Carbon nanotube and graphene reinforced magnesium matrix composites:A state-of-the-art review
Lava Kumar Pillari,Kyle Lessoway,Lukas Bichler
School of Engineering,The University of British Columbia-Okanagan,Kelowna,BC V1V 1V7,Canada
Abstract Magnesium(Mg)composites reinforced with carbon-based nanomaterial(CBN)often exhibit low density,enhanced strength,good conductivity,improved wear resistance,and excellent biocompatibility when compared to current industry Mg alloys.This review aims to critically evaluate recent developments in Mg-CBN composites and is divided into five sections: First,a brief introduction to Mg-CBN composites is provided,followed by a discussion of different fabrication techniques for these composites,including powder metallurgy,casting,friction stir processing,and selective laser melting.A particular focus is on the current processing challenges,including dispersion strategies to create homogeneous Mg-CBN composites.The effect of processing on the quantifying disorder in CBNs and distinguishing different sp2 carbon materials is also highlighted.Then,the effect of CBN on various properties of Mg-CBN composites is thoroughly analyzed,and the strengthening efficiency of CNTs and graphene in the Mg matrix is examined.Finally,the potential applications of Mg-CBN composites in various industries are proposed,followed by a summary and suggestions for future research directions in the field of Mg-CBN composites.
Keywords: Magnesium;Carbon nanotubes (CNTs);Graphene;Nanocomposites;Processing;Mechanical properties.
1.Introduction
Global trends in the automotive and aviation industries continue to drive vehicle lightweighting in order to meet new fuel standards,leading to reduced greenhouse gas emissions.One way to reduce vehicle weight is to replace conventionally used materials,including steel,aluminum (Al) alloys,and titanium (Ti) alloys,with light metal alloys without compromising material performance.Magnesium (Mg) and its alloys are some of the lightest structural materials,with a density of 1.74 g/cm3(Al: 2.7 g/cm3;Ti: 4.5 g/cm3;steel:8.05 g/cm3).Due to their low density,high specific strength,good castability,easy recyclability,and high damping capacity,Mg alloys continue to show promise for structural applications in the automotive and aviation sectors [1,2].In addition,Mg alloys have been also successfully applied for temporary bio-implants,primarily due to their excellent biocompatibility combined with good mechanical strength and tunable biodegradation [3,4].Recently,the use of Mg alloys for high hydrogen storage(capacity of up to 7.6 wt.%)created new opportunities for evolution in the Hydrogen economy[5].However,in comparison to currently used materials,Mg alloys often exhibit relatively poor ductility due to the limited slip systems at room temperature [6],relatively low strength levels,high degradation rate [7],poor hydrogenation kinetics[8],and inferior wear resistance [9].These shortfalls continue to hinder the wider commercialization of Mg alloys in various sectors.
One way to overcome the above limitations is to incorporate a suitable reinforcement (with superior properties) into Mg [10].In this direction,researchers have made significant efforts to produce Mg matrix composites with various reinforcements such as Al2O3[11,12],B4C [13],SiC [14],TiB2[15],TiC [16],and Y2O3[17].However,adding the micronsized reinforcements (carbides,ceramic fibers,and particulates) improved the strength of Mg,but ductility was ad versely affected by particle fracture and matrix/particle interfacial failure [18].Moreover,a higher fraction (>10%) of these reinforcements was generally required to achieve appreciable enhancements in strength.The high density of these reinforcements can increase the composite weight with these large volume fractions.
It has been reported that nano-sized reinforcements may appreciably enhance the properties of Mg alloy composites,but at a much lower reinforcement content (<3%) compared to micron-sized reinforcements [19].Carbon-based nanomaterials (CBNs) such as carbon nanotubes (CNTs) and graphene have attracted much interest as a suitable reinforcement for metal matrices owing to their ultralightweight,high theoretical mechanical properties combined with excellent thermal and electrical conductivities [20].Key properties of graphene,single-wall (SWCNTs),and multi-wall carbon nanotubes (MWCNTs) are shown in Table 1.The detailed information on the synthesis and structure of CBNs is beyond the scope of this review but can be found in [42–46].

Table 1Properties of carbon nanotubes and graphene.
Over the past two decades,CBNs have been incorporated into various metal matrices,including Al[47–49],Cu[50,51],Mg [52],and Ti [53,54],mainly to improve the mechanical properties of the matrix.In the case of Al and Cu composites,current research has significantly advanced,while in the case of Mg and Ti alloy systems,the development of CBNreinforced composites is still at an early stage.Fig.1 summarizes annual publication trends of articles in the field of Mg-CNT and Mg-graphene composites.Recently,graphene has received more attention than CNTs as reinforcement due to its unique desirable characteristics,including its two-dimensional(2D) morphology and its high surface area and aspect ratio[55].The earliest studies on Mg-CNT composites were carried out by a Swiss research group and published in 2004[55,56].A 9.3% improvement in Young’s modulus was observed by adding 2 wt.% CNT to Mg [56].The first report on Mg-graphene composites was by Chen et al.[57] in 2012,demonstrating a novel processing technique for the uniform dispersion of graphene nanoplatelets (GNPs) in the Mg matrix.The resulting composite of Mg-1.2 vol.% GNP showed a dramatic enhancement in the hardness of 78%.More recently,some works have shown that CBNs can improve corrosion resistance,biocompatibility,hydrogen storage properties,and antibacterial activity of Mg [58–62].
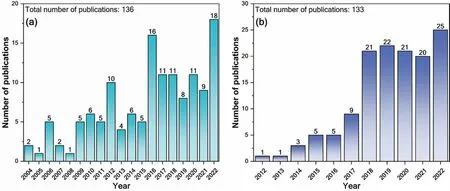
Fig.1.The year-wise trend in the publication of research articles in the field of (a) Mg-CNT and (b) Mg-graphene composites,the publication data is collected from the ‘Web of Science’ using the keywords of “Magnesium CNT composites” and “Magnesium graphene composites.”
Although the Mg-CBN composites showed superior properties over unreinforced Mg,there have been no successful attempts to use Mg-CBNs for large-scale industrial applications,mainly due to the difficulty of dispersing CBNs in Mg.The large surface areas of CBN and van der Waals forces between the carbon sheets or tubes in these reinforcements lead to the agglomeration or clustering of CBNs,causing deterioration of composite’s properties [19].Also,poor wettability between CBNs and metal matrices is another problem arising from their large surface tension difference [63–66],leading to poor interfacial bonding.Therefore,there is a compelling demand to develop a novel processing route that enables the uniform dispersion of CBNs in metal matrices while securing proper interfacial bonding.Considerable research efforts have been devoted to achieving the uniform distribution of CBNs in metal matrices (including Mg) using various processing strategies [19,47,52,67–69].However,researchers have not fully succeeded in uniformly dispersing CBNs in Mg,and further research is needed.Several general review articles are available on metal matrix composites(MMCs)reinforced with CBNs [19,20,70–75].To the best of the authors’ knowledge,only two review articles have been published exclusively on Mg-CBN (CNTs [76],graphene [77]) composites.However,a critical review assessing the results of all aspects of Mg-CBN composites in one place is still lacking.
As a result,this review endeavors to provide readers with an in-depth overview of state-of-the-art processing,properties,and potential applications of Mg-CBN (CNTs and graphene)composites.
2.Fabrication of Mg-CBN composites
The properties of Mg-CBN composites greatly depend on their processing route.Researchers have mainly used powder metallurgy (PM),casting,friction stir processing (FSP),and selective laser melting (SLM) to develop Mg-CBN composites.
2.1. Powder metallurgy
PM-based techniques have been most commonly used to process Mg-CBN composites owing to their simplicity and ability to disperse CBN easily (Fig.2(a,b)).This processing route involves three steps,(i) dispersing CBN in Mg powder,(ii) compacting the Mg-CBN powder mixture to form a green compact,and (iii) sintering the Mg-CBN green compact at elevated temperature in a controlled atmosphere or vacuum.

Fig.2.Contribution (%) of different processing techniques used by researchers to fabricate (a) Mg-graphene and (b) Mg-CNT composites.
2.1.1.CurrentdispersiontechniquesinthePMroute
Over the past two decades,researchers have attempted different dispersion techniques to optimize the dispersion of CBNs in Mg,primarily using wet mixing (ultrasonication and mechanical agitation) and ball milling [78–81].However,most researchers have used ultrasonication with mechanical agitation/stirring [52,82] and/or BM [83,84],called hybrid processing,to disperse CBNs in Mg.
2.1.1.1.Wetmixing.Wet mixing of CBNs by ultrasonication and/or mechanical agitation in solvents(e.g.,acetone,ethanol,isopropanol) is widely used for deagglomerating CBNs.Ultrasonication uses sound energy to agitate particles in a liquid medium,while mechanical agitation uses a rotating magnetic stir bar or motorized stirrers.Ultrasonication is typically performed either by a bath sonicator (40-50 kHz) or a tip horn(probe) sonicator (20-25 kHz) [85].In the bath sonicator,the sample is not in direct contact with the energy source,while in the probe sonicator,the energy source (probe/tip horn) is in direct contact with the sample.Thus,probe sonicators are generally more aggressive than bath sonicators,and the power delivered to the sample during probe sonication was estimated to be ∼10x higher than during bath sonication[86].Likewise,magnetic stirring generates a weak shear force compared to mechanical stirring,mainly due to the shape of the magnetic bar and relatively low rotational speeds[87].In addition to the type of wet mixing,the parameters of sonication(time,amplitude,and frequency) and mechanical agitation (time and rotational speed) are crucial,and they can be adjusted to achieve desired CBN size [88–91].
Fundamentally,during ultrasonication in a pure liquid medium,the liquid generally undergoes alternating compressive and expansive acoustic waves,which further causes the generation of spherical bubbles from existing impurities (e.g.,N2,O2).These bubbles can grow through a rectified diffusion mechanism,accumulating ultrasonic energy and oscillating with the applied field.Once these oscillating bubbles reach a critical size (usually tens of μm),they overgrow and then catastrophically collapse through a series of stages collectively known as cavitation (formation,growth,and implosive collapse of bubbles) [86,92].This rapid collapse of the bubbles causes the creation of local ‘hot spots’ (Temperature∼15,000 K [93]) and emanates liquid jets with high local velocities of ∼4000 m/s and pressure of ∼40-60 kbar [94].A slightly different cavitation mechanism was reported in a heterogeneous system containing solid particles (CBNs) [86,95].Similar cavitation bubbles were also observed in this system upon the passage of ultrasonic waves.But the cavitation at the CBN surface generated non-spherical bubbles,which directed high-speed liquid jets toward the CBN’s surface.These liquid jets were estimated to have velocities of 100-500 m/s[96],which induce separating [90,97] and scissoring forces[98,99] that cause shock wave damage to the CBN’s surface.Separating forces can overcome weak attraction forces,increasing interlayer/tube spacing.As a result,the agglomerated CBNs were seen to exfoliate into one or more layers of carbon atoms [85,100].Simultaneously,the scissoring forces were seen to break the continuous CBNs into smaller fragments of graphene/CNT,resulting in uniformly sized CBNs[101,102].In addition,the liquid jets generated by cavitation in the liquid medium can also cause fragmentation and surface damage to CBNs [93].A high-speed sequence of images captured during the ultrasonication of graphite in pure water is shown in Fig.3 [103].It clearly illustrates various steps during the exfoliation of graphite by ultrasonication: (i)formation of cavitation bubbles (Fig.3(a,b)),(ii) splitting of graphite flake (Fig.3(c)),and (iii) gradual exfoliation of the graphite flakes by continuous penetration of cavitation bubbles into the gap between the graphite layers (Fig.3(d-f)).

Fig.3.A high-speed sequence of images illustrating various steps during the exfoliation of graphite flake in water by ultrasonication.(a,b) formation of cavitation bubbles;(c)splitting of graphite flake;and(d-f)gradual exfoliation of graphite flake by continuous penetration of cavitation bubbles into the gap between graphite layers.Arrow indicates the place of splitting/exfoliation.Reproduced under terms of the CC-BY license from [103],Copyright 2020,Elsevier.
Commercially available CBNs are generally supplied in agglomerated states with highly entangled bundles and clusters.This is mainly due to the van der Waals forces,large surface areas,and high surface energies,which easily cause agglomeration of CBNs during their synthesis or when mixing CBNs in the Mg matrix[19].The binding energy between the graphene sheets in graphite is about 2 eV/nm2,and a force of 300 nN/μm2is required to exfoliate graphene [104].Similarly,the binding energy between parallel tubes is 500 eV/ μm,which requires energy of 37 kT/nm and 120 kT/nm to detach tubes from parallel tubes and a bundle,respectively [19,105].Sonication and mechanical agitation can easily overcome these binding energies,mainly due to the high local shear stresses which develop during processing.Considerable research has been devoted to addressing the dispersion of CBNs in the Mg matrix by wet mixing using different strategies.The various reports on the dispersion of CBNs in Mg by wet mixing are summarized in Table 2.
2.1.1.2.Ballmilling.Ball milling (BM) or mechanical alloying has proven to be a superior technique to homogeneously disperse/distribute CBNs in metal powders [19,20].Different types of mills,including planetary ball mills (FRITSCH GmbH and RETSCH),mixer mills (RETSCH),shaker mills(SPEX® SamplePrep),and attritor mills (Union Process,Inc.,Szigvari),are used to perform BM.A detailed description of the various mills available for BM,the process,and the various parameters of BM can be found in the preceding reviews[133–139].
Among the available mills,planetary ball mills have been commonly used to disperse CBNs in metal matrices.In the planetary ball mill,the milling vials are arranged eccentrically on a rotating sun wheel,and they are filled with metal powder along with the milling balls.The milling vials rotate in the direction opposite to that of the wheel,creating a centrifugal force that acts on powder to be milled.The BM process involves repeated welding and fracturing of powder particles trapped between the milling balls.The mechanical behavior of powder components decides the extent of welding and fracturing powder particles during milling [136].The Mg-CBN mixture is considered a ductile-brittle system;Mg particles are flattened while the CBNs are fragmented during the initial milling stages.The flattened particles with atomically clean interfaces are brought into intimate contact and mutually cold-welded,while the fragmented CBNs are embedded in the layers of the Mg phase.The cold-welded layers get convoluted and refined by fracture as the milling proceeds due to work hardening.The deformed particles are continually refined and homogenized by the interplay of cold welding and fracturing events,while the CBNs are uniformly dispersed in the Mg matrix [134,140].

The studies on the dispersion of CNTs/graphene in the Mg matrix by ball milling are summarized in Table 3.The ball milling parameters,dispersion quality,and structural integrity of CBNs are also highlighted in the Table.BM of the Mg-CBN mixture has been performed either in wet or dry mode.In the wet mode,the powder particles were milled in a liquid medium(e.g.,ethanol,isopropanol,acetone,toluene,etc.),also known as a process controlling agent (PCA).The PCA is mainly used to minimize the cold-welding and prevents metal powder oxidation and overheating of milling vials.The PCA adsorbed on particle surfaces minimizes cold welding by lowering the metal powder’s surface energy and preventing agglomeration [137].Stearic acid is often used as a PCA during the milling of Mg-CBN in dry mode,and the milling is carried out under an argon atmosphere [60,81].Many researchers have synthesized Mg-CBN composite powder by reactive BM under a hydrogen atmosphere to improve the hydrogenation and dehydrogenation rates of Mg [147,175–177].In general,the BM of Mg powder was performed for long periods (typically 12 to 24 hrs) to produce nanocrystalline structure and then followed by milling with CBN for a shorter period (e.g.,0.5 hrs) to distribute CBN uniformly in the Mg matrix [175–177].Optimizing the milling conditions influenced the formation of defects,surface area of Mg particles,created diffusion channels,and reduced diffusion distance.Such structural changes enhanced the hydrogen storage properties in the nanocrystalline material [189,190].
Du et al.[169] fabricated Mg-GNP (0.25,0.75,1.25 wt.%)composites by BM and conventional sintering.The hardness of Mg improved with increasing GNP content and achieved a maximum improvement of 51.6% with 1.25 wt.% GNP,which was attributed to a uniform distribution of GNP.Kumar et al.[171] prepared Mg-3Al-0.1,0.3,0.5 wt.% GNP composites via BM,microwave sintering followed by hot extrusion,and observed GNP clusters with the addition of more than 0.3 wt.% GNP.Similarly,Thornby et al.[156] observed agglomeration of CNTs in the Mg matrix with an addition beyond 0.5 wt.%.The literature on Mg-CBN composites shows that there is always an optimum amount of CBN above which the clustering occurs.The optimum CBN content depends on the BM parameters(speed,ball-to-powder weight ratio(BPR),and time),the initial size of Mg powder,and the properties of the CBN.The strategies currently employed to disperse CBNs in the Mg matrix by wet mixing and BM are discussed in the forthcoming sections.
2.1.1.3.CurrentdispersionstrategiesinthePMroute.In the PM route,the following approaches have been primarily used to disperse CBNs in Mg through wet mixing and BM: (i) hybrid dispersion (combination of sonication,agitation,and/or BM),(ii) surface modification (functionalization of CBNs using surfactants),(iii) surface coating of CBNs,and (iv) using hybrid reinforcement.
