Stress corrosion cracking of magnesium alloys: A review
Jiho Jing ,Xue Geng ,Xiobo Zhng,b,∗
a School of Materials Science and Engineering,Nanjing Institute of Technology,Nanjing 211167,China
b Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology,Nanjing 211167,China
Abstract Magnesium (Mg) alloys have been widely used in automobile,aviation,computer,and other fields due to their lightweight,high specific strength and stiffness,low pollution,and good electromagnetic shielding performance.However,the chemical stability of Mg alloys is poor,especially in the corrosive medium environment with high stress corrosion sensitivity,which causes sudden damage to structural components and restricts their application field.In recent years,owing to the increasing failure rate of engineering structures caused by stress corrosion of Mg alloys,it has become necessary to understand and pay more attention to the stress corrosion cracking (SCC) behavior of Mg alloys.In this paper,the SCC mechanisms and test methods of Mg alloys have been summarized.The recent research progress on SCC of Mg alloys has been reviewed from the aspects of alloying,preparation process,surface modification,corrosive medium,and strain rate.More importantly,future research trends in the field of SCC of Mg alloys have also been proposed.
Keywords: Magnesium alloys;Stress corrosion cracking;Alloying;Preparation process;Corrosion media;Surface treatment.
1.Introduction
As the lightest metal structural materials,Mg alloys exhibit many excellent properties,such as high specific strength and specific stiffness,excellent damping performance and electromagnetic shielding performance,good electrical conductivity and cutting performance,and easy recycling,thus they are considered as“green engineering material in the 21st century”[1,2].Now they have been widely used in automotive industry,3C electronic products,aerospace,military,and other fields[3–6].Owing to good biomechanical compatibility,biocompatibility,and biodegradability,they have also shown broad application prospects in biomedical materials over the past two decades [7,8].
Although many unique characteristics of Mg alloys make their application prospects broad,they are prone to chemical reactions with surrounding environment containing Cldue to active chemical properties;moreover,the oxide film formed on the surface of Mg alloys is often loose and porous,which cannot protect the substrate effectively [9–11].In addition,when Mg alloys are used as structural components,they will be subjected to the dual effects of stress and corrosion environment in the service process,which is prone to stress corrosion [12,13].Since most structural applications of Mg alloys are assumed to be temporarily or permanently exposed to corrosive environments,SCC is critical to the integrity and function of the component.SCC often leads to sudden brittle fracture,and has high concealment and unpredictability,which is easy to cause irreparable losses [14].In the biomedical field,Mg alloys are expected to become promising candidate materials for orthopedic implants and cardiovascular stents [15].However,the alloys are required to withstand stress during service and will be gradually degraded (corroded)invivo[16].Under the combined effects of these external loads and complex physiological environments,Mg alloy implants are extremely vulnerable to SCC during service,resulting in early failure,which not only affects the therapeutic recovery effect but also severely limits the application of Mg alloys in the biomedical field[17].Nevertheless,most of the current studies on biomedical Mg alloys focus on corrosioninvitroor biodegradabilityinvivo[18–41],and researches aimed at SCC is limited.In fact,although Mg alloys have been applied in industry and biomedical fields[42–45],the threat of SCC may occur under both stress and corrosion environment,which will reduce service life.Therefore,it is imperative to understand the SCC behavior of Mg alloys to improve the SCC resistance of Mg alloys,so that Mg alloys can be more widely used.Fig.1 depicts Mg alloys components that are commonly used in biomedical,automotive,and aerospace fields [46–49].It is difficult to determine the SCC mechanism because the SCC process of Mg alloys is not just a straightforward superposition of the two processes of stress and corrosion [50,51].Several reviews can be available on SCC of Mg alloy,and most of them have been published for over a decade [13,14,52,53].In the past decades,some progress has been made on the SCC of Mg alloys,especially in the biomedical field.Therefore,it is necessary and worthy to summarize these recent developments on the SCC of Mg alloys to better reveal its mechanism and progress.
In order to create Mg alloys with high SCC resistance and investigate new avenues for SCC resistance,it is important to understand the mechanism,influencing factors,protective measures,and recent research progress of SCC behavior of Mg alloys.This will help to advance the development and application of Mg alloys in a variety of fields.
2.SCC mechanism and test methods of Mg alloys
2.1. Stress corrosion crack propagation patterns in Mg alloys
SCC requires the material to have stress corrosion sensitivity when it is subjected to stress in a specific corrosive medium.Generally,there are two main mechanisms for crack initiation and propagation under stress corrosion conditions[52–57]: (1) Under the dominant effect of anodic dissolution mechanism,the oxide film on the surface of the alloy is broken due to tensile stress.The Mg alloy matrix is exposed,partially dissolved,then cracks appear.Under the action of stress,cracks continue to initiate and develop,and the whole process is continuously alternating;(2) The hydrogen atoms produced during the cathode reaction of Mg alloy penetrate into the Mg matrix and diffuse.When the hydrogen concentration reaches a certain value,it will induce cracks.
SCC of Mg alloys may be either transgranular (TGSCC)or intergranular (IGSCC) [57].The difference between the two forms depends on the composition of alloys,the corrosive mediums,and the conditions of the applied load.TGSCC is usually associated with hydrogen and microstructure of the alloy,while IGSCC is normally caused by microgalvanic corrosion and associated with continuous second phases distribution along grain boundaries [56].Padekar et al.[58] analyzed the fracture of Mg-Mn alloy and found that the fracture of the alloy had transgranular characteristics due to the presence of hydrogen in the process of SCC.Prabhu et al.[26] studied Mg-4Zn alloy and observed the fracture interface after solution treatment (T4).They found that the fracture mode of the alloy in simulated body fluid (SBF) was intergranular fracture,which was related to the occurrence of cracks after micropore consolidation.Nevertheless,in the slow strain rate experiment,Catar et al.[59] observed IGSCC and TGSCC in AZ31,AZ61,and AZ91 alloys,respectively,indicating that both kinds of SCC can occur together,as shown in Fig.2.

Fig.2.SCC crack propagation path of AZ31,AZ61,and AZ91 in NaCl solution [59].
