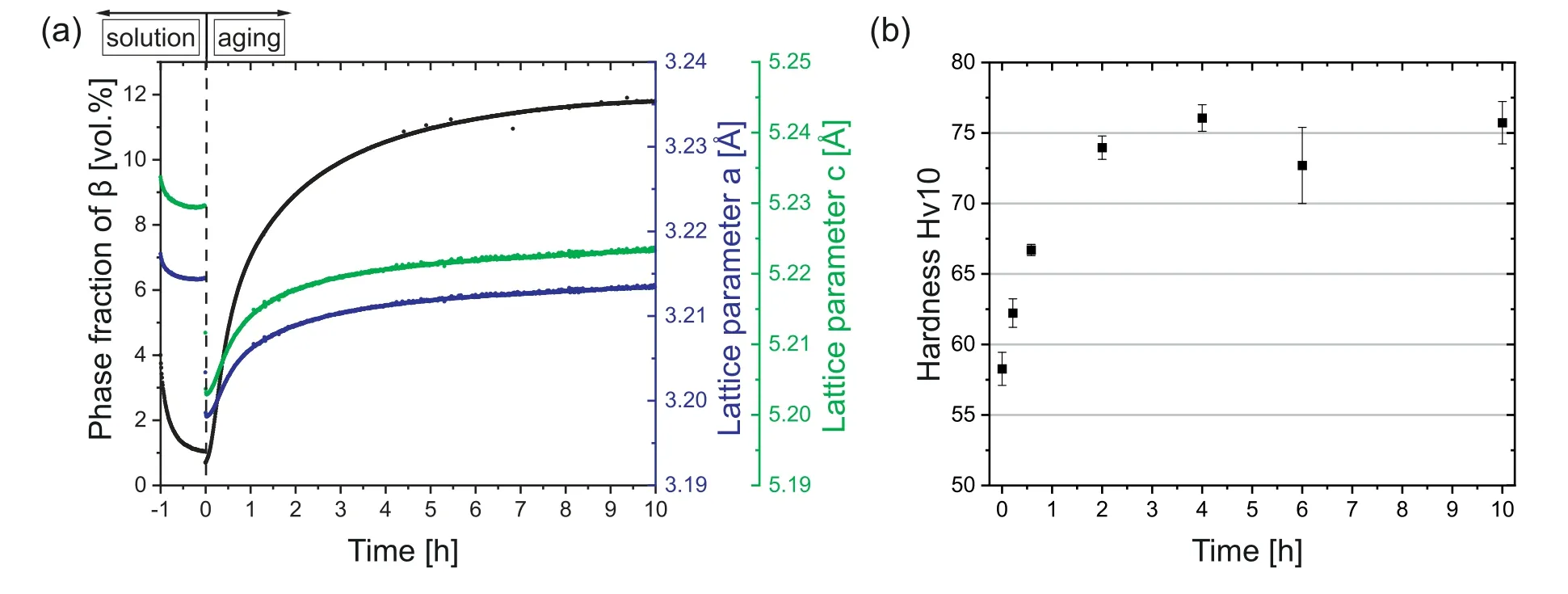
Fig.7.(a) Evolution of the phase fraction of β and the lattice parameters a and c of α-Mg during the solution heat treatment and single-step aging at 250 °C in the course of DA;(b) development of hardness with annealing time during single-step DA at 250 °C.
In Fig.8(a) the microstructure after the solution heat treatment is shown.The majority of segregations have dissolved after the solution heat treatment,leading to a more homogeneous microstructure.Fine-scaled Mn5Al8phase remains present,as its solution temperature is above the solidus temperature ofα-Mg [5,38].Additionally,the HEXRD study revealed that its phase fraction remains constant throughout all heat treatments and does not exceed 0.2 vol.%.Furthermore,remnants ofβ-Mg17Al12can be found,which is in agreement with the HEXRD investigation as well.

Fig.8.Evolution of the microstructure of the WAAM AZ91 alloy during DA at 250 °C.The secondary electron micrographs show the conditions (a) after the solution heat treatment,(b) after 35 min of aging,(c) after 4 h of aging and (d) after 10 h of aging.The orange arrow in panel (a) indicates β which is still present after the solution heat treatment.