2.1.1.3.1.DispersionofCBNsbyahybridapproach.Researchers have made significant efforts by combining ultrasonication and mechanical agitation to disperse CBNs in Mg.Rashad et al.[52,78,111–114] adopted a hybrid dispersion approach involving a combination of ultrasonication and mechanical agitation to disperse CBNs in the Mg matrix.In all these studies,as a first step,ultrasonication of CBNs was carried out in a suitable solvent to exfoliate and reduce the size of the CBNs.Then,mechanical agitation was performed to mix the exfoliated CBNs in the Mg powder.Uniform dispersion of CBNs in the Mg matrix has been reported using this approach.
Currently,research is increasingly focusing on employing hybrid dispersion techniques by combining wet mixing and BM to disperse CBNs in an Mg matrix.In this approach,the CBNs were first deagglomerated or exfoliated in a suitable solvent by wet mixing (ultrasonication or mechanical agitation).Then BM was performed with Mg powders to disperse CBNs.Say et al.[160] distributed CNTs in AZ61/AZ91 matrix by hybrid dispersion approach,including sonication of CNTs in ethanol for 1 h followed by BM of matrix powder and CNTs for 90 min.Uniform dispersion of CNTs was achieved,resulting in an improvement in the compressive strength of AZ61 and AZ91 alloys by 24% and 8%,respectively,with the addition of 0.5 wt.% CNT.In a study by Rashad et al.[84],CNTs and GNPs were first mixed separately in ethanol by ultrasonicator,and at the same time,AZ31 powder was mechanically agitated for 1hr.Then,the composite mixture was further agitated for 0.5hr,followed by BM for 4hr.The addition of 0.3 wt.% CNT and GNP improved the hardness of AZ31 by 34% and 22%,respectively.This improvement in mechanical properties was attributed to the effective dispersion of CBN in the Mg matrix caused by the hybrid dispersion approach.
2.1.1.3.2.SurfacemodificationofCBNs.Surface modification (functionalization) of CBNs using suitable surfactants(anionic,cationic,and neutral) is another approach widely used by researchers to disperse CBNs in the Mg matrix via wet mixing.The physical adsorption of surfactant molecules onto the surface of CBNs improves adhesion characteristics by lowering CBNs surface energy and thus reducing their tendency to agglomerate [191,192].Strano et al.[193] proposed a dispersion mechanism for isolating nanotubes from bundles with the combined assistance of ultrasonication and surfactant adsorption.The ultrasonication processing provided high local shear stress,mainly at the tube bundle ends,creating gaps in the bundles.The propagation of these gaps occurred through the adsorption of surfactant molecules and,ultimately separation of individual tubes from the bundle.A similar mechanism was also observed in graphene,where the interlayer space between the graphene sheets is suitable for surfactant adsorption[194,195].
Fukuda et al.[106,108] and Kondoh et al.[107] used a zwitterionic surfactant to deagglomerate CNTs and disperse them in the Mg matrix.First,the CNTs were coated with a zwitterionic-based solution using ultrasonication,then mixed with Mg powder.The zwitterionic surfactant coating provides attractive forces which were greater than the van der Waals forces,resulting in the complete separation of CNTs.Although the surfactant was efficacious in improving CBNs dispersion,it was challenging to subsequently eradicate the surfactant from the mixture even after thermal treatment.It is known that the presence of surfactants can deteriorate the properties of the Mg-CBN composites.
2.1.1.3.3.SurfacecoatingofCBNs.Surface coating of CBNs with MgO [68,196] and Ni [109,115,151,159] was another successful approach to achieve a good interfacial bonding and a uniform distribution of CBNs in an Mg matrix.The large difference in surface energy between Mg (785 mJ/m2[197];461-1094 mJ/m2[198]) and CBNs (CNTs: 45.3 mJ/m2[199];graphene: 46.7 mJ/m2and graphene oxide (GO): 62.1 mJ/m2[200]) is the main reason for their poor wettability of CBNs by liquid Mg,resulting in a weak interfacial bonding between Mg and CBNs.Thus,the coating of CBNs with MgO(1040 mJ/m2[201]) or Ni (1245-1730 mJ/m2[202]) improves the wettability of CBNs by Mg,as MgO and Ni have surface energy close to that of Mg.
Yuan et al.[68] have adopted a hybrid dispersion (sonication and mechanical stirring) to disperse CNTs coated with MgO in the AZ91 alloy matrix.Then,the composite mixture was consolidated by conventional sintering,and subsequently,the samples were processed by hot extrusion.Crystallographic analysis of the AZ91-3 wt.% MgO-coated CNTs composite confirmed the formation of two bonds at the MgO/CNT interface,including nanoscale contact and the diffused interface.It can be seen in Fig.4(a) that the interfacial distance between CNT and MgO is 0.3650 nm,confirming the nanoscale contact bonding.In addition,the Fourier-filtered image(Fig.4(b))shows a layer of disordered atoms (thickness <2.1 nm) at the interface of CNT/MgO,indicating the presence of an interface with a diffusion layer.Furthermore,the interatomic misfit at the Mg/MgO interface along MgO [011] and Mg [2110]was 6.5%,which represents a semi-coherent interface,indicating an excellent interfacial bonding between MgO and Mg(Fig.4(c)).It can be seen from Fig.4(d) that the plane (0002)of CNTs crossed the planes (20) and (011) of Mg at an angle of 98° and 50°,respectively.This reveals that CNTs are mainly incoherent with the Mg in the composite.Thus,the interfacial bonding between Mg and CNTs can be improved by coating the CNTs with MgO.
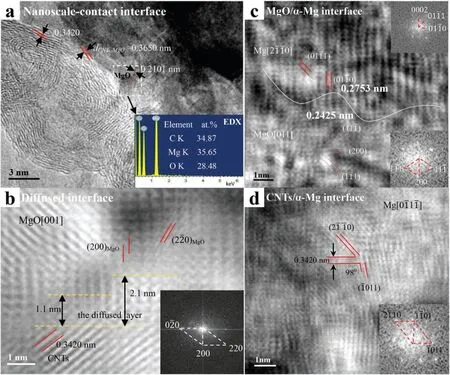
Fig.4.Interface structure of MgO/CNTs of AZ91-3MgO@CNT composite:(a)HRTEM image,showing the nanoscale-contact interface bonding between MgO and CNTs.The inset shows the EDX results,confirming Mg,O,C at the MgO/CNT interface;(b) Fourier filtered image,showing the presence of the diffused interface layer between MgO and CNTs.The inset shows the fast Fourier transform (FFT) patterns of MgO;(c) Fourier-filtered image of MgO/α-Mg interface,confirming the presence of MgO and Mg.The insets show the FFT pattern of α-Mg (top) and MgO (bottom);(d) Fourier-filtered image of CNTs/α-Mg interface,indicating that the CNTs are incoherent with α-Mg.The inset shows the FFT pattern of α-Mg.Adapted with permission from [68].Copyright 2016,Elsevier.
Ye et al.[115] coated the GNSs with Ni,then incorporated them in the Mg-9Al matrix by hybrid dispersion (mechanical agitation and sonication) and consolidated via PM and hot extrusion.The transmission electron microscopy (TEM)image of the Mg-9Al+Ni coated GNS composite clearly showed the formation of Mg2Ni at the Mg/GNSs interface(Fig.5(a)).In addition,it revealed a 1nm thick diffusion layer at the Mg/GNSs interface,leading to good interfacial bonding.This was further confirmed by the interatomic misfit,where the misfit between (1011)Mgand (112)Mg2Niwas calculated to be 1.7%,suggesting a semi-coherent interface(Fig.5(b)).The schematic representation of the interfacial structure of Mg-9Al+Ni-coated GNS composite is presented in Fig.5(c,d).Due to the good interfacial bonding,the Ni-coated GNS composite exhibited superior compressive properties to the GNS composite.It was observed that the formation of Mg2Ni intermetallic (at the Mg/CNT interface)also in Mg-Ni coated CNTs composite [151].
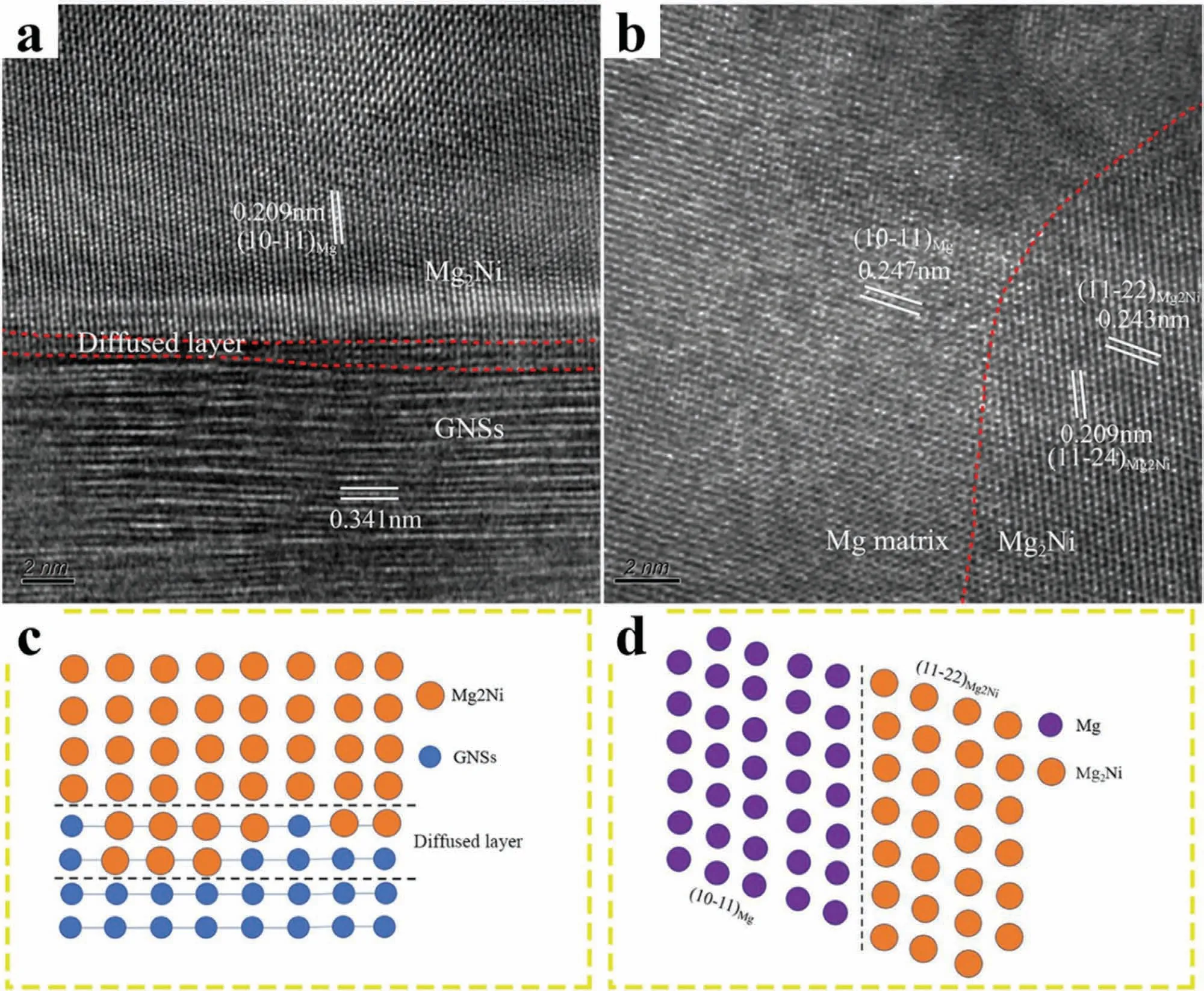
Fig.5.Interfacial structure of Mg-9Al+Ni coated GNSs composite: (a) HRTEM image of,confirming the presence of a diffused layer with 1nm thickness at the Mg/GNSs interface;(b) HRTEM image of Mg/Mg2Ni interface,showing that the Mg2Ni is semi-coherent with Mg matrix.Schematic illustration of the (c) Mg2Ni/GNSs interface,showing the interlace of Mg2Ni and GNS atoms;(d) Mg2Ni/Mg interface,showing the semi-coherent interface characteristics.Adapted with permission [115].Copyright 2020,Elsevier.
2.1.1.3.4.Hybridreinforcements.Some researchers have attempted to add hybrid reinforcements consisting of CBN and micron-size ceramic particles (Al2O3[149,155],B4C[203–205],SiC [143,157,158,168],ZrO2[204]) into an Mg matrix.The ceramic particles act as a carrier for CBNs to the Mg powder and reduce the agglomeration of CBNs.Li et al.[149] developed Mg matrix composites reinforced with CNTs-Al2O3hybrid reinforcement.First,the hybrid reinforcement was synthesized byin-situdepositing CNTs over Al2O3particles by chemical vapor deposition (CVD).Then,the CNTs-Al2O3mixture and Mg powder were milled for 24 h and consolidated using PM,followed by hot extrusion.The hybrid composite (Mg+4 wt.% CNTs-Al2O3) showed a 28.21%,19.35%,and 17.84% higher yield strength than Mg,Mg-4 wt.% Al2O3and Mg-4 wt.% SiC composite,respectively.Similarly,Zhou et al.[157] have used micronsize SiC particles as a carrier for CNTs and developed AZ61+5 vol.% SiC-CNTs composites by PM.The hybrid reinforcement (5SiC-CNTs) was pre-dispersed in a 1.5 wt.%sodium dodecylbenzene sulfonate solution with a sonicator to facilitate CNTs’ adsorption on the surface of SiC particles.Then,the sonicated mixture and Mg powder were mixed through shift speed BM and achieved good CNTs dispersion in the Mg matrix.The resultant composite (5SiC-1CNTs) exhibited the highest improvement of ∼47% (with respect to the AZ61 matrix) and ∼32% (with respect to the 5SiC composite) in ultimate tensile strength (UTS).
In a study by Rashad et al.[52],a hybrid dispersion approach was used and achieved good graphene dispersion in the Mg matrix,which also contained CNTs.As a result,the hybrid composite (Mg-1Al-0.6 wt.% CNT+GNPs) showed a fracture strain of 16.4%,which was 310% and 64% higher than the single GNPs (4.0%) and single CNTs (10%) reinforced composite,respectively.The CNTs were seen to intercalate between sheets of GNP during sonication and inhibited the aggregation of GNPs.Further,the CNTs acted as extended tentacles,providing better interaction or a higher contact area between Mg and hybrid reinforcement [206,207].Similar results were also observed with the hybrid reinforcement of GO+CNTs [129].
2.1.2.RamanspectroscopyofMg-CBNpowdermixture
Although several researchers have observed better dispersion of CBNs in the Mg matrix by BM,the harsh conditions in BM can destroy the structural integrity of CBNs.Wet mixing can also damage CBNs but to a significantly lower extent than BM.Raman spectroscopy has been extensively used to study the CBN damage to quantify the disorder insp2carbon materials [208–214].The Raman spectrum ofsp2carbon materials commonly exhibits two Raman active modes centered at ∼1580 and ∼2700 cm-1,called G and 2D-band (historically also called G′),respectively,where G denotes the ordered ‘graphitic’ structure [210].Additionally,three other bands called D,D′,and D+G,located at∼1350,∼1620,and ∼2940 cm-1respectively,are observed in the Raman spectra of disorderedsp2carbon materials,where D represents disorder or defect [212,213].The Raman spectrum of SWCNTs displays a 2D-band(∼2700 cm-1)similar to that of graphene.Despite the similarities,SWCNTs (diameter: 0.7-2 nm) show peculiar features compared to graphene,including radial breathing mode (RBM) at frequency ∼100-200 cm-1and splitting of the G-band into G+and G-peaks caused by the nanotube curvature (Fig.6(a))[213–217].However,the RBM,G+,and G-peak signals become weaker with increasing CNT diameter,and eventually the peaks broaden/smear out due to the diameter distribution within the individual DWCNT or MWCNT (Fig.6(b,c))[217,218].Thus,Raman spectra of MWCNTs and graphene look similar,and it may not be obvious to distinguish MWCNTs from graphene.The position of the D and 2D bands is dispersive and depends on the excitation laser energy,as shown in Fig.7(a) [212,219,220].

Fig.6.Comparison of (a) the Raman spectra of various SP2 carbon materials,from bottom to top: amorphous carbon,single-wall carbon nanohorns (SWNH),damaged graphene,SWCNT,highly ordered pyrolytic graphite (HOPG),single layer graphene;(b) RBM and (c) G-band splitting in Raman spectra of SWCNT,DWCNT,and MWCNT,showing the RBM,G+,and G- signals become weaker with increasing CNT diameter.(a) Adapted with permission from [213],Copyright 2010,American Chemical Society.(b,c) Reproduced with permission from [218],Copyright 2008,Materials Science-Poland.