It has been indicated that TGSCC and IGSCC are also related to grain size [57,60,61].Wang et al.[60] found that when the grain size was small,intergranular fracture occurred;when the grains were coarse,transgranular fracture occured;when the grains are bimodal,there were both transgranular fracture and intergranular fracture.Based on the above theories,the fracture models are shown in Fig.3.Fig.4 shows the experimental results of the above SCC crack propagation patterns of the Mg-Zn-Zr alloy.Casajús et al.[61] also proposed that the crack propagation in the recrystallization region of fine grains was IGSCC,while the crack propagation in the large grains was TGSCC.In addition,the transgranular cracks of as-cast Mg-7%Gd-5%Y-1%Nd-0.5%Zr alloy,which propagate along the coarse grains,has been observed by Wang et al.[62].However,Kannan et al.[63] found that finegrained AZ80 alloy exhibited TGSCC,while coarse-grained ZE41,QE22,and EV31A had both IGSCC and TGSCC.Generally,the TGSCC fracture paths of AZ80,ZE41,and QE22 are consistent with the hydrogen-containing mechanism and the as-cast Mg alloys were used in the experiment.Compared with the as-extruded Mg alloys,the grain size is larger,which belongs to the coarse grain and is prone to TGSCC.In fact,the IGSCC is related to the galvanic corrosion between the second phase and the matrix,while TGSCC is more related to the hydrogen embrittlement inside the grains.
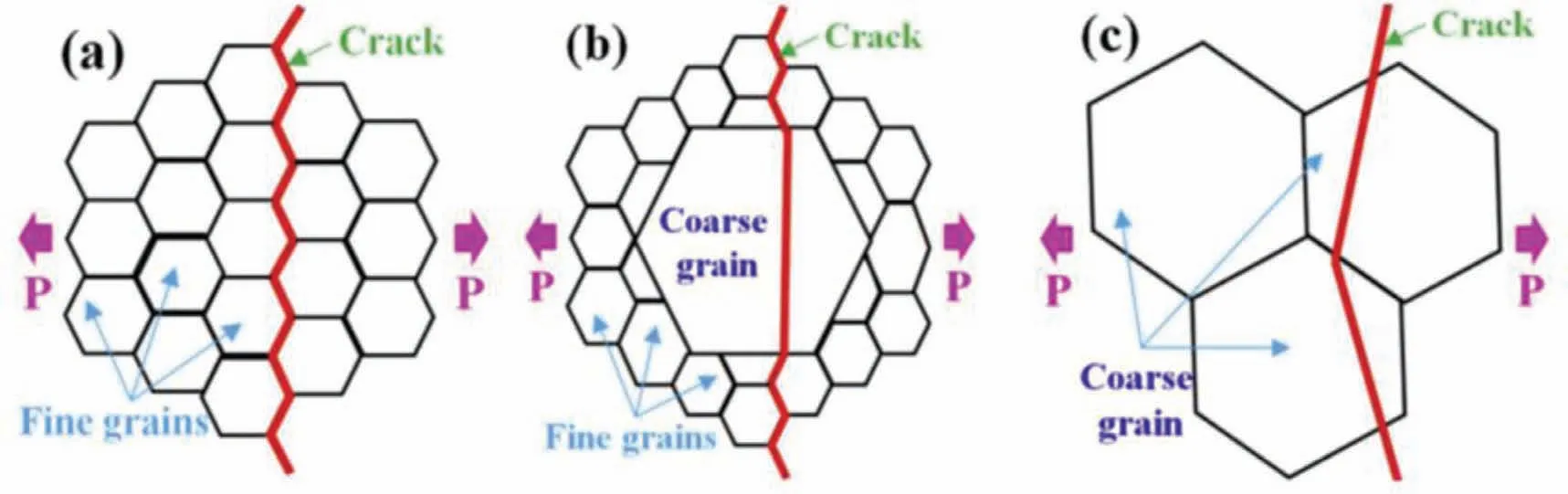
Fig.3.Schematic diagrams for the cracking modes of differently grain-structured samples during stress corrosion cracking process: (a) fine grains;(b) bi-modal grains;(c) coarse grains [60].
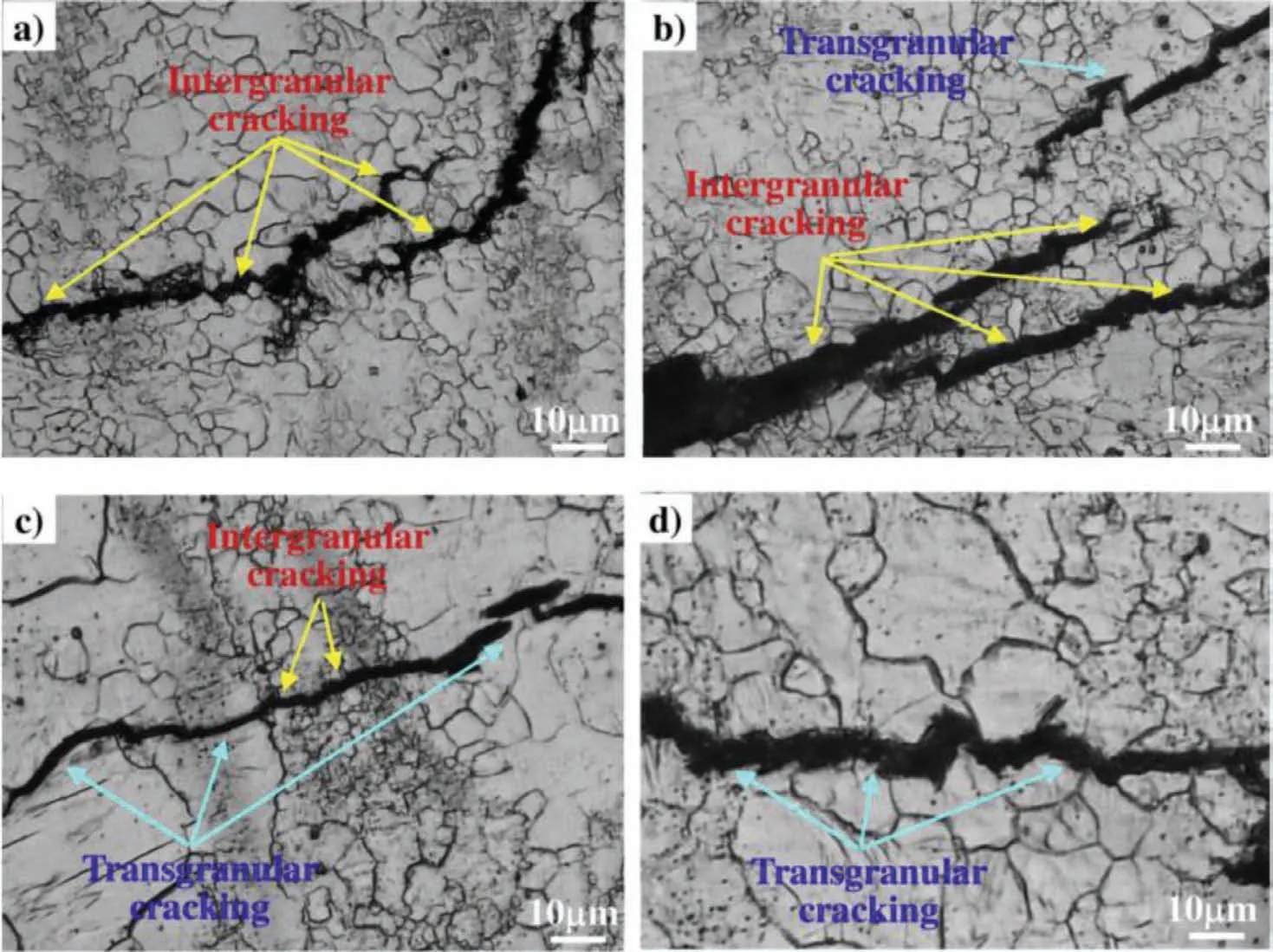
Fig.4.SCC crack propagation patterns of the Mg-Zn-Zr alloy (a) fine grain structure: intergranular cracking;(b) and (c) bimodal grain structure: the mixed type of fine intergranular cracking and coarse transgranular cracking;(d) coarse-grained grain structure: transgranular cracking [60].
2.2. Theoretical explanation of SCC of Mg alloys
For a long time,researchers have been committed to providing new insights into the SCC mechanism of Mg alloys.Since there are many factors that affect stress corrosion,such as composition,strain rate,corrosion environment,load,etc.The SCC mechanisms are complex and diverse,and it is a challenge to understand these mechanisms clearly [64,65].So far,the SCC mechanism of Mg alloys has been biased towards anodic dissolution and hydrogen-induced cracking.