3.3. Single-step aging at 250 °C
After the solution heat treatment an ensuing annealing step at 250 °C was conducted.The specimen was quenched directly to the annealing temperature,which resulted in the immediate decrease in the lattice parameters ofα-Mg in Fig.7(a).Furthermore,in the course of the annealing heat treatment an increase of the lattice parameters of theα-matrix is observed,which can be attributed to the precipitation ofβphase.With regard to the evolution of theβphase fraction,a rapid increase can be detected in the initial stage of annealing,while the value slowly saturates after longer holding times.This behavior is also reflected in the evolution of hardness during aging at 250 °C,as shown in Fig.7(b).First,a steep increase in hardness is visible,which eventually results in a plateau value after 2 h of annealing.In different studies in literature the peak hardness at 250 °C is reached after 5-20 h of annealing in the case of as-cast material [25–27,50–52].This large range can be explained by the different starting conditions of the material before annealing.Comparing the results in literature on as-cast material to the current study,enhanced precipitation kinetics are observed in this study.An explanation for this behavior will be given at the end of this section.
The SEM study at different holding times at 250 °C is illustrated in Fig.8.In the under-aged condition at a holding time of 35 min,it can be seen in Fig.8(b) that at this temperatureβis formed in a continuous as well as discontinuous manner,which was also found in literature[31,32].Discontinuous precipitation occurs at the grain boundaries and exhibits lamellar features,while continuous precipitation is visible as laths inside the grain.It should be noted that,in the present study,even after this short holding time discontinuous precipitation is already very prominent.At a holding time of 4 h,as shown in Fig.8(c),the fraction of discontinuous precipitation has increased,as have size,number and density of continuous precipitates.In Fig.8(d)the microstructure after a holding time of 10 h is displayed.Compared to the microstructure after 4 h no significant changes are visible,which indicates that the microstructure is stable at longer holding times.This also explains the constant hardness value observed after an aging time of 4 h.
Due to the complex temperature profile imposed by the WAAM process,microstructures substantially different from those known from conventional manufacturing techniques,such as casting,can develop.Owing to the higher cooling rates,the formation of metastable phases is possible as well.Hence,a microstructural investigation by means of TEM was performed additionally.In Fig.9(a) the microstructures of a sample which was solution heat-treated and subsequently annealed at 250 °C for 13 min is displayed.Alongside the rodshapedβ-Mg17Al12phase another phase is visible.This spherical phase,precipitated in rows,with a diameter of around 20 nm indicates a formation along dislocations.As it is also present at the end ofβrods,it is assumed that the spherical phase serves as nucleation sites for the continuous precipitation ofβ.By means of EDS an enrichment of the spherical phase in Al and Mn was verified,which is displayed in Fig.9(b).The presence of Al-,Mn-rich phases with sizes ranging from 20-200 nm has been demonstrated already by Zeng et al.[53]for the AZ91 alloy.The quasicrystalline phase was reported in the as-cast microstructure and was even stable after a solution heat treatment [53].Moreover,it was suggested that this phase acts as nucleation sites for continuous precipitation [53].

Fig.9.(a)TEM image of the microstructure after 13 min aging at 250 °C.The red circles mark exemplary areas,where β-Mg17Al12 nucleates at the nm-scaled Al-,Mn-rich particles.(b) EDS mapping of continuously precipitated β and Al-,Mn-rich particles.
Based on the experimental results presented in this work,the different precipitation response of as-built and as-cast material can have multiple reasons.Firstly,it was observed that,in as-built AZ91 material,discontinuous precipitation occurs rapidly in the initial stage of aging.Since grain boundaries are the primary nucleation sites for discontinuous precipitation,a finer grain size,i.e.an increased grain boundary area,also provides a higher amount of nucleation sites.As shown by Duly and Brechet [54],the nucleation rate at higher aging temperatures close to the solution temperature can be described by Fournelle and Clark’s mechanism,which states that the nucleation rate is inversely proportional to the square of the grain diameter.It is assumed that this holds true for the current study as well,since higher annealing temperatures are applied.Experimental evidence was provided by Sun et al.[55],who showed that the formation of discontinuous precipitates is faster for a finer initial grain size.The grain size of the as-built WAAM material in this study is significantly smaller than in conventional as-cast material.Consequently,this is one reason for the enhanced precipitation kinetics.