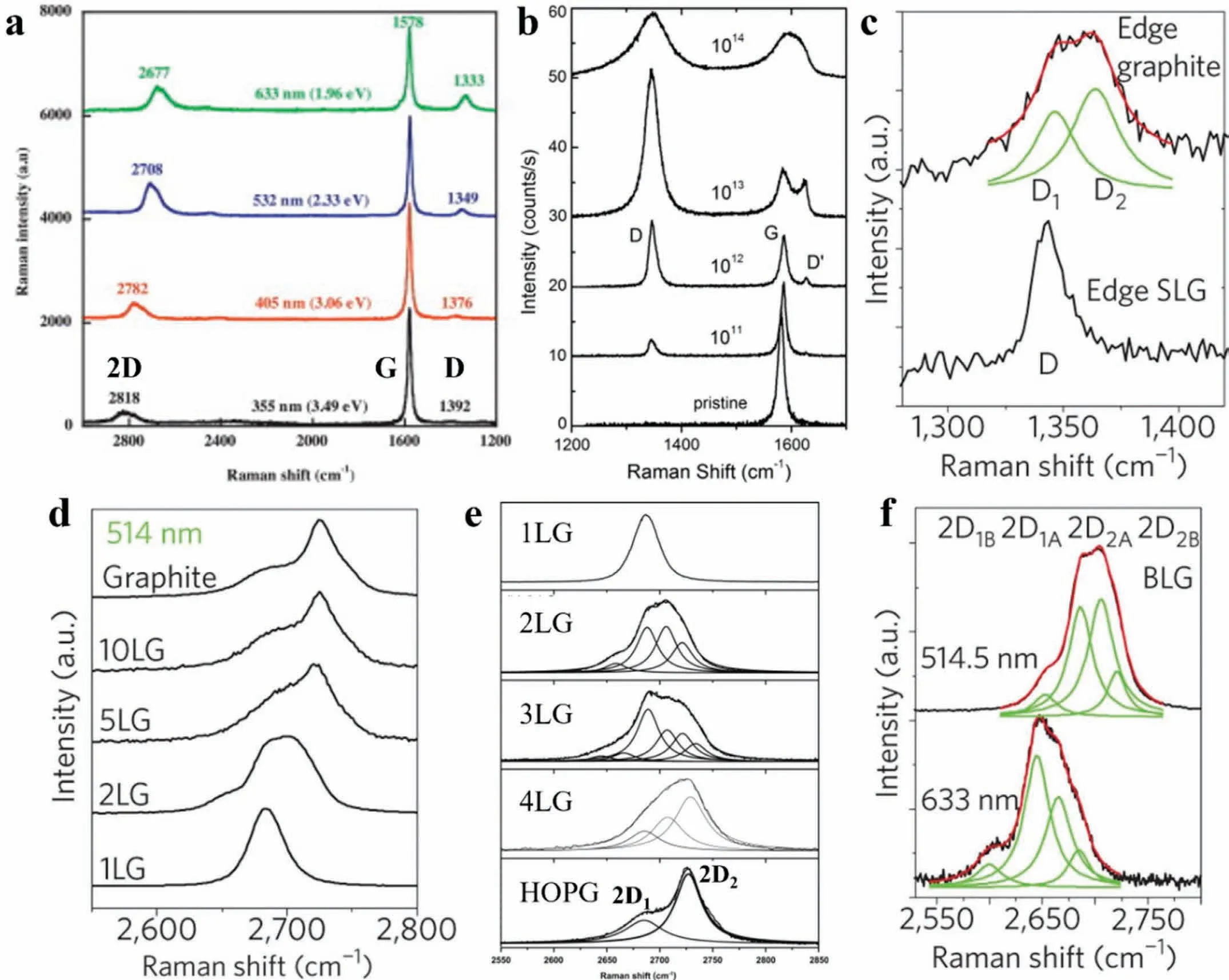
Fig.7.(a) Effect of excitation energy on Raman spectra of graphene,showing the upshift in D and 2D-band position with increasing excitation energy;(b)evolution of the D,G,and D′-bands of single-layer graphene (SLG) samples subjected to different ion doses (1011 to 1014 Ar+/cm2) of bombardment;(c)Raman spectra of graphite and SLG edges,showing the single D-peak at graphene edge,whereas graphite edge is composed of two peaks;(d) evolution of the 2D-band as a function of a number of layers,showing upshift in 2D-band position with an increasing number of layers;(e) Lorentzian curve fitting of 2D-band of graphene samples with the number of layers and highly oriented pyrolytic graphite (HOPG);(f) Lorentzian curve fitting of Raman 2D-band of bi-layer graphene (BLG) with the excitation wavelength,showing four Lorentzian components.(a) Adapted with permission from [212],Copyright 2014,Elsevier.(b) Adapted with permission from [221].Copyright 2010,Elsevier.(c,d,f) Adapted with permission from [222].Copyright 2006,American Physical Society.(e) Adapted with permission from [226].Copyright 2009,Elsevier.
The intensity of the D-band is proportional to the degree of disorder in the material,and theID/IGcan be used to quantify the disorder [216].Fig.7(b) shows the Raman spectra of single-layer graphene (SLG) samples subjected to different ion(Ar+)dose bombardments[221].The D-and D′-bands are absent in the Raman spectra of pristine graphene but re-appear in ion-bombarded (i.e.,defect-containing) samples.Further,theIDincreased with increasing bombardment doses from 1011to 1013Ar+/cm2,and a further increase in bombardment doses also caused peak broadening.Above 1014Ar+/cm2,the spectrum showed a decreasedID,indicating partial sputtering or full amorphization of graphene.It is interesting to note that the D-and D′-bands were absent in the Raman spectra of pristine graphene at its center (Fig.7(b)),but the bands appeared at its edge (Fig.7(c)) [222,223].Edges or sample boundaries are symmetry-breaking defects that can invoke D and D′-bands [224].Thus,theID′/IGgives insight into the number of edges that are present in the graphene [225].
Furthermore,the line shape of 2D-band provides information about the number of layers in graphene since graphene and other CBNs are susceptible to layer stacking (Fig.7(d)).For instance,single-layer graphene consists of a sharp singlepeak 2D-band,while bi-layer graphene (BLG) has a broader 2D-band spectrum,composed of four Lorentzian peaks such as 2D1B,2D1A,2D2A,2D2B(Fig.7(e,f)) [222,226,227].Likewise,tri-layer graphene requires at least six Lorentzian peaks to fit the 2D band.A further increase in the number of layers increases the number of double resonance (DR) scattering processes.Eventually,the line shape of the 2D-band converges to graphite,where only two peaks,2D1and 2D2,are observed (Fig.6(a) and Fig.7(e)) [213,226,227].Thus,the spectrum for more than five layers becomes difficult to distinguish from graphite.Another approach to determine the number of layers in the graphene is to use the value ofI2D/IG,full width at half maximum (FWHM),and position of the 2D-band.For example,the typicalI2D/IGvalue for monolayer,bilayer,trilayer,4-5 layers,and multilayer (5-10)graphene is >1.3,0.7-2.2,0.6-0.7,0.4-0.6 and <0.4,respectively.The FWHM of the 2D-band for monolayer,bilayer,and trilayer is in the range of 24-50,38-65,and 55-85,respectively[228].These quantitative indicators indicate that the 2D-band broadens,and its position upshifts with increasing layers in graphene,as shown in Fig.7(d) [222].
Zhou et al.[157] observed an increase inID/IGof CNTs from 0.27 to 0.29 with sonication,further increasing to 0.61 after BM.The results showed that sonication caused slight damage to the CNTs,due to the relatively low impact or shear forces than would be expected during BM.In another study,Song et al.[175] milled Mg+5 wt.% graphene at a speed of 400 rpm,45:1 BPR for 0.5 hr,and theID/IGof graphene increased from 0.728 to 1.255.Though the BM caused severe damage to the CBNs,it can also exfoliate graphite into graphene due to the relatively high shear forces [229].Thus,attempts have been made to combine the mechanical exfoliation of graphene and its dispersion in the Mg matrix by BM [174].Similarly,it has also been observed that BM can cut CNTs into shorter segments,and prolonged milling can destroy the tubular structure of CNTs altogether [230,231].Therefore,the BM parameters are crucial and must be optimized to achieve adequate dispersion of CBNs in the Mg matrix without destroying their structural integrity.For example,Zhou et al.[157] adopted a shift speed BM approach to fabricate AZ61+5SiC-CNT composites.This approach consisted of two steps: (i) low-speed ball milling (LSBM) at 150 rpm for 4 h,followed by;(ii)high-speed ball milling(HSBM)at 300 rpm for 1 h.The LSBM helped achieve uniform dispersion of the SiC-CNT mixture in the Mg matrix,and the good bonding between Mg and CNT/SiC was realized with HSBM.
2.1.3.ConsolidationofMg-CBNpowdermixture
The Mg-CBN powder mixtures have been typically consolidated by various PM techniques,broadly classified into pressureless sintering(PLS)and pressure-assisted sintering(PAS).In PLS,the powder mixture is pressed to form a green compact at room temperature and then sintered using microwave heating [152,232] or resistance heating [112,141].Whereas in PAS,the pressure and temperature are applied simultaneously during consolidation,and the resulting methods involve hot pressing (HP) [233,234],spark plasma sintering (SPS)[234–237],and hot isostatic pressing [238].Among these techniques,HP and SPS have been widely used to fabricate Mg-CBN composites.The simultaneous application of pressure and temperature enhanced densification by increasing the driving force for sintering by facilitating particle rearrangement and promoting diffusional atomic mobility during consolidation.Additionally,the applied pressure inhibited grain growth during sintering by decreasing the grain boundary mobility,resulting in a fine-grained microstructure [239,240].
The HP and SPS setup typically consisted of a uniaxial press coupled with a heating device.However,the sample was heated by a radiative heat transfer in HP,whereas SPS used the Joule effect (caused by a pulsed direct current) to heat the sample [241,242].In SPS,heat dissipation occurs at the particle boundaries,resulting in uniform heating and high heating rates (up to 600 °C/min) [237].Thus,fully densified compacts can be fabricated by SPS at lower temperatures and pressures with shorter sintering times than traditional techniques (PLS and HP).In some cases,secondary processing treatments,mainly extrusion[79]and rolling[121],have been performed on as-sintered materials to improve the dispersion of CBNs in Mg and reduce porosity in sintered compacts.Reports on the consolidation of Mg-CBN powder mixture by PLS and PAS processes are summarized in Tables 4 and 5,respectively.
Rashad et al.[52,78,84,111–114] have conducted many studies to fabricate Mg-CBN composites by PLS and extrusion.Also,Yuan et al.[123] fabricated AZ91-GNS(0.1,0.3,0.5,1.2 wt.%)composites by PLS followed by hot extrusion and T4 heat treatment.The composite with 0.5 wt.%GNS exhibited maximum improvement in yield strength(76.2%),UTS (55.8%),and microhardness (22.2%) compared to the base alloy.Islak et al.[252] have examined the effect of HPing temperature (450,500,550 °C) on Mg-5Al+CNT(0.25,0.5,0.75,1 wt.%) composites.It was observed that an increase in sintering temperature from 450 °C to 550 °C increased the relative density and hardness of all composites.It is well known that increasing sintering temperature improves the matrix/reinforcement interfacial bonding and interparticle bonding due to the higher diffusion rates,resulting in enhanced properties [259].However,higher sintering temperatures and longer sintering times can lead to abnormal grain growth that adversely affects the mechanical properties of the composites [260].Thus,sintering parameters are crucial and need to be optimized to use the full potential of CBN as a reinforcement in the Mg matrix.
Isaza et al.[261,262] have developed a sandwich approach using HP to fabricate Al-CNT composites.This approach was later adopted to develop Mg-CBN composites by HP,followed by rolling [263,264].In this approach,CNTs were first introduced into polyvinyl alcohol (PVA) solution using magnetic stirring and probe sonication.Then,the solution was dried to produce sheets of PVA reinforced with CNTs,followed by stretching these sheets to align CNTs in the stretching direction.Finally,Mg/Al and PVA-CNT composite sheets were alternatively stacked in a die followed by HP and rolled at 350 °C.The resultant composites exhibited good bonding between Mg layers without pores/voids at the interface of all composites,indicating the sandwich approach was successful in fabricating Mg-CNT composites.
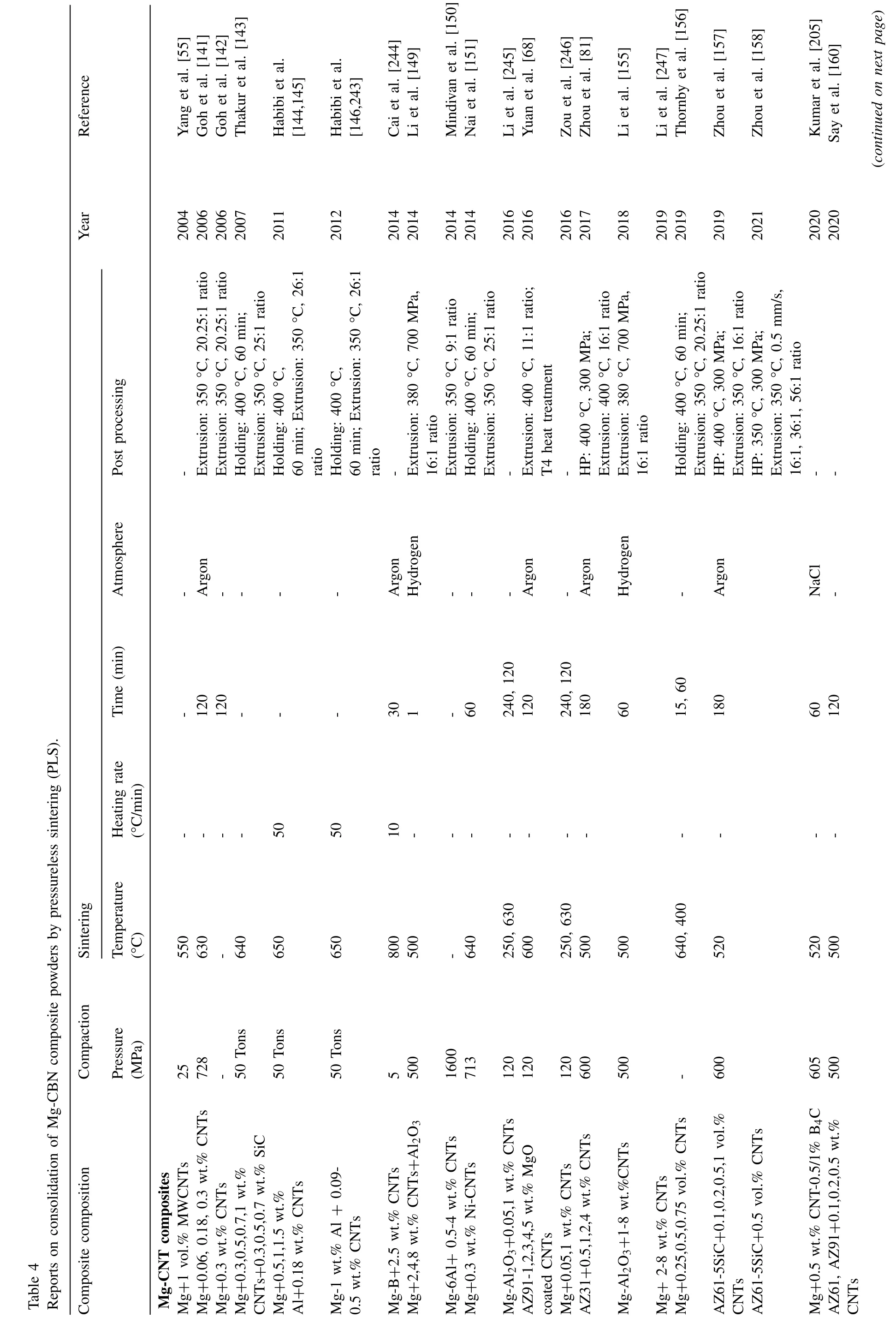
Meng et al.[121] tried a slightly different sandwich approach to fabricate Mg-0.25,0.75 vol.% GNPs.First,the GNPs were treated with H2SO4and HNO3(3:1 volume ratio),then the acid-treated GNPs were introduced into alcohol.The suspension (or slurry) of GNPs in alcohol was subsequently sprayed onto Mg foils.Then,the sprayed foils were stacked layer by layer in a die and HPed at 620 °C,50 MPa for 6h.Finally,the HPed composites were hot rolled to a total reduction of 80% (10% reduction per pass).The scanning transmission electron microscope (STEM) image of Mg-0.75 vol.% GNP composite captured with a high-angle annular dark-field (HAADF) detector,and the corresponding EDS maps are shown in Fig.8.Strong interfacial bonding was achieved between Mg and GNPs with no voids,as shown in Fig.8(a).EDS elemental mapping and HRTEM image further confirm the presence of Mg and GNPs at the interface (Fig.8(b,c,e)).EDS mapping also confirms the presence of O in the GNPs-rich region,indicating that the acid treatment successfully introduced O functional groups into GNPs(Fig.8(d)).Due to the robust interfacial bonding between Mg and GNPs,the composite with 0.75 vol.% GNPs exhibited a 31% improvement in UTS over pure Mg.