Two mechanisms dominate the occurrence of SCC in Mg alloys.One is anodic dissolution mechanism,including film rupture theory and slip dissolution theory [66–76].The other is mechanical crack propagation mechanism,mainly dominated by hydrogen embrittlement [77–95].In general,the cracks caused by anodic dissolution are continuous propagation,and the crack propagation mechanism dominated by hydrogen embrittlement is discontinuous.
2.2.1.Anodicdissolution
It is known that the possible anode and cathode reactions of Mg in solution are as follows [66]:
Anodic dissolution is an important mechanism of SCC on Mg alloys.According to this mechanism,the SCC of Mg alloys can be divided into three processes: 1) electrochemical corrosion leads to a local crack of the surface film;2) the crack tip is further directional dissolved so that the crack continues to expand deeply;3) when the crack size exceeds the critical size,the remaining parts become unstable and fracture occurs [52,67].The two main models for anodic dissolution are listed below.
(1) Film rupture theory
Mg alloys are prone to form a loose and porous corrosion film in the corrosive environment.However,this film cannot achieve an actual passivation film.It is more accurate to call it a quasi-passivated film [68].Under the action of stress,the quasi-passivated film deforms and breaks [69–71].Fig.5 shows the film rupture theory [72].The Mg matrix is exposed to the corrosive medium,forming a large cathode-small anode state [73],resulting in a large instantaneous current.And when the stress is concentrated at the crack tip,it will hinder the formation of a new quasi-passivated film.The corrosion galvanic group will continue to form between the exposed fresh metal matrix and the passivation film,accelerating the dissolution of Mg and expanding and deepening the pits.Finally,the nucleation of anodic dissolution and stress corrosion cracks was promoted.Huang et al.[74] hypothesize that the quasi-passivation film of AZ80 alloy may be broken in the slip stage under the action of tensile stress,which promotes anodic dissolution and SCC crack formation,but more accurate experimental observation is still needed to determine this stage.