Secondly,Lee et al.[49] found that a too short solution heat treatment leads to the acceleration of discontinuous precipitation.The HEXRD and microstructural investigation in the current study revealed that the solution heat treatment of 1 h was indeed too short to fully homogenize the sample.As a consequence,the Al concentration in the vicinity of the grain boundaries remained increased,which provided a higher chemical driving force for the nucleation of discontinuous precipitation [49].Thus,it led to a more rapid formation of discontinuous precipitates.
Whereas the two former arguments focused on particularities regarding the formation of discontinuous precipitates,the following argument is finally concerned with differences in the continuous precipitation kinetics.Zeng et al.[53] proved that nm-scaled Al-,Mn-rich phases can act as nucleation sites for continuous precipitation.Furthermore,Tamura et al.[56] showed that a higher local Mn concentration leads to enhanced formation of continuousβ.While the mechanism behind this was,however,not investigated,it can be speculated that a higher fraction of nm-scaled Al-,Mn-rich phases was present in these areas,increasing the number of nucleation sites for continuous precipitation.In the WAAM sample investigated in the present work,nm-scaled Al-,Mn-rich phases were found as well,as the TEM investigation disclosed.There is a possibility that differences in the production process have an influence on size,volume fraction or distribution of this phase,which could lead to a more rapid continuous precipitation.
In summary,the mechanisms leading to more rapid precipitation kinetics in this study are a higher number of nucleation sites for discontinuous precipitation due to a finer grain size,a higher chemical driving force for discontinuous precipitation due to a higher Al concentration in the vicinity of the grain boundaries and the possibility of an increased amount of nucleation sites for continuous precipitation due to the presence of nm-scaled Al-,Mn-rich phases.
3.4. Single-step aging at 325 °C
In Fig.10 the evolution of theβphase fraction for all aging heat treatments is displayed.For the CA and DA samples aged at 325 °C,a rapid increase inβphase fraction can be observed,comparable to the specimen aged at 250 °C.However,due to a higher Al solubility at more elevated temperatures,the phase fraction ofβis lower than for those,aged at 250 °C.The SEM images shown in Fig.11(a) and(b) (CA and DA respectively) demonstrate that the majority ofβformed at this temperature is continuously precipitated.This is in agreement with Duly et al.[31] and Braszczy´nska-Malik[32],who reported that just below the solution temperature continuous precipitation predominates.Due to the higher bulk diffusion at elevated temperatures,bulk precipitation is favored,which reduces the chemical driving force for discontinuous precipitation [31,32].The higher bulk diffusion also leads to a homogenization of the Al content at both sides of the grain boundary and thus to a reduction in chemical driving force [31].

Fig.10.Evolution of β-Mg17Al12 in terms of its phase fraction in the course of different heat treatments.

Fig.11.Secondary electron micrographs of (a),(c) and (e) CA samples as well as (b),(d) and (f) DA samples.The specimens were exposed to single-step heat treatments at 325 °C (a,b) or 250 °C (e,f).Panels (c) and (d) illustrate the microstructure after a double-step heat treatment.
3.5. Double-step aging
It is well known that peak aging of the AZ91 alloy generally takes around 10-50 h at 200 °C in order to yield a considerable hardness gain [25–27,51].Such a heat treatment duration is comparatively long.It was shown for Al alloys[57–59] as well as for other Mg alloying classes [60,61] that a double-step aging heat treatment can substantially shorten the necessary annealing time and increase the hardness response.Furthermore,double-step aging can have an influence on the type of precipitates formed[42].Consequently,doublestep aging was performed on the WAAM produced specimens in this work to study its effect.