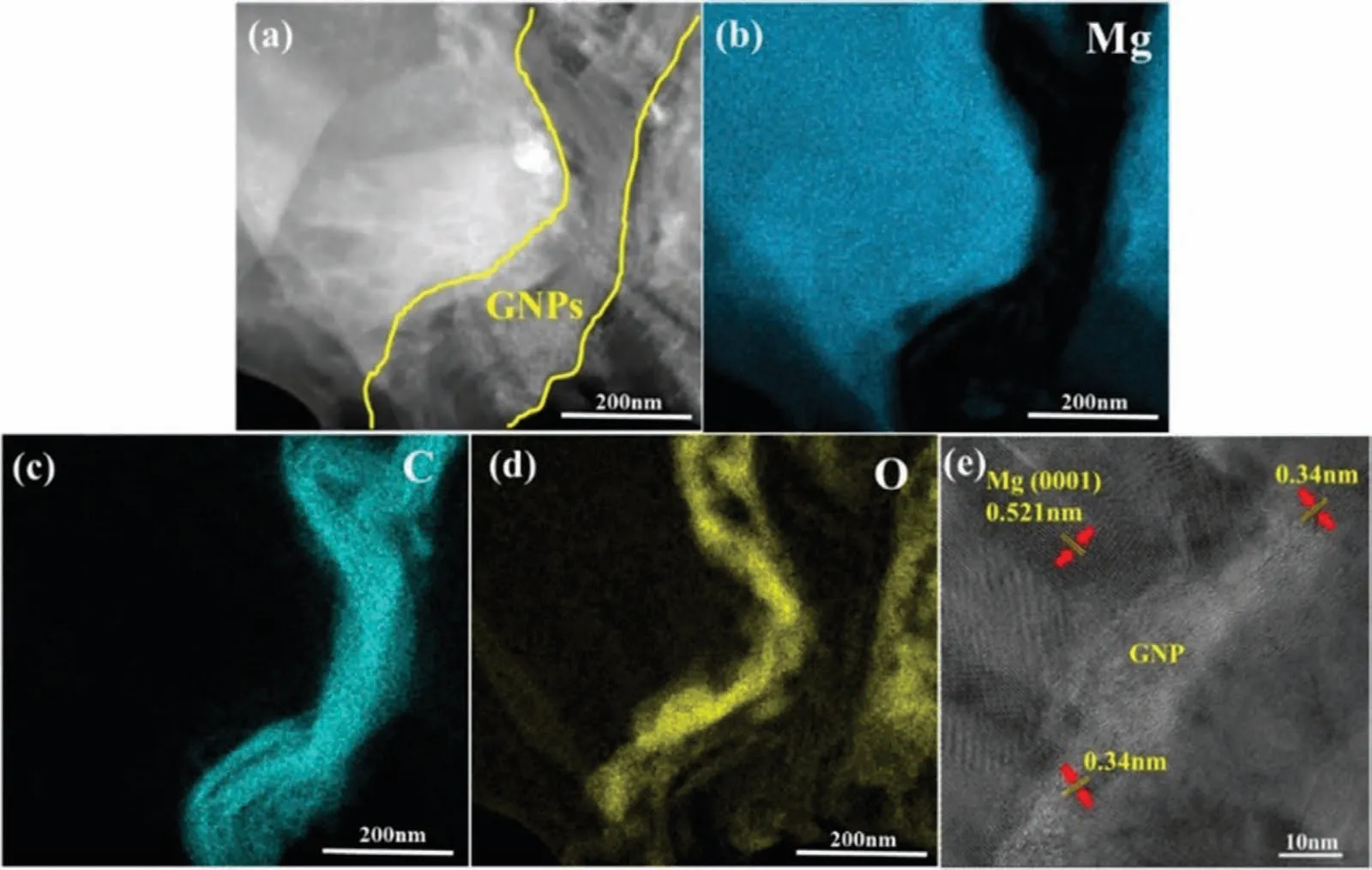
Fig.8.TEM analysis of Mg-0.75 vol.% GNPs composite fabricated by sandwich method followed by rolling: (a) STEM image captured with HAADF detector,showing the strong interfacial bonding between Mg and GNPs;EDS elemental mapping of (b) Mg,(c) C,(d) O;(e) HRTEM image of Mg/GNPs interface.Reproduced with permission from [121],Copyright 2018,Elsevier.
Yoo et al.[265] explored the fabrication of Mg-CNT composites through HP assisted by accumulative roll bonding(ARB).ARB is a severe plastic deformation (SPD) method invented by Saito et al.[266] in 1998.In the ARB method,two or more layers of material are rolled by a 50% reduction in thickness per pass.Then,the rolled sheets are sectioned into two halves,and the sectioned stripes are again stacked,followed by rolling.This process is repeated several times,resulting in the bonding of stacked sheets,thus forming a single bulk part [267].Recently,Zhang et al.[268] also fabricated Mg-CNT composites by ARB,in which the acid-treated CNTs were deposited on Mg plates by electrophoretic deposition (EPD).Then,the CNT deposited plates were stacked,and ARB was performed three times (50-60% reduction per pass) at 270 °C without the HP step.The microstructure of the roll-bonded composites showed a layered structure with uniformly distributed CNTs.
Li et al.[247] proposed a novelin-situsynthesis process to fabricate Mg-CNT composites.In this process,Mg-coated CNTs (CNTs@Mg) powders were synthesized via solid-state displacement reaction and then consolidated with the PM route (PLS followed by hot extrusion).An HRTEM analysis of these composites revealed the presence of nanoscale interfacial bonding contact and interfacial diffusion bonding(0.9 nm) between Mg and CNTs,indicating strong interfacial bonding in thein-situprepared composite.As a result,the 4 wt.% CNTs@Mg composite exhibited UTS of 33.4% and elongation of 31.3% higher than unreinforced Mg and 13.7%and 26% higher thanex-situprocessed composite with the same CNT content.Similarly,a study by Sun et al.[248]from the same research group demonstrated better mechanical properties with thein-situsynthesis of Mg-graphene composites thanex-situprocessed composites.Thein-situsynthesized CBNs formed finer structures (in size),fewer processing defects,less surface contamination,and were seen to be dispersed more uniformly in the matrix,resulting in stronger interfacial bonding.It should be noted that the tight interfacial bonding not only enhances the load transfer capability from the matrix to CBN but also decreases the electron or phonon scattering at the interface,leading to better mechanical strength and conductivity.
2.2. Casting
Casting or melt solidification is one of the most widely used routes in industries to manufacture components from composite materials,including a broad range of Mg alloys.The main advantage of casting is the capability to achieve the production of near-net-shape parts in a cost-effective manner.Additionally,casting processes lend themselves to manufacturing large parts of complex-shaped parts at high production rates,which is required by automotive and other consumerdriven industries.Many researchers have used casting technologies to fabricate Mg-CBN composites.Reports on the fabrication of Mg-CBN composites by casting route and key process parameters are summarized in Table 6.Generally,the casting of Mg-CBN composites includes the following steps:(i) melting of the Mg matrix,(ii) incorporation of CBNs into the Mg melt followed by stirring and/or sonication,and (iii)pouring of composite slurry into a mold followed by solidification under gravity or pressure.The following factors need considerable attention in preparing Mg-CBN composites by casting route: (i) achieving a uniform distribution of CBNs and (ii) attaining a good wettability between the Mg and CBN.These factors are influenced by the form or method of introducing CBNs into the Mg melt,dispersion technique,and casting process.CBNs are usually introduced into the Mg melt in the following forms: as-received [294,322],wrapped in Al foil [270],CBN-coated Mg chips [293,323],ball-milled Mg-CBN powder mixture [300],and composite master alloy or pre-form [279,292,316].Literature shows that primarily stirring [18,273] and ultrasonication [57,291] techniques have been used to mix CBNs in the Mg matrix.
It should be noted that the distribution of CBNs in the final cast composites can become non-uniform even when a uniform suspension is maintained in the slurry state.During the solidification of the Mg-CBN composite slurry,the solidification front can engulf or reject (push) CBNs,resulting in a kinetic redistribution of CBNs [329].The engulfing effect of the solidification front causes the CBNs to be distributed uniformly in the grains.Thus,the CBNs in the grain interior may act as heterogeneous solidification (nucleation) sites,resulting in a more refined microstructure.In contrast,the push effect forces CBNs to be clustered at the grain boundaries or dendritic regions,preventing adequate interfacial bonding between CBN and Mg and adjacent Mg grains,leading to poor mechanical properties [285].It has been reported that the dendrite size and the solidification rate affect reinforcement distribution in the final composite [330–332].
The studies on the solidification of MMCs suggest that smaller dendrite arm spacing (DAS) or a high solidification rate gives uniform distribution of reinforcement[285,331,333].At high solidification rates where the DAS is smaller than the reinforcement size,reinforcement is incorporated into the solid by geometrical trapping and becomes immobile,hence it does not result in segregation.In contrast,lower solidification rates lead to higher DAS due to decreased nucleation,resulting in reinforcement pushing and eventual clustering at the grain boundaries [334].Li et al.[285] investigated the effect of solidification rate on the CNT distribution and mechanical properties of resulting Mg-6Zn+1 vol.%CNT composites.The clustering of CNTs was observed when the solidification rate was low (Fig.9(a)),while CNTs were singly dispersed uniformly in grains under a high solidification rate (Fig.9(b)).As a result,the composite processed at higher cooling rates exhibited better tensile properties than those at lower cooling rates.This was attributed to the effective load transfer between Mg and CNTs caused by their good interfacial bonding in the composite processed at a high solidification rate (Fig.9(c)).Thus,it is necessary to control the solidification process to incorporate CBNs in grains and eliminate clusters in the Mg matrix.A change in the chemical potential of Mg melt due to the presence of CBNs,and thermal conductivity or heat diffusivity ratio of the Mg melt and CBNs also affects whether CBNs will be rejected or engulfed by the solidification front [335].Researchers have mainly used ultrasonic-assisted stirring [275–277],disintegrated melt deposition [278,313],and pressure infiltration casting [274,296]to manufacture Mg-CBN composites.Secondary processing of cast Mg-CBN composites was often carried out using extrusion to break the CBNs clusters,improving the distribution of CBNs in the Mg matrix and decreasing the composite’s porosity [158,303,317–319].
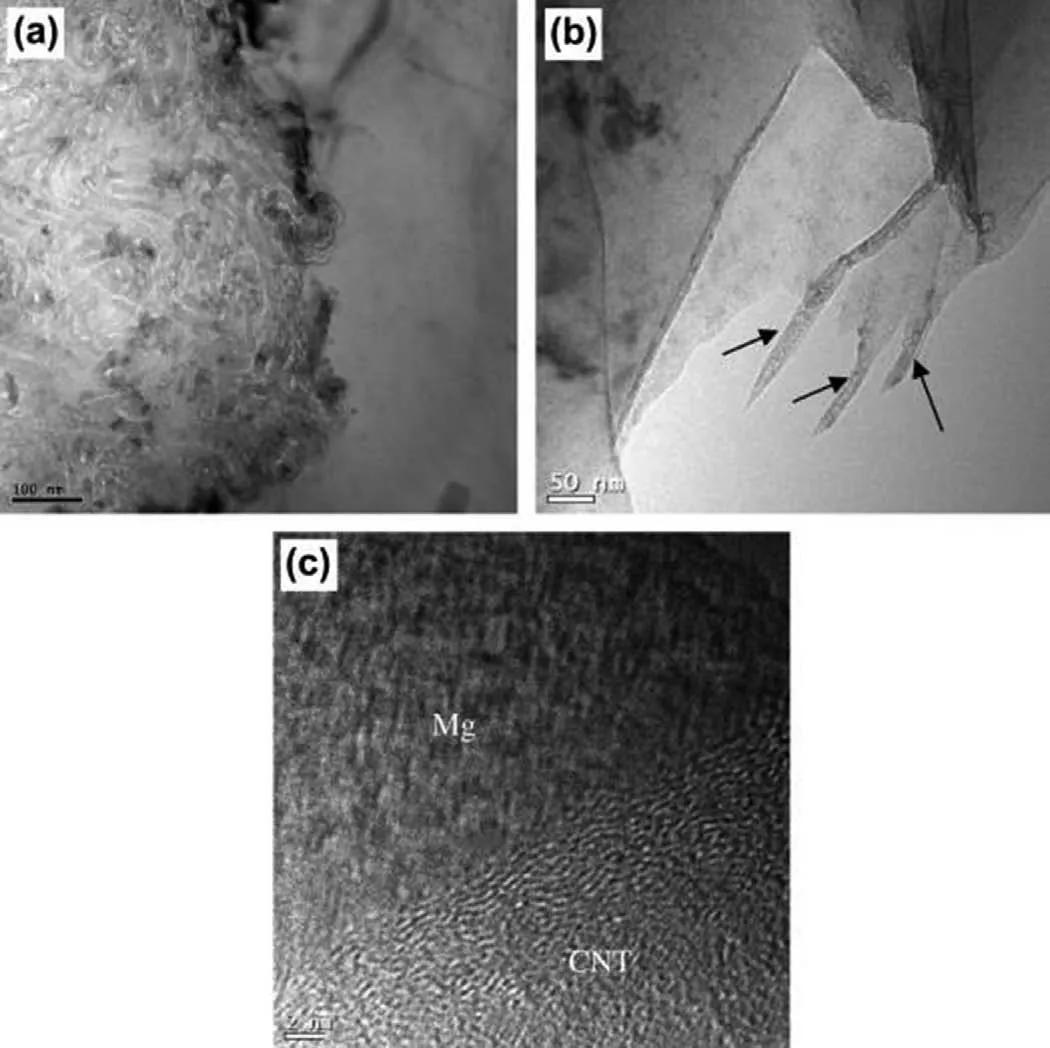
Fig.9.TEM images of the Mg-6Al+1 vol.% CNTs composite processed at:(a) lower solidification rate,showing the CNTs clusters;(b) higher solidification rate,showing individual dispersion of CNTs,which are indicated by the black arrows;and (c) HRTEM image showing the good interfacial bonding between Mg and CNT.Reproduced with permission from [285],Copyright 2014,Elsevier.
2.2.1.Ultrasonic-assistedstircasting(UASC)
Stir casting is commercially used to fabricate large-scale MMCs containing a uniform distribution of micron-sized reinforcement particles [336–339].In stir casting,a mechanically driven stirrer is used to create a vortex that helps distribute reinforcement in the molten matrix.However,mechanical stirring is inadequate to exfoliate or disperse nano-sized reinforcements (CBNs) in molten metal due to their large surface area,low wettability,and high van der Waals forces.Ultrasonic melt processing (USP) has proved to be an effective technique to deagglomerate and disperse nano-sized reinforcement particles (CBNs) in molten metal [57,291306].As discussed in Section 2.1.1.1,the intense cavitation and robust acoustic streaming generated during USP leads to exfoliation and uniform dispersion of CBNs in the Mg matrix.Also,USP has attracted significant interest from the industrial and academic communities as a promising technique for degassing [340–342] and grain refinement [343,344] of Al and Mg alloys.Despite its attractive benefits,including energy efficiency and environmental friendliness,achieving uniform distribution of reinforcement in large melt volumes with USP alone is challenging.Therefore,great efforts have been devoted to processing castings with a combination of stirring and USP to fabricate Mg-CBN composites to overcome individual shortfalls of these processes [292,293,310–312].
It can be seen from Table 6 that the majority of researchers have carried out stirring when the Mg melt is at a semi-solid or mushy state(usually ≤600°C),followed by USP in a fully liquid state before being poured into the mold [292,293,310–312].It is well known that the solid-liquid interfaces (dendrites)in a mushy state restrict CBN’s motion and arrest them between the dendrite arms [345,346].In addition,stirring the molten mixture in a semi-solid state can exert large forces on the CBNs,uniformly distributing CBNs in the Mg matrix without settling or floating.The USP of a semi-solid stirred mixture leads to better dispersion of CBNs in Mg melt due to the exfoliation or breaking of CBN clusters and enhanced CBN wettability [293,347–349].Another advantage of USP is the significant microstructural refinement caused mainly by the fragmentation of dendrites and enhanced heterogeneous nucleation under the action of cavitation while the melt is in a semi-solid state [344,347,350].Shi et al.[286] fabricated Mg-6Zn+0.5 wt.% CNTs composite by UASC,which showed a grain size refinement of 55% and UTS of 24.8%over an unreinforced matrix.Also,several other researchers have reported similar improvements in mechanical properties for Mg-CBN composites developed by UASC,indicating that UASC is an effective processing approach[275–277,283,316].
2.2.2.Disintegratedmeltdeposition(DMD)
DMD is a dynamic solidification process that combines the features of conventional casting and spray deposition processes,designed to circumvent each process’ limitations(mainly related to poor reinforcement dispersion and interfacial bonding) [10,351–353].DMD was developed by Gupta’s research group in the early 1990s to make Al or Mg matrix composites [354–357].In the DMD technique,the composite melt is superheated to a temperature of ∼750 °C,followed by mechanical stirring.Then the resultant composite slurry is released through a pouring nozzle which is located at the bottom of the crucible and disintegrated into fine dispersion of droplets by high-velocity jets of inert gas.The disintegrated composite droplets are subsequently deposited on a metallic substrate located at a certain distance from the pouring point,as shown in Fig.10 [358,359].Afterward,the obtained composite ingots were machined and extruded.Xiang et al.[313]fabricated Mg-0.1,0.25 wt.%GNP composites by DMD,followed by homogenization (at 400 °C for 4 h) and extrusion (at 350 °C with a ratio of 20.25:1).Mg-0.25 wt.% GNPs composite showed uniform distribution of GNPs,which predominantly occupied the grain interiors of Mg.The presence of GNPs in the grain interior was attributed to the engulfing effect of GNPs by the solidification front due to high solidification rates and dynamic solidification conditions realized during the DMD technique.The subsequent extrusion process was also seen to disintegrate GNP clusters further and improve GNPs distribution in the Mg matrix.Similar observations were also reported by Paramsothy et al.[270],where CNTs occupied both grain interiors and grain boundaries in AZ31 matrix composites fabricated by DMD and extrusion.