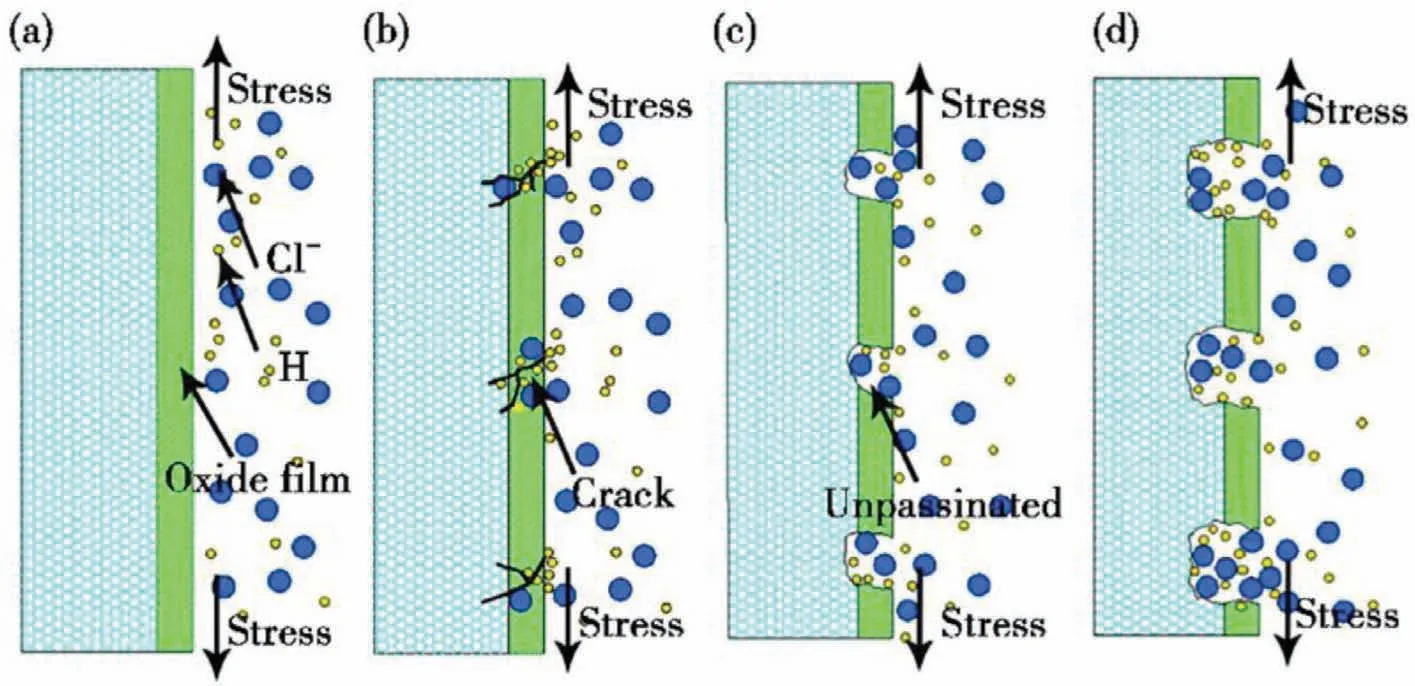
Fig.5.Schematic diagram of film rupture process: (a) oxide film formation;(b) oxide film rupture (Cl- or stress);(c) matrix dissolution (accelerate H entry);(d) accelerated matrix dissolution [72].
(2) Slip dissolution theory
According to the slip dissolution theory,the stress will promote the dislocation movement on the sliding surface and form a sliding step,and the surface protective film will be broken [67].Due to the destruction of the surface film,the exposed new substrate surface will be locally corroded as an anode.In these areas,the passivation behavior will occur again,which will hinder the corrosion process,but there is stress concentration in these areas.At this time,the newly formed passivation film will also crack again,and the substrate will continue to be partially dissolved.In this cycle,the stress corrosion crack will continue to nucleate and expand,eventually leading to failure [75].Fig.6 is a schematic diagram of the slip dissolution theory [75].This cyclic process will cause the crack to continue to expand and eventually lead to material failure [76].This theory is more persuasive than the film rupture theory.According to the film rupture theory,the quasi-passivated film will not form again.According to the slip dissolution theory,the passive film can be repaired,but it will be affected by stress concentration and fracture will occur again [14].Taheri et al.[77] reported that the film of Mg alloy was composed of outer Mg (OH)2layer and inner MgO layer,but the film was not stable.When there is a molar volume difference between the hydration products of MgO and Mg (OH)2,mechanical stress is easily formed inside,resulting in stress fracture of MgO film,and then Mg(OH)2self-recovery is formed at this fracture position.
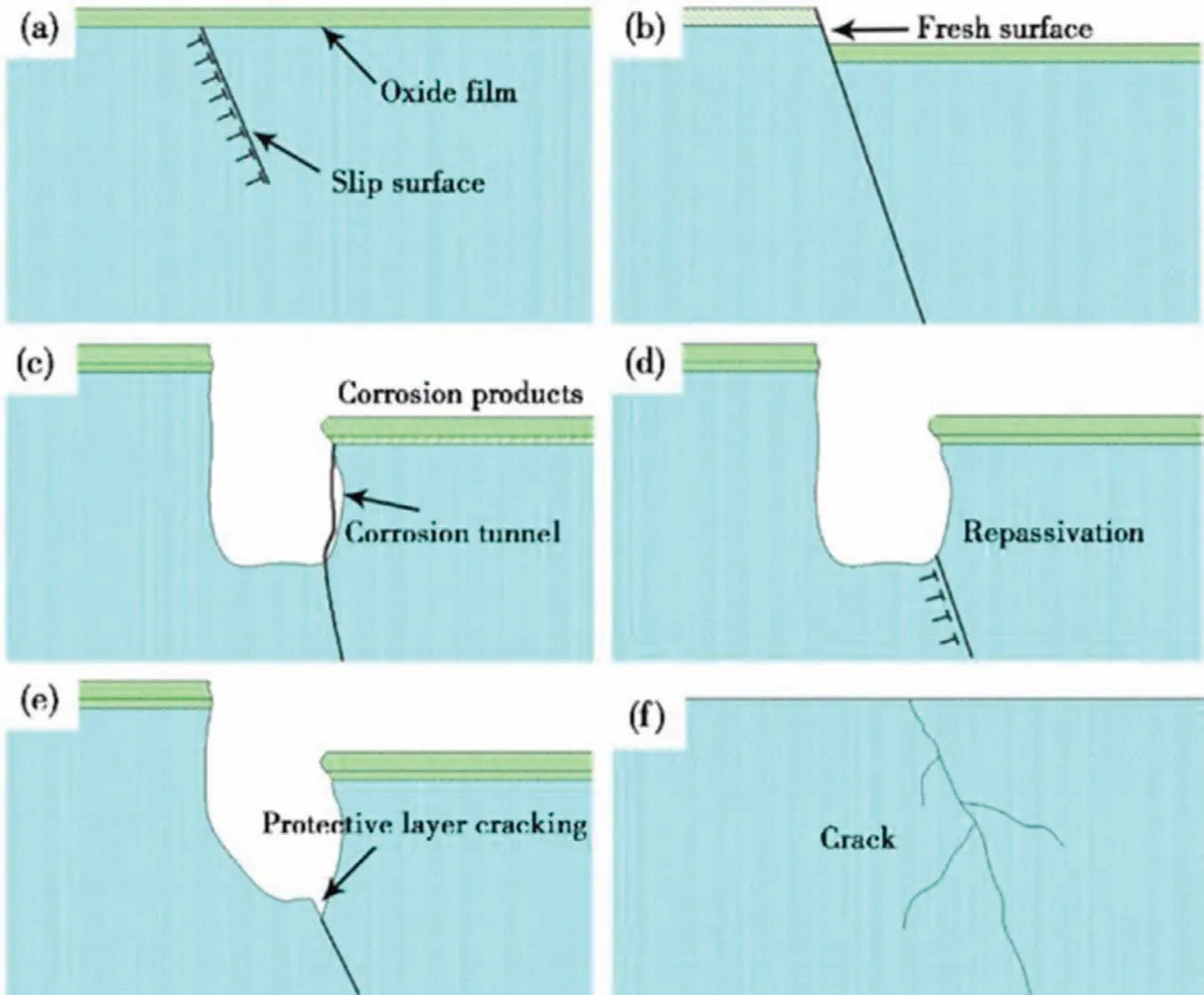
Fig.6.Mechanism of slip dissolution theory [75]: (a) dislocation slippage under stress;(b) rupture of the surface film,forming slip steps;(c) the matrix is corroded and corrosion pits are formed;(d) quasi-passivation of the exposed surface;(e) the film layer formed by quasi-passivation is broken again;(f) the crack extends into the matrix again.