The evolution of theβphase fraction throughout the double-step heat treatment,which was conducted either as CA or DA,is presented in Fig.10.During the first aging step at 250 °C for 3 h,a rapid increase inβphase is observed.Enhancing the temperature to 325 °C in the second aging step results in a sudden drop in theβphase fraction.Since in the course of the first step of the heat treatment at 250 °C the equilibrium phase fraction ofβat 325 °C was already exceeded,the second step at 325 °C leads to a rapid dissolution of the phase due to the higher diffusion rate and solubility.Subsequently,a constant value ofβphase fraction is reached,which can be assumed to be the equilibrium phase fraction at this temperature.
In Fig.11(c) and (d) the final microstructures after the double-step heat treatment for CA and DA,respectively,are displayed.Continuous as well as discontinuous precipitation is found in the microstructure,which formed during the first heat treatment step at 250 °C.However,the amount of each precipitation type is reduced with respect to the single-step heat treatment condition at 250 °C for CA and DA,shown in Fig.11(e) and (f).Moreover,the majority of coarse discontinuously precipitatedβlamellae have sectioned into smaller precipitates with either an elongated or a spherical shape after the second heat treatment step at 325 °C.As a spherical precipitate shape is more favorable in terms of the minimization of phase boundary energy,the long holding times at elevated temperatures just below the solution temperature ofβlead to an instability of the lamellar shape of theβprecipitates.As compared to the single-step heat treatment at 325 °C,the distribution of precipitates in the microstructure is considerably finer and the interparticle spacing lower in the doublestep heat-treated specimen.No additional phases are present.The same behavior was observed by Crawley and Lagowski[62] for a double-step aged AZ91 alloy,which was aged at 100 °C for 96 h in a first step,followed by aging at 140 °C for 192 h.
The hardness after the single-and double-step heat treatments can be compared in Table 3.Out of the three heat treatments,i.e.single-step at 250 °C and 325 °C and the doublestep heat treatment,the highest hardness could be achieved by a single-step heat treatment at 250 °C.Even after 2 h of aging at 250 °C,the hardness already exceeds the one obtained after a double-step heat treatment,as can be seen in Fig.7(b).Furthermore,the hardness of the double-step heattreated sample is higher than the one of the single-step heattreated sample at 325 °C.Asβis more finely distributed in the double-step heat-treated samples than in the single-step heat-treated ones at 325 °C,the hardness is elevated in the former.Moreover,the volume fraction ofβin the double-step heat-treated samples (except for the directly aged one) is also increased,which also contributes to the enhanced hardness.Hence,it can be concluded that a double-step heat treatment(first step at 250 °C for 3 h/second step at 325 °C for 2.5 h) does increase the hardness as compared to a single-step heat treatment at 325 °C for 2.5 h,as the equilibrium phase fraction at this temperature is reached more rapidly andβis more finely distributed.However,a single-step heat treatment at 250 °C for the same amount of time would still be more beneficial,since an even higher fraction of second phase can be reached.
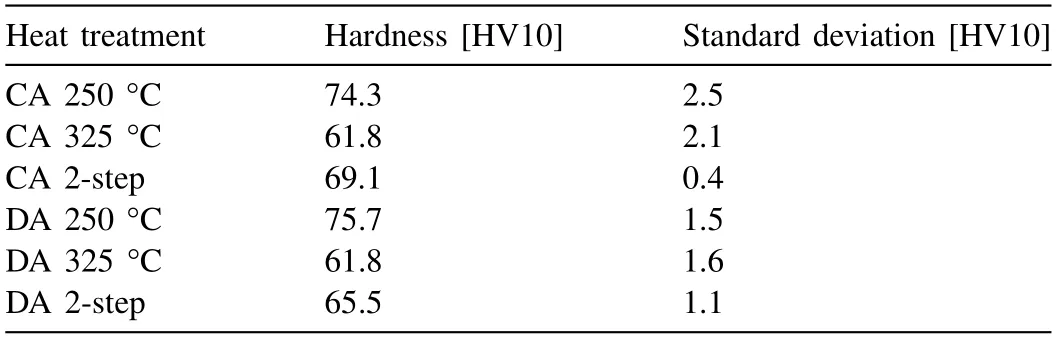
Table 3Mean hardness values and standard deviation for the different heat treatments.