2.2.3.Pressureinfiltrationcasting(PIC)
In the PIC technique,the Mg melt is pushed into the preform of CBNs using either pressurized inert gas [55,321] or mechanical pressure exerted by a piston (up to 100 MPa)[274,280],which leads to infiltration of Mg melt into spaces in pre-form or mold,thus forming a composite material[360,361].Unlike stir casting,CBNs do not have the freedom to move during the PIC;thus,the floating or settling of CBNs can be avoided [362].Therefore,the distribution of CBNs in pre-form is crucial as it decides their distribution in the final composite,and care must be taken in preparing pre-forms.Usually,pre-forms for DMD have been prepared by the PM route.The first study on the PIC of Mg-CNT and Mg-graphene composites was conducted by Yang et al.[55]in 2004 and Cevik and Gundogan [321] in 2017,respectively.In a study by Yang et al.[55],the pre-form consisted of 25 vol.%Al2O3coated with CNTs prepared by CVD,and the resultant pre-form was used to make an Mg hybrid composite by PIC.It was found that the hybrid composite with CNTs exhibited ∼20% enhancement in high-temperature (227 °C) shear modulus over Mg-25 vol.% Al2O3composite.This improvement was attributed to well-dispersed CNTs in the Mg matrix.Park’s research group has done considerable research on the processing of Mg-CNT composites by PIC and demonstrated that PIC is a feasible technique for making Mg-CBN composites [272,274,280,289,290].Xiang et al.[310] fabricated Mg-6Zn composites reinforced with 0.7,1,and 1.6 vol.%graphene by the multi-step dispersion route,including WM(pre-dispersion),semi-solid stirring,ultrasonic processing,and pressure casting,followed by hot extrusion (Fig.11).The TYS and UTS of Mg-6Zn increased by 165% and 35%,respectively,with the addition of 1.6 vol.% graphene.The improved strength with higher plasticity in the composite was attributed to heterogenous bi-modal microstructure induced by well-dispersed and inhomogeneously distributed graphene.

Fig.11.Schematic illustration of the multi-step dispersion method used to fabricate Mg-6Zn alloy reinforced with graphene composites.Reproduced under terms of the CC-BY license from [310],Copyright 2016,Springer Nature.
2.3. Friction stir processing (FSP)
FSP is a solid-state material processing technique derived from friction stir welding invented by The Welding Institute(TWI),UK,in 1991 [363].FSP was developed by Mishra et al.[364] in 2000 to refine the microstructure of 7075 Al alloy.In general,FSP consists of a non-consumable rotating tool with a specially designed pin at the bottom of its shoulder.During FSP,the tool is rotated at high speed while an axial force plunges the pin into workpiece until the shoulder contacts the workpiece and traverses in the desired direction.The workpiece in the processing region plasticizes and softens under the action of frictional heating.Due to the tool’s simultaneous linear and rotational motion,the softened material flows around the pin from the adjoining side to the trailing side,which leads to material mixing.Usually,the tool is held at an angle(0.5-3°)to the workpiece,providing forging action at the trailing edge of the shoulder [365].
The synergistic effect of severe plastic deformation,frictional heat generation,and material mixing during the FSP process leads to refinement and homogenization of the stirred microstructure [366,367].It is well known that the grain refinement of Mg during FSP is mainly due to dynamic recrystallization (DRX).Researchers have reported three recrystallization mechanisms for Mg during FSP: continuous DRX,discontinuous DRX,and twinning-induced recrystallization [368–372].The predominant recrystallization mechanism during FSP depends on temperature,degree of deformation,and deformation rate [373].The mixing of materials during FSP offered the possibility of introducing reinforcement particles into the processed zone,thus making a composite.Researchers have tried different approaches to incorporate reinforcement particles in the workpiece during FSP,such as groove filling [374],drill hole filling [375],surface coating [376],sandwich [377],and direct friction stir processing (DFSP) tool method [378].In groove and drill hole filling approaches,several grooves or holes were machined on the workpiece surface,then filled with reinforcement particles.In the surface coating method,the reinforcement particles were mixed with a volatile solvent and coated on the workpiece surface,while the particles were placed between the sheets or plates of a workpiece,followed by FSP in the sandwich approach.Contrary to all these approaches,reinforcement particles were not incorporated into the workpiece prior to processing in the DFSP approach but were introduced during FSP through a hole designed within the rotating tool.To prevent scattering of inserted reinforcement particles during FSP,grooves or holes filled with particles are sealed either by a thin sheet or a capping pass with a pin-less tool[374,379,380].
The first study on MMCs fabrication using the FSP technique was also by Mishra et al.[381] in 2003,where Al-SiC surface composites were fabricated successfully by FSP through a surface coating approach.Later FSP was adopted to make various MMCs[382],and the first study on FSP of Mg-CNT composites was by Morisada et al.[383] in 2006.Their study showed a ∼42%improvement in hardness of AZ31 with the addition of CNTs.To the best of the authors’ knowledge,the earliest report on Mg-graphene composites by FSP was published by Arab and Marashi [384] in 2019.Literature on the processing of Mg-CBN composites by FSP and the key processing parameters are summarized in Table 7.It can be seen in Table 7 that researchers used either the groove or holefilling approaches to fabricate Mg-CBN composites.The FSP parameters (tool tilt angle,rotation speed,traverse speed) and the pin’s geometric characteristics (size,shape) have a significant effect on heat generation,working volume,and material flow,which in turn influenced the reinforcement distribution in the matrix,microstructure,and properties of the composite[379].
Arab et al.[388]fabricated AZ31-CNT composites through the groove filling method,where CNTs were filled into the grooves,followed by a capping pass with a pinless tool and then filling passes with a pin.They investigated the effect of pin shape,number of FSP passes,and speed ratio (rotational over the traverse speed) on the composite’s microstructure and tensile properties.It was observed that the tool with the stepped pin (square or cylindrical) refined the microstructure effectively in comparison to the cylindrical pin without steps.Each step in the pin acted as a shoulder to the one below,and material flow in the stepped channel was transported downward,averting material from flashing out.Furthermore,the additional downward force provided by steps in the pin enhanced the movement of plasticized material around the pin,leading to enhanced mixing of CNTs.Also,the vibratory and shattering effect caused by the stepped pins dispersed CNT clusters in the matrix,which provided grain refinement via additional nucleation sites and inhibited grain growth.According to the Zener equation (1) [397],for a given reinforcementcontaining material,the resultant grain size of the composite greatly depends on reinforcement size and its content.Thin CNTs exert higher Zener pinning (drag) forces than thicker CNTs;thus,the tiny CNTs formed during FSP effectively impede grain growth by pinning the grain boundaries.Suárez et al.[398] observed a decrease in grain size of Ni from 47.6 to 4.9 μm upon adding 3 wt.% CNTs,but the addition of CNTs above 3 wt.% led to an increase in the grain size to 5.3 μm,which was attributed to CNTs’ re-agglomeration at higher fractions.Re-agglomeration of CNTs leads to increased size and interparticle spacing,thus decreasing grain boundary pinning ability.In addition to reinforcement size,content,and distribution,reinforcement morphology also significantly affects grain growth rates in composite [399].
WhereRc: Zener limit or critical radius of a matrix,K:proportionality constant,r: radius of reinforcement,f: volume fraction of reinforcement,andm: exponent forf.
Arab et al.[388] observed higher grain refinement when the composite was processed for more FSP passes at lower speed ratios.The increase in FSP passes caused more accumulated strains and promoted further DRX and grain refinement.Also,the fragmentation of CNT clusters and their distribution improved with increasing FSP passes,forming a finer microstructure.On the contrary,Rayes and Danaf[400]observed grain coarsening with increasing FSP passes in Al 6082 alloy.It was attributed to the extra thermal cycles that the alloy experienced and the simultaneous occurrence of continuous DRX with each FSP pass.More grain refinement at lower speed ratios was explained by equation (2),showing the relationship between peak temperature (Tp) in the stir zone,melting temperature of a workpiece (Tm),rotational speed(ω),and traverse speed (ʋ) [401].Equation (2) indicates thatTpincreases with either increasingωor decreasing ʋ due to increased frictional heat.Thus,higherωand lower ʋor higher speed ratios (ω/ʋ) lead to higher peak temperature in the stir zone and longer exposure time,consequently resulting in coarser grains and poor mechanical properties.
Morisada et al.[383] studied the effect ofυ(25,50,and 100 mm/min) on CNT dispersion in the AZ31 matrix.Uniform distribution of individual tubes was observed at a lowerυof 25 mm/min and of CNT clusters at 50 and 100 mm/min.The presence of CNT clusters in samples processed at higher traverse speeds was mainly due to insufficient heat flow orTpcaused by the short exposure times.Similar findings of increased grain size with the increasingωor decreasingυwere also observed by Mahallawy et al.[402] and Rayes and Danaf [400].In contrast,Sharma et al.[391] reported a reduction in the grain size of AZ31+0.3 vol.%GNP+1.6 vol.%CNTs composite with an increase inω.This discrepancy may be due to the material composition,tool geometries,and other FSP parameters.Thus,along with a suitable tool,a good balance of FSP parameters is needed to obtain a uniform dispersion of CBNs and maximum grain refinement in Mg-CBN composites.
WhereTmis the melting temperature,K,andαare constants.
2.4. Selective laser melting (SLM)
SLM is an additive manufacturing process invented in the mid-1990s by Dr.D.Schwarze and Dr.M.Fockele of the F&S Stereolithographietechnik GmbH,Paderborn and Dr.W.Meiners and Dr.K.Wissenbach from the Fraunhofer ILT (Institute for Laser Technology),Aachen,Germany [403,404].SLM is also known as the powder bed fusion process,which uses a high-intensity laser beam to melt and consolidate selective regions of powder in a layer-by-layer fashion according to the 3D computer-aided design (CAD) model [404].In SLM,the 3D CAD model of a final product is first created using software (AutoCAD,CATIA,SolidWorks,etc.).The final CAD model is then converted to STL (stereolithography)format,compatible with the SLM machine [405].The conversion involves the tessellation process of forming several triangular facets of XYZ.The STL files are further processed to generate slice data,where the individual facets are converted to respective line segments.Later,these line segments are connected to create 2D contours (thickness of 30–100 μm),where each contour represents physical layers that the SLM machine prints [406,407].Next,the 2D sliced model is uploaded to the SLM machine control computer,followed by a thin layer of powder deposition on a substrate plate in an inert gas-filled build chamber.The laser beam,controlled by an f-θlens and X-Y scanning mirrors,melts the selected powder areas and forms the first layer.Then,the substrate plate lowers a certain distance (next layer thickness),followed by powder deposition and laser scanning.These processing steps are repeated until all layers of the component are printed [408].The schematic illustration of the typical SLM setup is shown in Fig.12.

Fig.12.Schematic illustration of a typical SLM setup.Reproduced with permission from [408],Copyright 2018,Elsevier.
The SLM process has recently attracted significant attention from the research community and has been intensively studied to develop MMCs.This is mainly because SLM processing of composites minimizes reinforcement agglomeration compared to casting,as the feedstock in SLM is powder.The SLM technique also facilitates the production of near-net-shaped composites with high density,avoiding postprocessing such as machining.Furthermore,SLM involves high heating and cooling rates,resulting in a more refined microstructure [408,409].SLM technique has been used to fabricate various MMCs based on Al,Co,Fe,Ti,and Ni[410,411].Research on the production of Mg-based composites by SLM is still at an early stage,and only a few research articles have been published [412–416].To the best of our knowledge,the first attempts at SLM of Mg-CNT and Mg-graphene composites were made by Wu et al.[412] in 2018 and Shuai et al.[415,416] in 2020,respectively.The main challenges with SLM of Mg and its alloys were identified as: (i) oxidation of Mg powder and (ii) the evaporation of Mg.Mg has a high affinity for oxygen,which causes the rapid formation of MgO during SLM,even at meager oxygen contents.The oxide subsequently reduces the bond strength between powder particles,thus inhibiting their coalescence and part densification [417].The oxidation of Mg can be minimized by purging the SLM chamber with inert gas,the addition of rare earth (Be) [418],passivating (Al,Zn) [419],and transition elements (Cu,Fe,Mn,Ni) to the powder blend[417].
The evaporation of Mg is caused by its low laser absorption energy or high reflectivity,high vapor pressure,and narrow temperature range between melting (650 °C) and boiling point (1090 °C) [417].Thus,high-energy inputs are required to melt the Mg powder completely and obtain high-density components.However,after the Mg powder melts,the melt pool absorbs higher laser energy than the powder layer,causing the melt pool to reach the boiling point quickly.In addition,the evaporation of Mg powder is accelerated by the increase in vapor pressure from 0.13 kPa (620 °C) to 51 kPa (1027 °C) [420].The evaporation of Mg leads to defects(pores,hot cracks,etc.) and flocculent deposits (cauliflowershaped grains[420])on the SLM-scanned powder layer[421].Previous studies have indicated that adding suitable alloying elements to Mg extends the range between melting and boiling point [417,422,423],thereby avoiding Mg vaporization during SLM.Wu and Wang [412] fabricated AZ31BCNTs composite by SLM and addition of 1.5 wt.% CNTs enhanced laser absorption energy of AZ31B by 7.9%.As a result,the complete melting of AZ31B powder was obtained at a low laser input energy density of 42 J/mm3.Rodríguez et al.[424] also observed an improvement in the laser absorption energy of Al with CNT addition,which was mainly attributed to the high laser absorptivity of CNTs caused by their large surface area to volume ratio.
Shuai et al.[415] fabricated AZ61+MgO-coated reduced graphene oxide (rGO;1,2,3,4 wt.%)composites by SLM.The hardness and compressive strength of AZ61 were enhanced by ∼27% and 30% with the addition of 4 and 3 wt.% CNTs,respectively.This improvement in the mechanical properties of AZ61 was attributed to grain refinement and the strong interfacial bonding between Mg and rGO provided by the MgO.The grain size of the AZ61 decreased from 20.3 to 8.4 μm with 4 wt.% CNTs addition,corresponding to a 58.6%refinement over unreinforced AZ61.Furthermore,MgO/rGO interfaces were indiscernible and showed a distortion area and nanoscale contact,indicating the strong interfacial bonding of MgO/rGO.Moreover,a semi-coherent structure was observed betweenα-Mg (1102)//MgO (200),and a planar distregistry of 7.5% was detected.Semi-coherent structures are known to be associated with strong interfacial bonding between the matrix and reinforcement.Raman spectra of SLMed composites showed the presence of D and G-bands,and there was no significant change in theID/IGratio,indicating the rGO did not undergo any structural changes during SLM.This study showed that the MgO coating acted as an interfacial bridge and enhanced the interfacial bonding between Mg matrix and rGO.
In another study by Shuai et al.[416],AZ61+TiO2-coated GO composites were made by SLM,where TiO2was coated on the GO via hydrothermal reaction.It was observed that TiO2was reduced by a magnesiothermic reaction during SLM.Then,the reduced Ti reacted with Al in AZ61 and C in GO,resulting in interfacial compounds TiAl2and TiC.Due to synergistic strengthening by grain refinement and good interfacial bonding (caused by MgO,TiAl2,and TiC)in AZ61-TiO2/GO composite,the hardness and compressive strength of AZ61 improved by 16.5% and 10.1%,respectively,compared to AZ61-TiO2-GO composite (without hydrothermal reaction).Similar results were also reported by Tao et al.[425],where ZK30-0.9 wt.% GO composite prepared by SLM showed ∼19% higher hardness over unreinforced matrix.These preliminary studies demonstrated that SLM is a potential process for fabricating Mg-CBN composites.However,further research is needed to understand CBNlaser interactions and the effect of CBN on the microstructural evolution and strengthening in SLMed Mg-CBN composites.
3.Properties of Mg-CBN composites
Generally,Mg has been reinforced with CBNs to improve its mechanical,tribological,corrosion,and biomedical compatibility.The various properties of Mg-CBN composites are summarized in Table 8.The summary table shows that the addition of CBN significantly improved the properties of Mg.However,it should be noted that the fabrication of Mg-CBN composites with low porosity is crucial to obtain improved properties,especially in the PM route,since the PM processing of composites is performed in a solid state.The porosity of Mg-CBN composites produced by PLS (conventional and microwave sintering) and PAS (HP and SPS) as a function of CBN content is presented in Fig.13.The porosity levels in PLSed composites are high (∼13.5%) compared to PASed composites (∼4%).It is well known that the simultaneous application of pressure and temperature in PAS techniques results in an enhanced consolidation with lower porosity.

Fig.13.The porosity of Mg-CBN composites fabricated by pressureless and pressure-assisted sintering.The data points for this plot were taken from articles mentioned in Table 8.
Usually,researchers have used a CBN content of ≤1 wt.%in the PM route.Increasing the CBN content generally increases porosity in MMCs,which has been attributed to the agglomeration of CBNs and the large melting point difference between the metal matrix and the CBN [47].The presence of high melting point CBN at the grain boundaries of Mg limits atomic diffusion in the PM processing,resulting in reduced sinterability and ultimately leading to a higher porosity.Similarly,in the casting route,the increased porosity with CBN addition is caused by the poor wettability of CBNs by liquid Mg.Regardless of the metal matrix,porosity has shown adverse effects on the mechanical,thermal,electrical,and tribological properties of MMCs [150,426].Therefore,in addition to the uniform distribution of CBN,the fabrication of Mg-CBN composites with a lower porosity is essential to achieve the expected properties.This section discusses the effect of CBN addition on various properties of Mg and its alloys.