In summary,the anodic dissolution theory mainly focuses on the local corrosion of the passive film and the substrate,but this theory cannot prove the stress corrosion behavior without the formation of the passive film.Therefore,this theory requires to be further enriched and improved for the lacks in certain application universality and persuasion.
2.2.2.Hydrogenembrittlement
Hydrogen embrittlement mechanism is mainly divided into the following types [78,79]:
•Hydrogen-enhanced decohesion (HEDE);
•Hydrogen-enhanced localized plasticity (HELP) theory;
•Hydrogen adsorption-induced dislocation emission (AIDE)theory;
•Delayed hydride cracking (DHC) theory.
Usually,AIDE and DHC theories are often used to explain TGSCC of Mg alloys [80].Both HELP and AIDE are related to hydrogen-promoted dislocation motion and local micro-void coalescence.Among them,HELP mainly occurs local nano-void coalescence process,and smaller and shallower dimples in fracture surface are regarded as evidence of the existence of HELP [79–81].AIDE is a continuous process in which adsorbed hydrogen promotes dislocation motion to generate cracks and merges with voids in front of the crack tip.It can determine the crystallographic path of the cracks.Theoretically,there are also shallow nano-dimples on the fracture surface.According to hydrogen embrittlement theory,hydrogen evolution reaction occurs at the cathode during the corrosion process and hydrogen atoms are produced[82].Some hydrogen atoms form H2,and the others become absorbed hydrogen.The hydrogen atoms,adsorbed on the surface of the alloy,are induced to diffuse into the defects due to the presence of stress,and the hydrogen atoms gather together to cause cracks [83].Under the action of stress,the cracks will continue to expand into the alloy,and eventually stress corrosion embrittlement occurs.Many studies have shown the importance of hydrogen embrittlement in SCC process [84–96].
(1) Hydrogen-enhanced decohesion (HEDE)
HEDE believes that hydrogen atoms are easier to diffuse and accumulate in the lattice gap and crack tip under the induction of applied stress,which reduces the binding energy between metal atoms and leads to the separation of adjacent metal atoms [83,84].Zhou et al.[84] studied the SCC behavior of ZK60 alloy under tin electrolyte layer condition,and found that under stress the place where the diffused hydrogen atoms gathered in the interior was the preferred crack initiation location.Winzer et al.[54] concluded that the fracture ofβparticles in Mg-Al alloy was dominated by HEDE mechanism based on fracture analysis,and the fracture of these particles promoted the initiation and propagation of stress corrosion cracks.When HEDE and DHC are dominant mechanisms,cleavage fracture was often observed in fracture surfaces[81].The existence of hydride is a method to distinguish the two mechanisms.Tuchscheerer et al.[85]studied the SCC sensitivity of AZ31 alloy,X-ray diffractometry did not determine a detectable amount of Mg hydrides in the embrittled AZ31 structure.Therefore,no hydride is regarded as one of the dominant characteristics of HEDE mechanism.
(2) Delayed hydride cracking (DHC)
DHC theory holds that when the hydrogen concentration formed in the corrosion exceeds the critical value,brittle hydrides are formed,and fracture occurs under stress,which becomes the crack source [69,81,85,86].Due to the existence of stress,these brittle hydrides are easy to crack,and there will be a continuous accumulation of hydrogen.This will again generate hydrides,and eventually cause crack nucleation and propagation.The above process can be summarized as Fig.7.The premise of this theory is that brittle hydrides must be generated,and the generated brittle hydrides fracture under stress to form crack sources.Typically,researchers link TGSCC to the DHC mechanism.However,when the hydride is formed in the second phase distributed along the grain boundary,the crack of the alloy will appear as IGSCC.Therefore,TGSCC is not conclusive evidence of DHC mechanism.In fact,SCC cracks are related to local hydrogen concentration.When the hydrogen concentration in the grain is high,TGSCC will be formed.On the contrary,when the hydrogen concentration along the grain boundary is high,IGSCC will be formed [81].

Fig.7.Schematic diagram of hydride delayed cracking theory: (a) hydrogen accumulates at crack tip under stress;(b) aggregation of hydrogen to form brittle hydrides;(c) brittle hydrides fracture under stress,forming a new crack source;(d) forming new brittle hydrides and repeating the previous process.
Zeng et al.[87] found the existence of hydride phase by observing the fracture surface of AZ31 alloy,and confirmed the DHC mechanism.Chen et al.[88] believed that the increase of crack length after vacuum annealing can prove the existence of hydride in SCC of AZ91 alloy.After vacuum annealing at 480 °C,the crack length was obviously increased.Wang et al.[89] reported that the hydrogen aggregation reaction near theβphase in AZ91 alloy formed brittle hydride after cathodic charging.The local hydrogen aggregation formed hydrogen high pressure,broking the brittle hydride and accelerating the formation and expansion of SCC.The DHC mechanism can explain the transgranular cleavage fracture behavior of Mg-Al-Zn,Mg-Al-Mn,and Mg-Zn-Zr [55,84,90]alloys under stress corrosion.However,with the help of secondary ion mass spectroscopy technology,Chen et al.[78] have found that when the hydride preferentially forms in the second phase distributed along the grains,IGSCC cracks appear in AZ91 alloy due to local hydrogen high pressure and brittle hydride fracture.Merson et al.[56] considered that the SCC mechanism of ZK60 and AZ31 alloys was likely related to DHC by testing the diffusion hydrogen concentration and the fracture surface morphology of brittle cleavage during SCC.In addition,there may be many intermetallic compounds in Mg-RE alloys,but due to the strong affinity between RE and H atoms,the main hydride is MgH2when it reacts with hydrogen.And the external hydrogen pressure determines the type of hydride of Mg alloy,H diffusion controls the growth of MgH2[91].