3.6. Direct aging versus conventional aging
The evolution of the formation ofβin the course of theinsituHEXRD experiments for CA and DA is shown in Fig.10.At an aging temperature of 250 °C the precipitation response is equal for DA and CA.The volume fraction ofβafter a holding time of 10 h converges to the same value and kinetic differences are marginal.Moreover,both specimens exhibit the same hardness,as can be seen in Table 3.The SEM investigation revealed a consistent behavior in the formation of the microstructure after the application of the two heat treatment types (see Fig.11(e) and (f)).
After holding for 2.5 h at 325 °C,theβcontent in the CA specimen is 5.3 vol.%,while it is 4.5 vol.% in the DA specimen (Fig.10).This small difference is reflected in the microstructures presented in Fig.11(a) and (b),in which the amount ofβis clearly higher in the CA specimen.However,the hardness does not show a similar trend.Both specimens obtain a hardness of around 62 HV10 (Table 3).This can be ascribed to the comparably low volume fraction ofβformed and its coarse nature.
In the case of the double-step heat treatments the volume fraction ofβafter the first step is identical(Fig.10).Nonetheless,after heating to 325 °C,different plateau values were reached.For CA 6.5 vol.%βfinally remained and for DA 5.1 vol.%.Furthermore,by comparing the microstructures in Fig.11(c) and (d) the higher amount ofβin the CA specimen is clearly visible and,consequently,the reason for the higher hardness of the CA specimen (Table 3).The fraction ofβpresent after the second step is assumed to be the equilibrium amount at this temperature.
The formation of continuous precipitation is similar to the precipitation process known for age-hardening Al alloys,i.e.quenched-in vacancies are needed in order to form continuous precipitates [32].In a recent investigation Pogatscher et al.[63] found that directly aged Al-Mg-Si samples exhibit faster precipitation kinetics during artificial aging at lower temperatures than specimens,which were previously naturally longterm aged.At medium aging temperatures both types showed equal kinetics,while at high aging temperatures the kinetics of the naturally pre-aged samples were faster.This was attributed to the formation of vacancy-rich co-clusters during natural aging,which act as “vacancy-prisons” rendering vacancies immobile at low temperatures.However,at high temperatures vacancies in the directly aged samples are quickly annihilated,while vacancies in the naturally aged samples are set free leading to enhanced precipitation.In the AZ91 alloy no precipitation sequence occurs[30].However,the formation of clusters cannot be completely ruled out.The CA specimens in this investigation underwent natural aging for 9 weeks after the solution heat treatment.In Fig.10 slightly higher kinetics at 325 °C and slightly lower kinetics at 250 °C can be observed for CA specimens,which would be in agreement with the “vacancy-prison mechanism” [63].However,if this mechanism is present in the WAAM AZ91 alloy,it is not very pronounced,as it is not reflected in the hardness and only marginal differences can be observed duringinsituHEXRD.A more likely explanation for the differentβcontent produced during aging at 325 °C is the difference in Al content throughout the specimen,as shown in Section 3.1.As it can be assumed that during the second step of the doublestep heat treatment the equilibrium fraction ofβis reached,a difference in Al content in the CA and DA specimen is obvious.The measurement position during theinsituHEXRD experiment was random.Consequently,it is possible that positions with locally different Al content were probed.As the differences between DA and CA in kinetics,microstructure and hardness can most likely be ascribed to the local variations in Al content,it can be concluded that no significant difference between CA and DA exists.
4.Conclusions
The objective of this study was to gain a deeper insight into the age-hardening response of a WAAM AZ91 alloy and to compare for the first time different aging techniques in the course of the heat treatment.For this purpose,first the influence of the WAAM process on the formation of the microstructure and precipitates was analyzed for the as-built condition of the AZ91 alloy.Then,the age-hardening response during various heat treatments was studied by means ofinsituHEXRD as well as microstructural and hardness investigations.From these experiments the following conclusions can be drawn:
•In the as-built condition a fine equiaxed grain structure with a random grain orientation was apparent throughout the whole specimen.HEXRD and SEM confirmed the presence ofβ-Mg17Al12phase as well as Mn5Al8phase at the grain boundaries.