3.1. Mechanical properties
Most studies on Mg-CBN composites have investigated the effect of CBN on the mechanical properties of Mg.The maximum improvement in various room temperature mechanical properties of Mg-CBN composites reported in individual studies is presented as a function of CBN content in Fig.14(a-f).The absolute value of Mg-CBN composite’s mechanical properties is also shown as an inset in respective plots (Fig.14).The highest improvement in room temperature mechanical properties of Mg with CBN addition reported so far in literature is as follows: hardness of 78% [57],EMTof 89%[113],TYS of 165%[310],UTS of 76%[322],CYS of 426%[164],and UCS of 209%[159].These significant levels of improvement in mechanical properties were attributed to: (i) the uniform distribution of CBN in the Mg matrix via melt ultrasonication [57,310] and (ii) good interfacial bonding caused by adding Cu[113]or coating CBNs with Ni[159,164].Limited research has been carried out on the high-temperature mechanical properties of Mg-CBN composites.Early studies show a great improvement in the mechanical properties of Mg with CBN addition,even at elevated temperatures [274,296].It should be noted that available literature points to an optimum CBN content at which the composite’s properties exhibit the greatest improvement,while above this level deterioration in mechanical properties occurs due to CBN agglomeration.The optimum CBN content reported in individual studies is presented in Fig.15.It can be seen that the optimum CBN content varied between 0.05 wt.% to 5 wt.%,but the majority of the studies reported optimal values less than 2 wt.%.
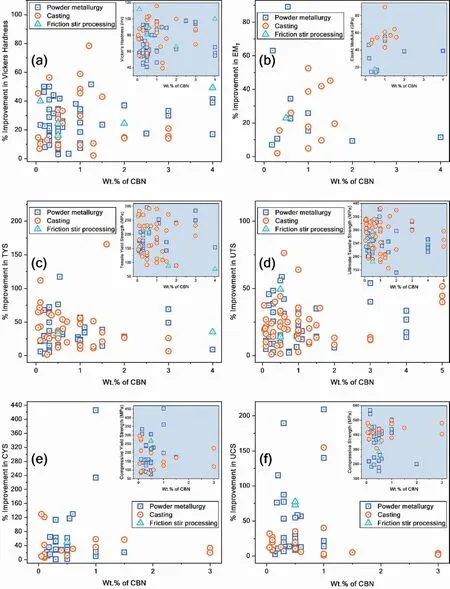
Fig.14.Percentage improvement in various room temperature mechanical properties of Mg-CBN composites at optimum CBN content reported in the individual studies: (a) Vickers hardness,(b) EMT,(c) TYS,(d) UTS,(e) CYS,and (f) UCS.Inset shows the absolute value of mechanical properties.The data points for this plot were taken from articles mentioned in Table 8.
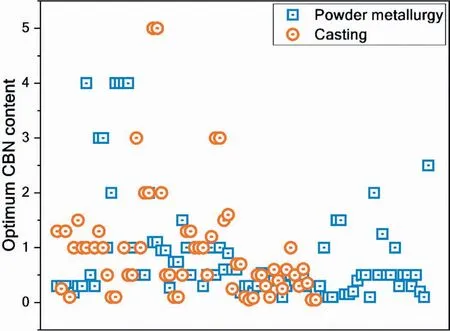
Fig.15.Optimum CBN content reported in individual studies summarized in Table 8.
The following mechanisms were identified to contribute to the strengthening of Mg-CBN composites: (i) load transfer,(ii) dislocation,(iii) Orowan,and (iv) grain refinement strengthening mechanisms [78,157,248,254].Researchers have observed bridging/pulled-out CNTs (Fig.16(a)) and GNPs (Fig.16(b)) in the tensile fracture surface,indicating strengthening by the load transfer mechanism in Mg-CBN composites.Fig.16(d) shows the dislocations at the Mg/GNPs interface shown in Fig.16(c).These dislocations were generated due to the strain caused by a mismatch in thermal expansion or modulus between the Mg and GNPs.The generated dislocations can restrict the motion of other dislocations leading to improved strength of the composite.The grain size of Mg-CBN composites fabricated by various techniques as a function of CBN content is presented in Fig.17(a).It shows that the grain size of Mg decreased with CBN addition,suggesting grain refinement strengthening in Mg-CBN composites.The average grain size of Mg and Mg-CBN composites processed by PM was 32 μm and 20 μm,casting was 73 μm and 33 μm,and FSP was 11 μm and 4 μm,respectively(Fig.17(b)).It can be seen from the inset in Fig.17(a)that the highest grain refinement in Mg grains with the addition of CBN was ∼87% [52],∼95%[326],and ∼78% [390] in PM,casting,and FSP techniques,respectively.Researchers often quantified individual contributions of the abovementioned mechanisms in strengthening metal-CBN composites using theoretical models [19,47,72].

Fig.16.SEM image of the tensile fracture surface of (a) Mg-1.5 wt.% GNPs and (b) Mg-4 wt.% CNTs composite,showing the bridging and pulled out CBNs;(c) TEM image of Mg-1.5 wt.% GNPs,showing the interfacial structure between Mg and GNPs,and (d) Fourier filtered image of region b in Fig.(c),showing the presence of dislocations at the interface between Mg and GNPs.(a) Adapted with permission from [247],Copyright 2019,Elsevier.(b-d)Adapted with permission from [248],Copyright 2020,Elsevier.

Fig.17.(a) Grain size of Mg-CBN composites at optimum CBN content reported in the individual studies,inset shows the percentage grain refinement,and(b) average grain size of Mg and Mg-CBN composites processed by various techniques.The data points for these plots were taken from articles mentioned in Table 8.
The strengthening efficiency of CBNs (CNTs and graphene) in Mg matrix composites under tensile and compressive load is presented in Fig.18(a,b).The strengthening efficiency of CBNs can be determined using Eq.(3) [60,247].It can be seen from Fig.18(a,b) that graphene’s strengthening efficiency is higher than CNTs under both tensile and compressive loads.Graphene with sheet morphology and a high specific surface area of graphene (2630 m2/g [32]) provides a large contact area with the matrix compared to tubular CNTs (single-wall: 1315 m2/g,multi-wall: 50-850 m2/g[30]).Thus,graphene offers a good interface with sufficient load transfer between matrix and reinforcement,leading to better strengthening over CNTs.Also,dislocations are known to tangle or accumulate during plastic deformation when composites are subjected to tensile loading,resulting in plastic strain fields around graphene.Xiang et al.[310] calculated the strain fields around the CNTs and graphene using the shear lag model.For the same amount of CNTs and graphene,larger plastic strain fields can be generated near the edges of graphene than CNTs,as graphene has a larger edge area than CNTs.The larger plastic strain fields around graphene can hold higher applied loads,indicating higher load transfer efficiency.Therefore,graphene exhibits a higher strengthening efficiency compared to CNTs.
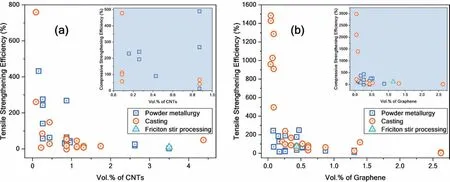
Fig.18.Strengthening efficiency of CBNs at optimum CBN content reported in individual studies (a) CNTs and (b) graphene in Mg-CBN composites under tensile load.Inset in Fig.(a) and Fig.(b) show the compressive strengthening efficiency of CNTs and graphene,respectively.The tensile and compressive yield strength of Mg and Mg-CBN composites were taken from reports mentioned in Table 8.
Whereσcandσm: TYS or CYS of composite and matrix,respectively andVf: volume fraction of CNTs or graphene.
The CBNs in as-cast composites showed higher strengthening efficiency than in PM and FSPed composites,which can be attributed to better interfacial bonding and low porosity in cast composites.It is worth noting that the strengthening efficiency of CBNs decreases at higher loading content,which was attributed to a reduction in the effective content of CBN participating in the strengthening process caused by the CBN clustering.There is a large scatter in the mechanical properties and CBNs strengthening efficiency in Mg-CBN composites,as can be seen in Fig.14 and Fig.18(a,b),respectively.This can be due to using CBNs with varied sizes and surface areas to make Mg-CBN composites,as summarized in Table 8.However,it can be observed that thinner CNTs and graphene with a lower number of layers exhibit higher strengthening efficiency[47,60,107].The weak van der Waals forces between graphene layers or between the walls of nanotubes allow easy sliding,leading to a decrease in loadbearing capacity with an increase in the number of layers in graphene or CNTs.Munir et al.[60] observed higher compressive strength and strengthening efficiency using coarser graphene (15 μm) with a smaller thickness (5 nm) than using finer graphene(5 μm)with thicker graphene(9 nm)at the same content.This indicates that graphene’s thickness plays a crucial role in improving the mechanical properties of metal matrices compared to its size.
In addition to the above-mentioned mechanical properties,a few studies reported Mg-CBN composites’ creep [156,296]and fatigue behavior [269,282].Thornby et al.[156] assessed the creep behavior of Mg-CNT composites using a nanoindentation test at room temperature and 0.5 vol.% CNT composite exhibited higher creep resistance than monolithic Mg.The dominant creep mechanism in Mg and its composites at ambient temperature was identified as a dislocation-controlled creep through dislocation glide.In another study,Park et al.[296] observed decreased minimum creep rate in hybrid composite (AS52-20 vol.%ABw+5 vol.% CNT) at 150 °C compared to 25 vol.% ABw(aluminum borate whisker) composite,indicating CNTs enhanced the creep resistance.Srivatsan et al.[282] observed a 40% higher endurance limit for 1 vol.% CNT composite over the unreinforced AZ31 matrix.In contrast,Goh et al.[269] reported deterioration in fatigue resistance by adding 1.3 wt.% CNT,attributed to the presence of voids in the composite caused by CNT clusters.
3.2. Corrosion and biocompatibility properties
Mg and its alloys have emerged as a promising material for bone-tissue applications owing to their tunablein-vivodegradation,eliminating the need for secondary surgeries.In addition,the density (1.74-2.0 g/cm3),elastic modulus (41-45 GPa),and fracture toughness (15-40 MPa.m1/2) of Mg are closer to those of natural bone (1.8-2.1 g/cm3,3-20 GPa,3-6 MPa.m1/2,respectively) compared to other conventional biomaterials,which can alleviate the stress shielding [4].Despite these well-matched properties,Mg’s high degradation rate and inadequate strength can cause premature implant failure,limiting its extensive application in load-bearing implants.The degradation of Mg-based implants can be explained by reaction 4,which produces a large amount of H2gas at the implant region.The accumulation of H2gas at the implant region can delay tissue or bone healing [427] and block the bloodstream [428].Therefore,pure Mg is generally alloyed with various alloying elements such as Al,Ca,Ce,La,Mn,Nd,Sr,Zn,and Zr to modify the mechanical properties and corrosion resistance[4,429].However,these alloys exhibit uneven degradation,and some alloying elements (e.g.,Al) can release undesired metal ions,potentially causing health problems.Furthermore,the electro-potential difference between Mg and alloying elements or intermetallic phases can trigger micro galvanic corrosion,further increasing the degradation rate.Mg-based composites with various reinforcements,such as Al2O3[430],HA (hydroxyapatite) [431],TiO2[431],MgO[432],ZnO [433],etc.,have also been explored to improve the corrosion resistance of Mg.However,galvanic corrosion between Mg and reinforcement can occur,so proper reinforcement selection is crucial to achieve high corrosion resistance.
In addition to the reinforcements mentioned above,researchers have recently attempted to introduce CBNs to Mg matrices and study their effect on biocompatibility,corrosion,and biological properties.However,limited research has been done on these properties of Mg-CBN composites,as summarized in Table 8.Say et al.[160] fabricated AZ61 and AZ91 Mg matrix CNT (0.1,0.2 and 0.5 wt.%) composites by PLS route and investigated the corrosion behavior of composites in 3.5% NaCl aqueous solution by potentiodynamic scanning test.Composites with 0.2 wt.%of CNTs exhibited the highest corrosion resistance in both alloys and increased CNT content to 0.5 wt.% deteriorated the corrosion resistance.Similar observations were also seen in AZ61/AZ91-graphene composites,where maximum corrosion resistance was observed with 0.2 wt.%graphene and decreased at 0.5 wt.%graphene [182].Many researchers have observed improvement in the corrosion resistance of Mg at lower concentrations of CBN and degradation at higher concentrations [82,117,118,182].The deterioration of corrosion resistance of composites at higher CBN concentrations was attributed to the galvanic corrosion and accelerated by the presence of voids,micropores,and cracks.The defects can easily absorb the corrosion solution;thus,higher porosity in composites leads to an increased corrosion rate [61].These results indicate that porosity plays an important role in the corrosion resistance of Mg matrix composites.
Munir et al.[60] fabricated Mg-graphene (0,0.1,0.2 and 0.3 wt.%) composites with two different graphene systems,G15 (particle size: 15 μm,thickness: 5 nm) and G5 (particle size: 5 μm,thickness: 9 nm) by BM followed by PLS.The degradation behavior of composites was investigated in Hank’s solution using electrochemical testing and hydrogen gas evolution.The corrosion rate (CR) of Mg decreased with graphene addition,and the lowest CR was observed with 0.3 wt.% of G15 and 0.1 wt.% of G5,which were∼56% and ∼39% lower,respectively,compared to pure Mg.Notably,the G5 composites exhibited lower CR than the G15 composites at the same concentration,attributed to agglomeration and more defects in the G-15 composites.The defect regions in graphene,such as vacancies and broken edges,act as preferable sites to which neighboring graphene sheets attach and form clusters in the Mg matrix.Due to the high electrical conductivity of graphene,the graphene clusters can induce galvanic corrosion between the graphene and the Mg matrix by forming a conductive network.This is another reason for composite corrosion resistance degradation at higher graphene concentrations.
A hydrogen evolution test showed that 0.1 wt.% graphene composites exhibited the lowest H2evolution rates compared to pure Mg,and G15 graphene composites exhibited lower H2evolution rates than G5 composites with the same graphene concentration.Interestingly,the unique structure of graphene allows for H2gas absorption,resulting in an overall decrease in the measured H2evolution in the composites.The absorption of H2gas by graphene can occur by the following mechanisms: (a) physisorption,in which H2molecules absorb onto graphene sheets due to the presence of van der Waal’s attraction forces;and (b) chemisorption,in which the chemical bond forms between H2atoms and C atoms in graphene.The XRD and DSC analysis of the corroded sample of Mg-0.1 wt.% G15 reveals the presence ofβ-MgH2(magnesium hydride) in addition to the characteristic peaks of Mg(OH)2,MgO,and Mg peaks.It was reported that theβ-MgH2phase has excellent potential in solid-state H2storage applications [434],thus the presence of MgH2in 0.1 wt.% G15 graphene composite was the main contributor to the lowest H2evolution rate.
The schematic illustration of the corrosion mechanism in Mg-0.1 wt.% G15 composite is presented in Fig.19.The release of Mg ions and H2gas formation occurs during the initial stages of degradation (reaction 4) and leads to the formation of corrosion products,including Mg(OH)2and MgO.Then (C4H9)2Mg phase is formed through the reaction of C in graphene with corrosion products,and finally,this phase is decomposed into stableβ-MgH2on graphene sheets under H2pressure.Also,the in vitro cytotoxicity of Mg-GNP composites was studied using human osteoblast-like cells (SaOS2).The results indicate that Mg with 0.1 wt.% G15 graphene showed a 12% increase in cell viability ratio (CVR) compared to pure Mg.The increase in CVR was attributed to lower H2evolution rates,indicating that the low concentration of graphene was not toxic and didn’t change the pH of the cell culturing medium.

Fig.19.Schematic illustration showing the corrosion mechanism in Mg-0.1 wt.% G15 graphene composites.Reproduced under terms of the CC-BY license from [60],Copyright 2020,Elsevier.
Saberi et al.[61] fabricated Mg-3 wt.% Zn-1 wt.% Ca alloy-graphene(0,0.5,1 and 2 wt.%)composites manufactured by wet mixing and PLS.Corrosion testing of the composites in simulated body fluid (SBF) solution showed that the corrosion resistance of Mg alloy increased with adding graphene content up to 1 wt.% and degraded with a 2 wt.% addition level.This improvement can be attributed to the excellent anti-permeability of graphene and increased charge transfer resistance of composite with graphene addition.Electrochemical impedance spectrometry (EIS) analysis of composites revealed that the charge transfer resistance of Mg alloy increased by ∼87% (104 to 195Ωcm2) with 1 wt.% graphene,indicating that graphene acted as a barrier to block ionic transport(e.g.,chloride ions)in the Mg matrix or slowed down the electrolyte infiltration to the surface of the Mg matrix.Furthermore,the antibacterial activities of Mg-graphene composites were assessed by the disk diffusion method and cultureforming unit (CFU) testing.It can be seen from Fig.20(a)that increasing the graphene content to 2 wt.% increased the inhibition zone diameter from 0.1 to 1.8 mm and 0.1 to 3.1 mm againstE.coliandS.aureusbacteria,respectively.Similarly,the plate count technique CFU results show that the 2 wt.% graphene sample had high antibacterial activity,as seen in Fig.20(b).