(3) Hydrogen-enhanced localized plasticity (HELP)
HELP is that hydrogen at the crack tip can reduce the dislocations’ elastic interaction and dislocation movement resistance,which makes the local crack easy to aggregate and propagate [14,92].The crack growth will then occur by a more localized microvoid-coalescence process,as shown in Fig.8,which mainly involves a pore-coalescence process [85].When the diffusion rate of hydrogen reaches a high level,hydrogen can move with the dislocation.Crack paths could be transgranular or intergranular depending on whether locally high hydrogen concentrations were present within grain interiors or adjacent to grain boundaries.Researchers used the embedded atom method for atomic calculation of crack propagation,and found that hydrogen facilitated the dislocation motion at the crack tip [93].Kuramoto et al.[94] reported that the accelerated diffusion of hydrogen caused by dislocation movement led to the brittle SCC of AZ31 alloy.Zhou et al.[84] found that there was a high concentration gradient of hydrogen in ZK60 alloy in 0.1%NaCl+0.05%Na2SO4+0.05%CaCl2solution,which provided a preferred way for hydrogen diffusion in Mg alloy.Moreover,the local corrosion of hydrogen accumulation position is more serious.This is because the HELP mechanism can promote local micro-hole coalescence and lead to local plastic deformation,which provides a way to form and expand SCC.
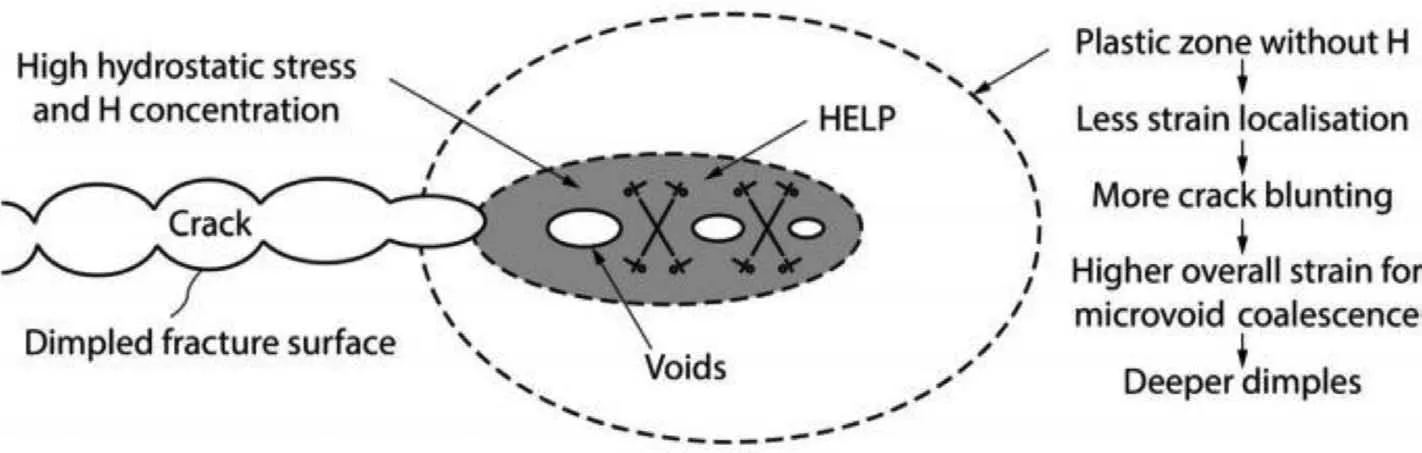
Fig.8.Schematic diagram illustrating the HELP mechanism [85].
(4) Adsorption-induced dislocation emission (AIDE)
AIDE mechanism is related to dislocation emission.The high concentration of hydrogen atoms at the crack tip weakens the binding energy between metal atoms,and the high stress in the front of the crack tip promotes dislocation emission from the crack tip forward [81].In the process of dislocation emission,the crack propagates due to the aggregation of local defects in the plastic deformation zone at the tip [95].Therefore,AIDE also requires hydrogen to diffuse and adsorb into the crack tip or hole to weaken the atomic bond,so as to promote dislocation emission from the crack tip.And large strain is required to push through the micro-voids to promote crack propagation.When AIDE occurs,Nano-scale dimples are observed on the fracture surface.In addition,the cracks generated by AIDE mechanism can be either IGSCC or TGSCC,depending on the location of dislocation emission and cavity formation [96].
It can be seen from the above that the SCC mechanism of Mg alloys is extremely complex and difficult to distinguish the specific mechanism.Usually,the SCC mechanism is affected by several mechanisms,and the different service environments will also lead to the change of SCC mechanism.Yang et al.[73] found that the SCC of AM50 alloy in distilled water was mainly hydrogen embrittlement,while in NaCl and Na2SO4solutions,hydrogen embrittlement and anodic dissolution acted together.Therefore,single mechanism cannot fully explain the SCC behavior of Mg alloys.In addition,the specific mechanism of hydrogen embrittlement is not clear.Hydrogen diffusion in Mg alloys is a dynamic process,which is difficult to monitor synchronously.However,hydrogen has an essential influence on the SCC behavior.Consequently,it is necessary to focus on the effect of hydrogen on SCC behavior of Mg alloys in the future.