•As a result of the layer-by-layer build-up during WAAM,the phase fraction ofβ-Mg17Al12is alternating along the building direction,i.e.the specimen height.This is caused by an alternating Al content due to the evaporation of Mg in the liquid state,which leads to an increased Al content close to the fusion line.
•Furthermore,the overall content ofβphase varies along the building direction.After the deposition of a layer,βis present as segregation in the microstructure.However,due to the high cooling rates,the equilibrium amount ofβphase is not reached.Hence,the heat input caused by the deposition of the subsequent layers causes either the precipitation or the resolution ofβ.Close to the top,βis resolved since the temperature rises above the solution temperature ofβduring further deposition.When the temperature is lower than the solution temperature,βphase is precipitated in the form of discontinuousβ.The formation of discontinuousβincreases with increasing distance to the top layer.
•As Al diffuses only slowly in Mg,a solution heat treatment for 1 h was insufficient to resolve allβsegregations and to completely homogenize the alternating Al content throughout the sample.
•During single-step annealing at 250 °C,both discontinuous and continuousβare formed.Enhanced precipitation kinetics were observed as compared to cast material,since a hardness plateau was already reached after 2 h.Possible reasons for this behavior are the finer grain size of the matrixαgrains,which increases the nucleation sites for discontinuous precipitation as well as the comparably short duration of the solution heat treatment investigated in this work.The latter enables a higher driving force for discontinuous precipitation due to the enhanced Al content in the vicinity of the grain boundaries.Another contribution may be made by the finely dispersed nm-scaled Mn-,Alrich precipitates.These precipitates can act as nucleation sites for continuous precipitation.
•No significant difference between conventional aging and direct aging within a temperature range of 250-325 °C was observed.
•In the investigated temperature range a double-step heat treatment at 250°C for 3 h followed by aging at 325°C for 2.5 h slightly improves the hardness as compared to singlestep aging at 325 °C for 2.5 h.However,a considerably higher increase in hardness is achieved by only applying the pre-aging step,i.e.single-step aging at 250 °C for 3 h,as a significant proportion ofβphase is resolved during the second aging step at 325 °C.
In summary,the age-hardening behavior for WAAM AZ91 material significantly differs from as-cast material,which was quantified exactly in this study.
Declaration of competing interest
None.
Acknowledgements
We acknowledge DESY (Hamburg,Germany),a member of the Helmholtz Association HGF,for the provision of experimental facilities at PETRA III.The beamline P07 (HEMS) is operated by Helmholtz-Zentrum Hereon and we would like to thank Peter Staron,Norbert Schell and Andreas Stark for assistance in using the beamline and the dilatometer.Beamtime was allocated for proposal I-20210560 EC.The research leading to this result has been supported by the project CALIPSOplus under the Grant Agreement 730872 from the EU Framework Programme for Research and Innovation HORIZON 2020.Furthermore,we gratefully acknowledge the financial support of the European Research Council (ERC)under the European Union’s Horizon 2020 research and innovation program (Grant No.771146 TOUGHIT).Parts of this research were funded within the AIT’s strategic research portfolio 2022 and by the European Commission within the framework INTERREG V-A Austria–Czech Republic in the project “ReMaP“ (Interreg project no.ATCZ229).The technical expert assistance by A.Birgmann and critical discussions with S.Gneiger are greatly appreciated.
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Carbon nanotube and graphene reinforced magnesium matrix composites:A state-of-the-art review
- Stress corrosion cracking of magnesium alloys: A review
- Simultaneous enhancement of mechanical properties and corrosion resistance of as-cast Mg-5Zn via microstructural modification by friction stir processing
- Regulating local coordination environment of Mg-Co single atom catalyst for improved direct methanol fuel cell cathode
- Hydrogen-induced optical properties of FC/Pd/Mg films: Roles of grain size and grain boundary
- Improving corrosive wear resistance of Mg-Zn-Y-Zr alloys through heat treatment