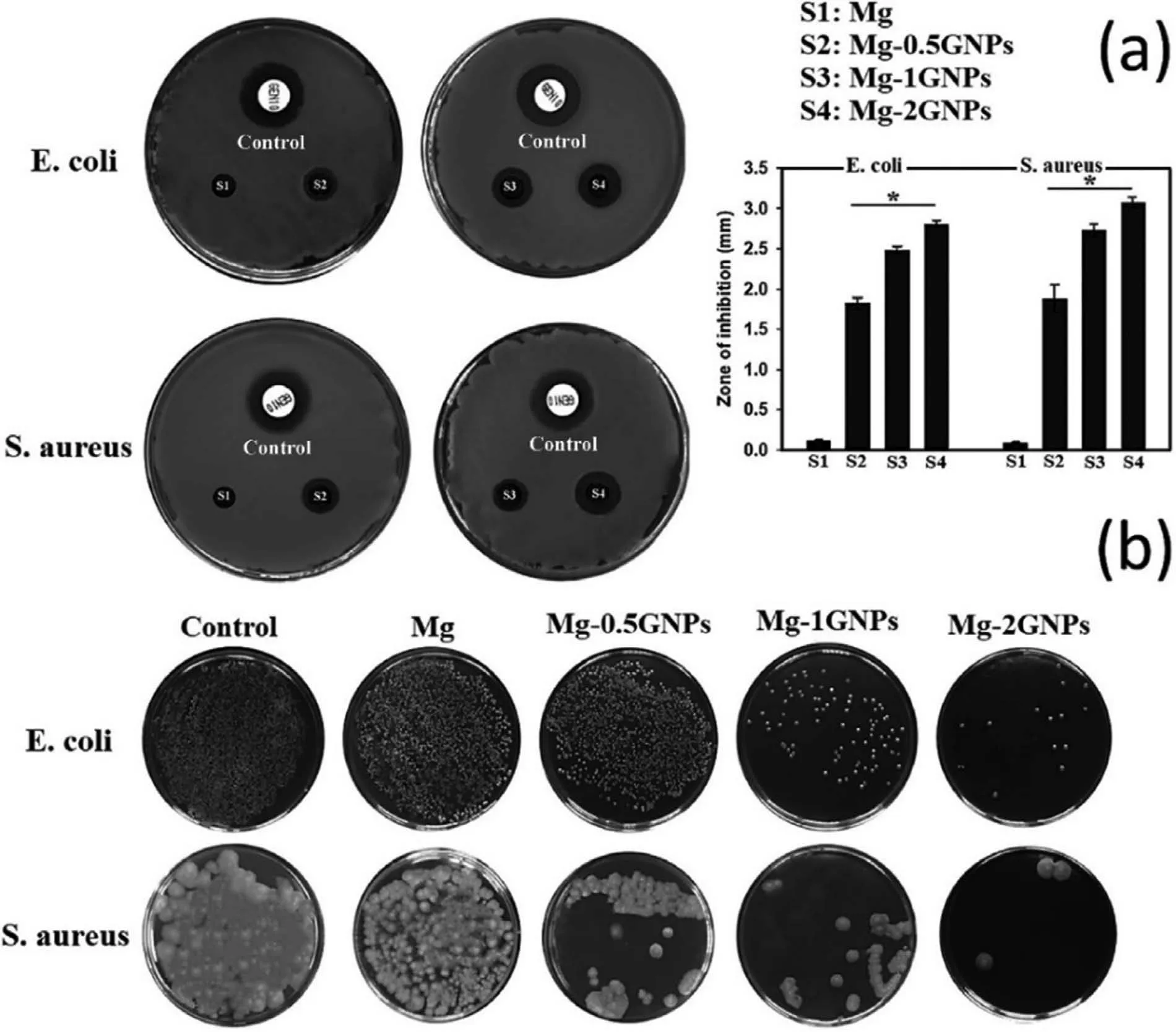
Fig.20.The antibacterial activity of Mg-graphene composites against S.aureus and E.coli bacteria was assessed by (a) disk diffusion method and (b)culture-forming unit testing.Reproduced with permission from [61],Copyright 2020,Elsevier.
The inhibition of bacteria growth with the graphene addition was explained via various physiochemical interactions that can drive their antimicrobial performance.The unique sheet morphology and high surface area of graphene allow more direct contact with bacteria through van der Waals forces,hydrogen bonding,and electrostatic interaction [435].Mainly,three antimicrobial mechanisms have been proposed for graphene,as shown in Fig.21: (i) trapping or wrapping of the bacteria by the flexible graphene sheet,inhibiting their growth [436],(ii) nano knives by the graphene’s sharp edges that can induce membrane stress,resulting in bacterial death [437,438],and (iii) oxidative stress with or without the release of reactive oxygen species (ROS) [438,439].The generation of oxidative stress occurs either due to the accumulation of intracellular ROS such as hydrogen peroxide (H2O2),hydroxyl radicals (OH),superoxide anions (O2-),etc.,or through charge transfer from the cell membrane to the graphene.In both cases,oxidative stress can inhibit cell growth and cause death.In addition to these mechanisms,nanoscale dewetting occurs between the graphene and bacteria molecules at the cell membranes and is caused by the hydrophobicity of graphene,resulting in the forcible removal of the lipid bilayer onto the graphene surface and resulting in bacteria death [437].The cell biocompatibility test using the MG63 osteoblast cell line showed that Mg alloy’s cell viability and proliferation improved with graphene addition (0.5 and 1 wt.%).

Fig.21.A schematic representation of feasible antimicrobial mechanisms in Mg-graphene composites against E. coli bacteria.Adapted with permission from[61],Copyright 2020,Elsevier.
More recently,Abazari et al.[440] studied the effect of MgO-coated CNTs on the biocompatibility properties of Mg alloy ZM31.The addition of CNTs improved the CVR of ZM31 alloy.However,irrespective of culture time,the ZM31 composite reinforced with MgO-coated CNTs exhibited higher CVR than the unreinforced matrix and CNTreinforced composite.The higher CVR with MgO-coated CNTs was attributed to a lower corrosion rate and slower Mg ion release,thus,lower pH.Similar results were also observed by Shuai et al.[415],where AZ61 composite reinforced with 3 wt.% MgO-coated rGO exhibited the slowest degradation and better cytocompatibility than unreinforced AZ61 regarding proliferation,differentiation,and cell viability.These preliminaryin-vitrostudies suggest that CBNs do not produce toxic reactions and allow cell lines’ adhesion and proliferation,thus improving cytocompatibility.Although all the studies on Mg-CBN composites werein-vitro,a limited number of studies have focused on investigating thein-vivobehavior of CBNs [58,441].Wang et al.[442] investigated GO’s biocompatibility in mice.Thirty mice were divided into three test groups called low,middle,and high doses,injected with 0.1,0.25,and 0.4 mg GO,respectively.The test groups were raised for 1,7,and 30 days,then the heart,spleen,kidneys,liver,stomach,brain,and lungs were collected for analysis.Low (0.1 mg) and middle (0.25) dose of GO did not pose a toxicity risk in mice,while a high dose (0.4 mg)showed chronic toxicity.The granuloma formation was observed mainly in the lung,spleen,kidney,and liver in 0.4 mg GO-injected mice.Similar results were reported by Zhang et al.[443],where no pathological changes in the examined organs were observed when the mice were treated with 1 mg/kg body weight of GO.In addition,GO exhibited excellent biocompatibility with red blood cells.However,significant pathological changes such as granuloma formation,pulmonary edema,and inflammation throughout the lung were observed at the high dosage of 10 mg/kg body weight of GO.These pathological changes were attributed to a high accumulation of GO in the lungs and slow excretion from the lungs.
The CBNs in Mg-CBN composites can cross the cell membranes and interact with DNA (Deoxyribonucleic acid) and RNA (Ribonucleic acid) due to their nano size and high surface area.Most reports on the interaction of CBN with DNA/RNA have been explored for drug or gene delivery[444–446],DNA intercalation [447],and biosensor applications [448].It has been reported that the adsorption of DNA on CBNs makes them effective materials as drug and gene delivery carriers.In bone tissue engineering applications,the desired DNA can be attached or coated to metallic implants that have the potential to promote bone formation in the early stages of bone healing and induces new tissue formation.Lu et al.[444] demonstrated that graphene could protect DNA from cleavage during cellular delivery,indicating good biocompatibility between graphene and DNA.There is no literature on studying the interactions between CBN and DNA/RNA in Mg-CBN composites,which thus warrants future research.In addition to CBN’s toxicity and its interactions with various biological molecules,the biodegradation of CBNs needs to be considered while designing Mg-CBN composites for biomedical applications.Literature suggests that the biodegradation of CBNs primarily occurs by peroxidase enzymatic processes leading to natural degradation by oxidation in biological systems [58,449,450].Kurapati et al.[451] demonstrated that both single-layer graphene (SLG)and few-layer graphene (FLG) can be degraded by human myeloperoxidase (hMPO) or by hMPO secreted with activated neutrophils.However,it was observed that the degradation of SLG is drastic and more uniform compared to FLG.The higher degradability of SLG can be attributed to better aqueous dispersion and high negative surface charge,leading to greater interactions with cationic hMPO.The same group of researchers [452] demonstrated that GO can be degraded completely within 24 h using hMPO,and its degradation is proportional to the degree of oxidation of GO.Therefore,the biodegradation of CBNs largely depends on their physiochemical surface characteristics,and using the functionalized CBNs at small concentrations can be biodegraded completely.However,systematicin-vivostudies are needed to better understand CBN’s nanotoxicity,its interaction with various organs,biodegradation,and to assess the by-products generated during the degradation of Mg-CBN composites.
3.3. Tribological properties
The inferior tribological properties of Mg continue to limit broad application in various industries,including automotive,aerospace,and biomedical.In addition to good strength and corrosion resistance,higher wear resistance is required for Mg and its alloys for applications where parts are in repetitive rolling or sliding motion with a counter body in contact.For instance,different Al automotive components have been replaced with Mg,but Mg is not a suitable candidate for the cylinder bore,bearings,piston,and gears,as these are exposed to continuous wear [117,290,453].Similarly,under physiological conditions,inevitable micro-movements occur between the Mg implant and surrounding tissues or bone.Therefore,the naturally forming oxide layer on the Mg implant is mechanically removed due to the friction between the implant and bone,leading to early degradation of the implant.Furthermore,the wear debris produced due to excessive wear can cause infection and osteolysis,eventually resulting in implant failure [185].Therefore,surface modification of Mg via optimization of its surface microstructure,physical deposition coating,and chemical conversion coating has been used to enhance the corrosion and wear resistance [454,455].
In addition to corrosion and wear protection,the coatings should provide improved biocompatibility,bioactivity,and antibiotic ability for biomedical applications,which is a challenge [454].Moreover,Mg is hard to coat due to the formation of an oxide (e.g.,MgO) layer caused by its high reactivity,and thus Mg needs extensive precleaning treatments.Also,galvanic corrosion is possible between Mg and the coating material,resulting in premature implant or automotive component failure.Furthermore,the mismatch in CTE between Mg and coating material can lead to thermal stresses at the interface and subsequent spallation of the coating.Additionally,the coatings are not reliable in terms of their thickness,durability,and interfacial bonding strength for automotive applications [185].Reinforcing the Mg with suitable reinforcement,such as Al2O3[456],CaB6[457],ZrO2[458],TiB2[459],SiC [460],etc.,was another approach to enhance the wear properties of Mg.However,counter-abrasion is a major drawback in conventional composites where the loosely bonded hard ceramic particles can detach from the composite and cause counter-abrasion during repeated sliding [461].
Thus,CBNs are currently being explored to improve the wear resistance of metal matrices due to their self-lubricating behavior [20,47,462].It was demonstrated that the presence of graphite particles in a metal matrix leads to an evolution of a lubricant film between the abrading contact surfaces.The lubricant film hinders direct contact with sliding surfaces,decreasing wear [20].However,the poor mechanical properties of metal-graphite composites are the main disadvantage,and sometimes the composite can have inferior mechanical properties to the matrix[463].Vahedi et al.[392]studied the effect of reinforcement (graphite and graphene) on the wear resistance of AZ31 Mg alloy.It was observed that graphene-based composites exhibited higher wear resistance than graphitebased composites.A similar observation was also made in Al matrix composites [464].The superior wear properties of graphene composites were attributed to their high hardness since the wear resistance of a composite is directly proportional to its hardness according to the Archard equation(Eq.(5)) [465].The tribological performance of materials is usually evaluated in terms of coefficient of friction(COF)and wear rate.
WhereV,K,P,L,andHare the volume of wear loss,wear coefficient,applied load,sliding distance,and hardness,respectively.
Limited research has been carried out on the Mg-CBN composite’s tribological properties compared to mechanical properties.It can be seen from Fig.22 that the addition of CBN decreased the wear rate and COF of the Mg matrix by a maximum of ∼66% [79] and ∼60% [118],respectively.Also,it is worth noting that the Mg-graphene composites showed the highest decrease in wear rate and COF compared to Mg-CNT composites (Fig.22).The superior wear properties of Mg-graphene composites were attributed to their better mechanical properties compared to Mg-CNT composites,as discussed in Section 3.1.Furthermore,the sheet morphology of graphene can easily cover the contact surface of the composite by forming a uniform and continuous lubricant film compared to one-dimensional CNTs,thus preventing direct contact effectively between the sliding surfaces.Turan et al.[127]investigated the synergistic effect of CNTs and graphene on the tribological performance of AZ31 matrix composites.The results showed that the hybrid composite (0.15 wt.%CNTs+0.15 wt.% graphene) exhibited better wear properties compared to 0.3 wt.% CNTs composite,attributed to higher hardness and forming continuous lubricant film in the hybrid composite.In a study by Lee et al.[290],AS52 matrix composites with 15 vol.% of SiC short fibers and varying CNT concentrations (0,5,10 vol.%) were fabricated by PIC and their wear properties were investigated.All hybrid composites exhibited superior wear resistance compared to the unreinforced AS52 matrix,with a maximum improvement of ∼22%in COF and ∼13% in wear rate with the addition of 10 vol.%CNTs.The worn surface of AS52-15 vol.% SiC composite was rough and had deep grooves,indicating severe adhesive wear.However,the worn surface of the hybrid composite(15 vol.% SiC+10 vol.% CNTs) showed a smooth surface without deep grooves,suggesting a minimization of adhesive wear with CNT addition.Tube-like CNTs provided easy rolling or sliding by forming a self-lubricating layer on the sliding surface.A similar observation of enhanced wear properties with hybrid composites was reported in graphene+TiB2[321] and CNT+Al2O3[385]-based composites.

Fig.22.Percentage decrease in wear rate and COF of Mg-CBN composites as a function of CBN content(the data for these plots are taken from Table 8).
In another study,Wu et al.[79] fabricated AZ31+0.5%Ni-coated graphene composites by VHP followed by hot extrusion and studied the effect of Ni coating on the mechanical and tribological properties of AZ31.The addition of graphene and Ni-coated graphene decreased the wear rate of the AZ31 matrix by ∼44% and ∼66%,respectively.The higher mechanical properties caused by the uniform dispersion of graphene and good interfacial bonding between Mg and graphene lead to superior tribological properties in Nicoated composite.Huang et al.[297] fabricated AZ61-CNT(0,0.1,0.5,and 1 wt.%) composites by stir casting followed by aging heat treatment.The resultant composites’ tribological performance was analyzed in as-cast and age-hardened conditions.The aged composites had better wear properties than as-cast composites,mainly because of the higher strength caused by the dissolution of the weaker Mg17Al12phase.It is worth noting that the wear mass loss and COF of the AZ61 matrix in as-cast and aged conditions decreased with increasing CNT content up to 0.5 wt.% CNT and there was no significant improvement with increasing the concentration to 1 wt.% CNT.Other researchers have also reported a similar trend of stabilization or deterioration of wear properties at high CBN concentrations [82,118,150].The degradation in composites’ wear properties at higher CBN contents was associated with lower strength caused by the presence of cracks and voids due to the CBN’s agglomeration.
3.4. Thermal and electrical properties
Heat dissipation is becoming increasingly important in electronics due to the continuous development of highperformance and increased miniaturization of electronic devices.Excessive heat generated during the operation of electronic components must be removed effectively to prevent them from overheating and subsequent failure.Thus,heat sinks made of Al or Cu,or W-Cu or W-Mo are often used to conduct and dissipate heat away from the active components(e.g.,light-emitting diodes,liquid crystal displays,integrated circuits,etc.).However,the large mismatch in CTE between the heat sink and printed PCB or other electronic materials can lead to thermally induced stresses at the interface leading to brittle failure of the component.Thus,it is demanded that heat sink materials should have high thermal conductivity,good mechanical strength,low CTE,and be lightweight[466,467].Mg and its alloys have attracted tremendous attention as a potential heat sink or electronic packaging materials by considering their light weight compared to conventional materials.Currently,research is focused on further improving mechanical strength without deteriorating the conductivity of Mg.Although adding alloying elements and/or traditional reinforcements were seen to enhance the mechanical properties,they generally decreased the conductivity of Mg composites[468].Their negative CTE and excellent thermal and electrical conductivity have made CBNs a potential reinforcement for Mg matrices for thermal management and electronic applications (Table 1).To date,only a limited number of studies have been carried out on the thermal and electrical properties of Mg-CBN composites.