2.3. SCC sensitivity test methods
2.3.1.Slowstrainratetest(SSRT)
During SSRT experiment,flake or round bar samples are fixed on a special testing machine,and then stretched at a constant slow strain rate (∼10-5s-1–10-7s-1).SSRT test sample size can be designed according to ASTM G129–00(2013) standard [97].The sample’s stress corrosion sensitivity was assessed by thoroughly examining the fracture time,elongation after fracture,yield strength,tensile strength,and fracture morphology [98].The SSRT is of high sensitivity and can be used to study the mechanism of SCC by continuing the experiment until the samples completely break.SSRT can also accurately distinguish SCC fracture and mechanical fracture zone by fracture surface analysis [23,24].SSRT is one of the most used SCC sensitivity test methods,and the most important thing is to select the appropriate strain rate[23,90,98].Fig.9 is a common SSRT device [99].
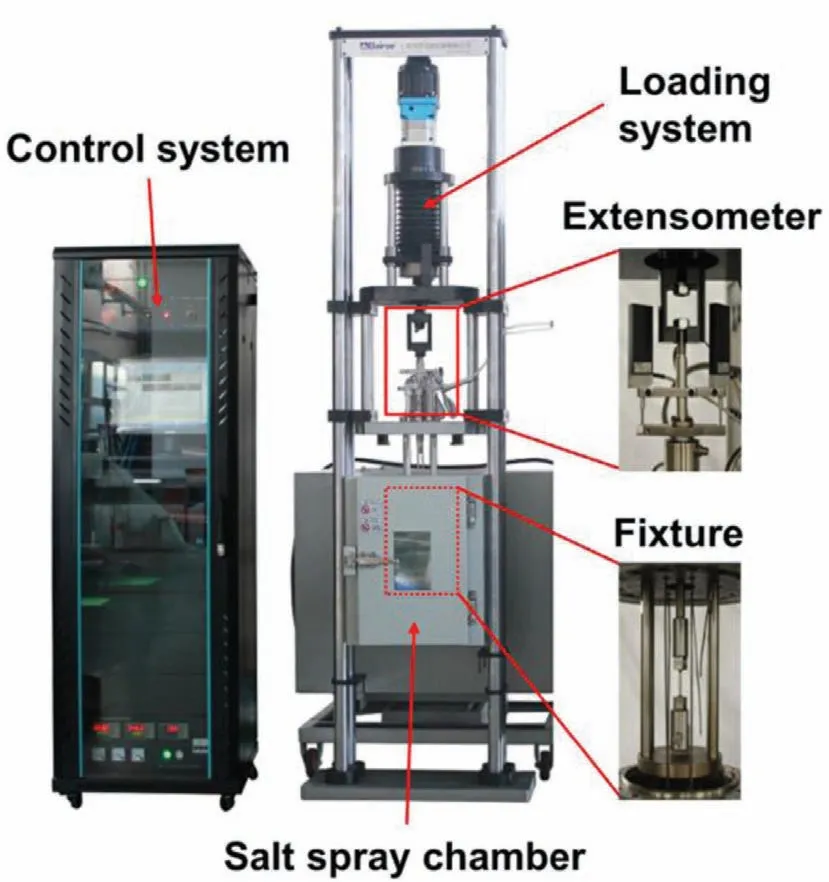
Fig.9.SSRT experimental device [99].
However,SSRT also has some limitations.Since the crack initiation time is unknown,this method usually underestimates the SCC crack growth rate [55].Moreover,since the strain rate is not an easily determined parameter,the results cannot be reasonably used for engineering design.And in the process of SSRT,there are many external factors that are difficult to fully control [98].In addition,the experimental equipment of this method is more complicated,and the shortening of the experimental period also brings about a shorter incubation period of stress corrosion crack,so it is difficult to obtain the information of SCC crack propagation process.
2.3.2.Constantloadtest(CLT)
CLT is a method to test stress corrosion performance.The sample is usually cylindrical or rectangular.By fixing one end of the sample,the other end is added with weights or springs as a constant static load,and the sample is immersed in the test solution [100].Finally,the threshold of stress corrosion fracture of the sample is estimated by drawing the initial loading stress and the fracture time of the sample [101].Therefore,CLT can accurately measure the stress date before the initial crack is generated.Fig.10 is a common CLT device schematic diagram.It applies a load to the sample through the reaction force of the helical compression spring,and the load can be adjusted by the tightness of the nut.Besides,a clock having external battery connection with banana clip is fixed to the upper and lower cups of loading spring.When specimen fails,the banana clip disconnects and clock stops,enabling to determine the exact time of failure [100].Moreover,after the crack is generated,the effective cross-sectional area of the sample decreases and the stress increases,and the sample may break prematurely.Usually,if no failure occurs,the test is typically terminated after any selected time.In this case,the approximate value of the crack propagation velocity is obtained by dividing the crack length by the test time.However,since crack initiation may not occur at the beginning of the experiment,this method is not accurate [102].
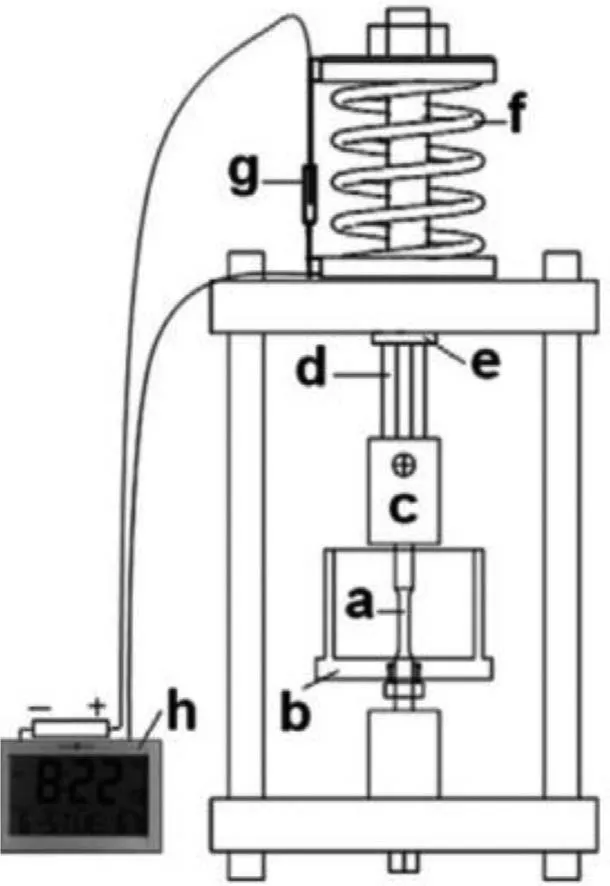
Fig.10.Schematic of constant load test rig showing: (a) test specimen,(b)corrosion cell,(c) grip member,(d) pull rod,(e) flat locator,(f) loading spring,(g) banana clip,and (h) clock [100].