Goh et al.[141] fabricated Mg-CNT (0,0.06,0.18,and 0.30 wt.%) composites by BM followed by PLS,and the CTE of composites was determined in the temperature range of 50-400 °C.Increasing the CNT content decreased the CTE of Mg and 0.3 wt.% CNT composite showed the lowest CTE of 25.90 × 10-6/ °C,which was ∼9% lower than unreinforced Mg.Similarly,Kumar et al.[172] observed a ∼10%reduction in CTE of Mg-3% Al alloy with 0.3 wt.% rGO addition.The decreased CTE with CBN addition was ascribed to its lower CTE and high thermal stability,impeding the Mg expansion.Sun et al.[203] observed ∼7% improvement in the thermal conductivity of Mg-1%B4C composite with 0.5 wt.% graphene.In a study by Zhang et al.[277],fabricated AZ91D-CNT (0,0.5 and 2 wt.%) composites by UASC followed by solution treatment (413 °C for 24 h) and extrusion at 300 °C for eight passes.The composite with 2 wt.%CNT exhibited 22% and 18% improvement in thermal conductivity over matrix in solutionized and extruded conditions,respectively.Du et al.[315] also reported ∼5% and ∼3%improvement in thermal and electrical conductivity of ZK60 alloy,respectively,with 0.04 wt.% graphene addition.In contrast,Mindivan et al.[150] observed a decrease in the electrical conductivity of the Mg-6 wt.%Al matrix with the addition of CNTs,which was attributed to increased porosity.
3.5. Electromagnetic interference (EMI) shielding properties
Electromagnetic interference (EMI) is an unwanted noise or disturbance in an electrical circuit or path that occurs when one device’s electromagnetic radiation interferes with another’s operation.EMI can degrade an electronic device’s performance or cause operational malfunction.Recently,EMI has been identified a source of public pollution besides air,noise,and water pollution [469–472].Protection from EMI pollution can be achieved by blocking/shielding the electromagnetic field,modifying the radiation path,or by reducing the emissions from the source [473].Among these,EMI shielding is an effective way to mitigate EMI pollution.In EMI shielding,a material is used to enclose an electronic device entirely to prevent electromagnetic radiation from escaping or entering the device.The shielding material must be strong,lightweight,and should exhibit good conductivity.Metallic materials,ceramics,and polymer-based composites are the most common candidates for EMI shielding applications [474].Due to their good conductivity,metallic materials such as nickel,copper,steel,zinc,and aluminum are used as EMI shielding materials [475].However,the high density of metallic materials and ceramics and polymers’ low strength and conductivity limit their widespread use in EMI shielding applications.Mg and its alloys have gained research attention as EMI shielding materials due to their low density,high specific strength,and recyclability [476].Several effective approaches,including alloying [477],heat treatment [477,478],and the addition of reinforcements [479],have been explored further to improve conductivity,mechanical and EMI shielding properties.
Recently,researchers also studied the effect of CBNs on the EMI shielding behavior of Mg and its alloys [255,268].It is known that when EM waves irradiate a shielding material,the following processes occur: absorption (A),reflection (R),multiple reflections (M),and transmission (T).Usually,to evaluate the individual contribution of these processes,the corresponding surface reflection coefficients are measured by a vector network analyzer (VNA) using Eqs.(6)–(10)[480,481].The EMI shielding performance of materials was assessed generally in terms of total shielding effectiveness(SET),and it is expressed in decibels (dB),as shown in Eq.(11).The shielding caused by multiple reflections (SEM)is ignored when theSETexceeds 15 dB [482].Sun et al.[255] produced laminated Mg-CNT composites by VHP and hot extrusion at 400 °C.The electrical conductivity of laminated Mg increased from 11 to 20 Ms/m with 0.5 wt.% CNT addition and there was no significant change with 0.7 and 1 wt.%CNT.Further,the EMI shielding performance of composites was analyzed by VNA in the frequency range of 8.2-12.4 GHz,known as X band.TheSETof bulk Mg increased from 20 to 40 dB after VHP and extrusion,representing a 100% improvement.This was primarily attributed to an increased number of laminated interfaces in the sample,which repeatedly contributed to the internal reflection and absorption of EM waves [483].Furthermore,the SE of Mg laminate further increased from ∼40 to ∼58 dB with 0.5 wt.%CNT addition.This can be due to the expansion of internal reflection paths caused by increased electrical conductivity and augmented micro-nano interfaces with CNT addition.The presence of CNTs at the interface act as a conductive network,which can convert electromagnetic energy into leaking heat or current,thus improving the absorption mechanism.Additionally,the microstructural refinement with the addition of CNTs leads to a larger area of grain boundaries.These boundaries can contribute to repeated reflection and absorption of EM waves,thus enhancing overall SE [484].
WhereSEA: shielding effectiveness of adsorption,SER:shielding effectiveness of reflection
Zhang et al.[268] fabricated Mg-CNT composites using ARB,and the EPD time was varied from 0 to 8 min to get the different concentrations of CNTs.The electrical conductivity of unreinforced Mg was ∼22 × 106S/m,and there was no significant change in its electrical conductivity with the addition of CNTs.The non-improvement in electrical conductivity with CNT addition was attributed to the presence of oxide layers and defects created during ARB.Moreover,theSETof Mg in the 8.2-12.4 GHz frequency was increased from 82-92 dB to 86-94 dB and 87-95 dB with 4 and 8-min EPD,respectively,indicating the increase in CNT content enhanced theSET.
4.Potential applications
Mg-CBN composites are still in the research stage,and presently,there are no commercial applications or products based on these materials.However,a combination of good mechanical properties,high wear resistance,improved thermal and electrical properties,and lightweight makes Mg-CBN composites suitable for applications in various industries,including the automotive,aircraft,space,biomedical,electronics,and sports industries.The Ashby plot comparing Mg-CNT and Mg-graphene composites to other materials in terms of tensile strengthvs.density is presented in Fig.23.Mg-CBN composites outperformed cast Mg alloys,Al alloys,Al-SiC composites,and most conventional materials in terms of a combination of density and tensile strength,indicating that Mg-CBN composites could be an alternative material for various applications.This section briefly describes the potential applications of Mg-CBN composites.
Mg alloys have a long history in the automotive sector,dating back to the 1930s when they were used for engine blocks.Since then,there has been a steady stream of Mg use in automotive applications covering a wide range of powertrain and body structure components [485].Over the past twenty years,many large automotive companies,including Ford,Kia Motors,Audi,Mercedes Benz,Jaguar,etc.,have replaced Al and steel with Mg in various parts of their products.Mg alloys are currently used in seat frames,fuel tank covers,gearboxes,steering wheels,roofs,door panels,engine front covers,cross beams,and driver’s airbag housings.Mg alloys are also used in aerospace applications,and they can be found in thrust reverser cascades for the Boeing 737,747,757,and 767,as well as in jet engine fan frames,optical imaging devices carried by satellites,helicopters,and aircraft transmission casings[485–487].The mechanical properties and thermal conductivity of Mg alloys commonly used in commercial applications are compared to Mg-CBN composites in Table 9.It can be seen that Mg-CBN composites showed superior properties and low density compared to traditional Mg alloys,indicating that Mg-CBN composites have a high potential for automotive,aircraft,and aerospace applications.

Table 9The properties of Mg-CBN composites in comparison to commonly used Mg alloys in commercial applications [488–490].
Recently,Mg alloys are gaining more attention for biomedical applications owing to their lightweight and natural biodegrading ability.However,the high degradation rate of Mg limited its extensive use in biomedical applications.In the past two decades,extensive research has been done to control the degradation of Mg in the physiological environment [491].Preliminaryin-vitrostudies on Mg-CBN composites showed improved corrosion resistance,cell viability,and antibacterial activity at low concentrations of CBN [60,61].Additionally,the addition of CBN showed no significant change in cytotoxicity,suggesting that CBNs are safe biomaterials at low contents (≤1 wt.%).The mechanical properties of Mg-CBN composites are compared to human cortical bone and commonly used metallic biomaterials in Table 10.Mg-CBN composites exhibit better mechanical properties than Mg alloys,without detrimentally increasing their density.In addition,the mechanical properties of Mg-CBN composites can be tailored by changing the CBN content.Thus,substituting Mg alloys with Mg-CBN composites for bone implant applications can provide higher load-bearing capabilities and retain their mechanical and physical properties in the human body for a long time.However,thein-vivobehavior of Mg-CBN composites must be studied before translating these materials into clinical applications.Furthermore,Mg-CBN composites can be a candidate material for electronic applications (e.g.,heat sink,shielding material,electronic packaging material,etc.) and sports applications (e.g.,tennis rackets,bicycle frames,etc.)owing to their lightweight,coupled with low CTE,improved EMI shielding effectiveness,excellent mechanical properties,and conductivity.The different fabrication techniques,beneficial properties,and potential applications of Mg-CBN composites in various industries are depicted in Fig.24.
Fig.24.A schematic illustrating the processing techniques,key properties,and potential applications of Mg-CBN composites.
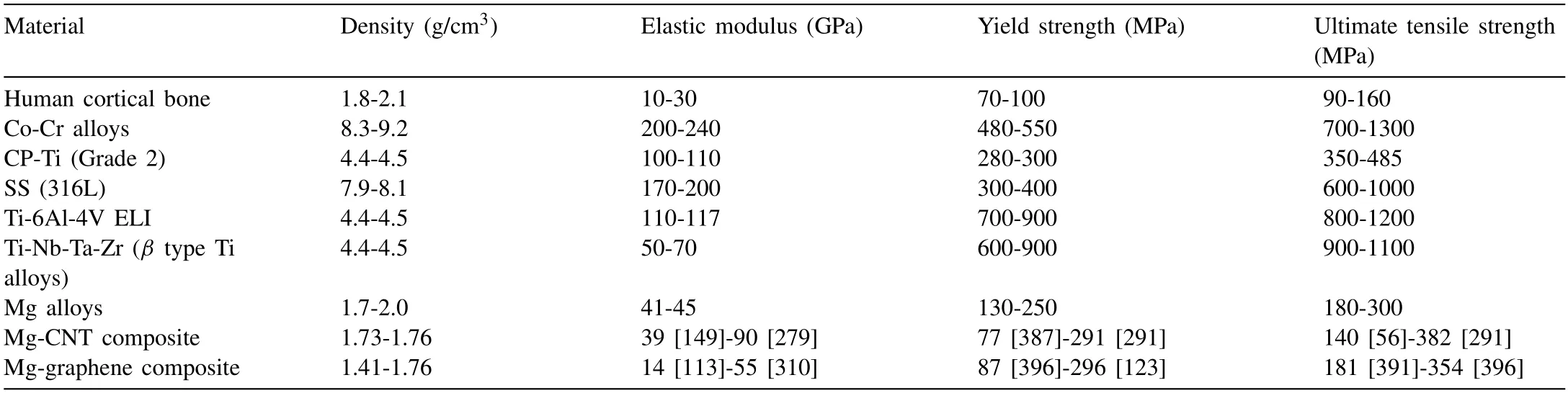
Table 10The mechanical properties of Mg-CBN composites in comparison to human cortical bone and commonly used metallic materials in biomedical applications[58].
5.Summary and future research directions
Increased interest in lightweighting,miniaturization,and high-strength components in the automotive,aerospace,and electronic industries is driving the growth of Mg-based composites.Recently,CBNs (CNTs and graphene) have gained great attention as reinforcement due to their ability to simultaneously enhance the mechanical strength,conductivity,and wear resistance of the Mg matrix.Literature shows that incorporating an optimum concentration (<2 wt.%) of CBN significantly improved the properties of Mg irrespective of the processing technique considered.This review article brings insight into the recent advancements in the processing techniques,properties,and potential applications of Mg-CBN composites.Key points from the current review are summarized below while outlining future research directions in the field of Mg-CBN composites.
•Researchers used various fabrication techniques to make Mg-CBN composites,including PM,casting,and FSP.Among these techniques,the PM route has been commonly used to fabricate these composites due to its ability to disperse CBNs uniformly in the Mg matrix.However,the PM route is not viable for making Mg-CBN composites on a large scale,which is required for commercialization.In this regard,the casting route is known for manufacturing large parts of intricate shapes at high production rates.But,a limited amount of research has been carried out on casting Mg-CBN composites.Thus,future research should focus on developing Mg-CBN composites on a large scale using the casting route.
•More recently,additive manufacturing of Mg-CBN composites has been attempted,and the preliminary studies indicate promising results.AM technology can solve some of the current processing challenges associated with traditional manufacturing techniques as AM can rapidly fabricate Mg-CBN composites directly from powder.So far,only the SLM technique has been used to produce these composites [412,415,416].Therefore,researchers should explore the fabrication of these composites using other AM techniques,such as direct energy deposition and binder jet 3D printing,etc.Also,CBN addition increased the laser energy absorption of Mg during SLM.Systematic studies are needed to understand CBN-laser interactions and laser absorption mechanisms in Mg-CBN composites.
•The majority of research work on Mg-CBN composites has been focused primarily on improving the dispersion of CBNs in the Mg matrix and investigating their effect on Mg’s properties.So far,Mg matrix composites with a maximum of 5 wt.% CBN have been fabricated to avoid the agglomeration of CBNs at higher contents.Future research should focus on manufacturing Mg composites with a larger concentration of CBN without agglomeration.During the last two decades,mainly the following approaches have been attempted to disperse CBNs in Mg using wet mixing and BM in the PM route,(i) hybrid dispersion (combination of sonication,agitation,and/or BM)[52,78,111–114],(ii) surface modification (functionalization of CBNs using surfactants) [106–108,193],(iii) surface coating of CBNs [68,109,115,151],(iv) using hybrid reinforcement [143,149,203,204] and (v)in-situsynthesis of Mg-CBN composites [247,248].Similarly,melt ultrasonication and stirring have been used in the casting route to fabricate Mg-CBN composites.Encouraging results have been observed by stirring when the Mg melt is at a semisolid (usually ≤600 °C),followed by melt ultrasonication in a fully liquid state before being poured into the mold[292,293,310–312].Secondary processing of these composites was often carried out using extrusion or FSP to break CBN’s clusters and distribute them in the Mg matrix.Among all these dispersion approaches,in-situsynthesis of Mg-CBN composites showed promising results as it provided tight interfacial bonding with fewer processing defects.But,there is a paucity of literature on thein-situprocessing of Mg-CBN composites.Future research should continue to explore thein-situsynthesis of composites and look at new dispersion strategies to circumvent the current processing limitations,such as CBN’s agglomeration and poor interfacial bonding between Mg and CBN.
•Literature shows an excellent improvement in the mechanical properties of Mg and its alloys by incorporating CBN.For instance,the highest improvement in various room temperature mechanical properties of Mg reported so far in literature is as follows: hardness of 78% [57],TYS of 165% [310],UTS of 76% [322],EMTof 89% [113],CYS of 426% [164],and UCS of 209% [159].The strengthening in Mg-CBN composites is mainly contributed by load transfer,dislocation,Orowan,and grain refinement strengthening mechanisms.It can be concluded from this review that graphene has a higher strengthening efficiency compared to CNTs under both tensile and compressive loads.However,few studies have evaluated the mechanical properties of Mg-CBN composites at elevated temperatures [274,296].Similarly,limited research is available in the open literature on Mg-CBN composites’ creep and fatigue behavior.The preliminary results show that CBN enhanced Mg alloy’s creep resistance [156,296] and fatigue resistance [282].A systematic study on the effect of CBN on precipitation behavior (precipitate size and morphology),high-temperature creep,and fatigue behavior of Mg alloys must be conducted to see if these composites are suitable for high-temperature applications.
•Most studies reported improved corrosion resistance of Mg with the addition of small content of CBN,while deterioration at higher concentrations was observed[82,117,118,182].This was attributed to the galvanic corrosion owing to the presence of voids,micropores,and cracks,caused by CBN’s agglomeration.It is demonstrated that the graphene coating enhanced the corrosion resistance of Mg [492,493].Hence,future research efforts should focus on coating the Mg-CBN composite with graphene and evaluating its effect on these composites’ corrosion and biocompatibility properties.
•More recently,in-vitrobiocompatibility and biological properties of Mg-CBN composites have been studied[60,61].The results suggest that adding CBN enhanced Mg’s cell viability,cytocompatibility,and antibacterial activity,which can subsequently promotein-vivobone tissue formation.Literature on the toxicity of CBNs suggests that CBNs exhibit size (number of graphene layers,lateral size,morphology,etc.) and dose-dependent toxicity to organs and cells.But,to date,all studies have been conductedinvitroand must be assessed these composites underin-vivoconditions to understand the CBNs’ biodegradation and their interaction with various biological molecules,e.g.,cells,various organs,proteins,and DNA.
•A limited amount of research has been carried out on evaluating the thermal and electrical properties of Mg-CBN composites,and they need to be explored.
•The EMI shielding effectiveness of Mg-CNT composites is better than that of unreinforced Mg.This improvement is mainly attributed to the expansion of internal reflection paths caused by increased electrical conductivity and micro-nano interfaces [255,268].However,the shielding mechanism in these composites has not been fully understood,and there is no literature focusing on assessing the shielding effectiveness in Mg-graphene composites.Therefore,more efforts are needed to better understand the EMI shielding mechanisms in Mg-CBN composites.
Declaration of competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Stress corrosion cracking of magnesium alloys: A review
- Simultaneous enhancement of mechanical properties and corrosion resistance of as-cast Mg-5Zn via microstructural modification by friction stir processing
- Effect of wire-arc directed energy deposition on the microstructural formation and age-hardening response of the Mg-9Al-1Zn (AZ91) alloy
- Regulating local coordination environment of Mg-Co single atom catalyst for improved direct methanol fuel cell cathode
- Hydrogen-induced optical properties of FC/Pd/Mg films: Roles of grain size and grain boundary
- Improving corrosive wear resistance of Mg-Zn-Y-Zr alloys through heat treatment