2.3.3.Constantextensiontest(CET)
CET has also been widely used in SCC sensitive test.Sometimes referred to as a constant total strain test,the sample is deformed by stretching or bending,and the deformation state is fixed by means of an external frame or bolt,so that the total strain of the sample is constant during the test.Fig.11 shows the most common samples,including bending beams,U-shaped,and C-rings [103–105].Compared with other test methods,this experiment is simple,low cost,and can qualitatively obtain the SCC sensitivity of the alloy.This method is suitable for long-term and large-scale experiments in containers,which can significantly shorten the experimental cycle.This method can also visually observe the crack propagation process.Although this method is simple and inexpensive,there are some drawbacks.The sample must be loaded prior to exposure to the environment,and this preloading may cause deviations in the experimental results.Moreover,the determination of threshold stress and failure time mainly depends on subjective judgment,so there is still deviation between true stress threshold and failure time.During test process,the stress in the crack propagation process is more complex.It can be increased or reduced,so the stress threshold test is not accurate.In addition,CET may not observe the stress corrosion fracture [102].The applied load can only be measured indirectly through strain changes.The bifurcation or passivation of the crack will affect the calculated value of crack growth rate.Furthermore,elastic relaxation of the loading system during crack propagation leads to increased displacement and higher load than expected [102].

Fig.11.The schematic of (a) bending beam samples,(b) multi-point bending samples,and (c) C-ring samples [103,105].
2.3.4.Linearincreasingstresstest(LIST)
The LIST device is equipped with a synchronous motor,which increases the strain and accelerates the stress corrosion process by continuously increasing the load [106].The specific device is shown Fig.12.The essence of LIST is the same as SSRT,but the former is loading control and the latter is displacement control [106].LIST is also more suitable for studying SCC in corrosive environment.This method is especially suitable for determining thresholds [107].LIST combined with electrochemical detection technology can measure the threshold stress of SCC relatively directly [108,109].
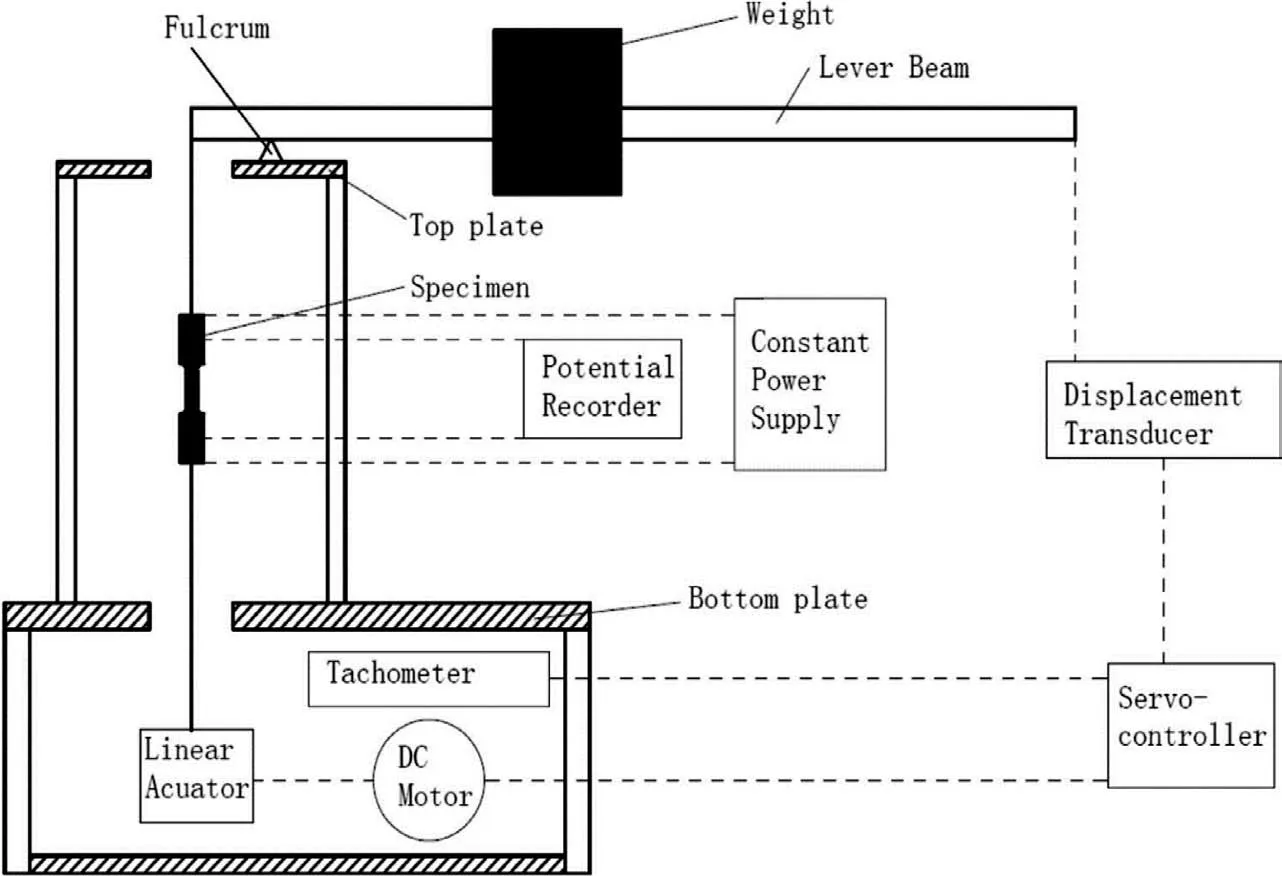
Fig.12.Device schematic of LIST [107].
In the process of LIST,cracks propagate and fracture rapidly after reaching threshold stress.Therefore,LIST requires less time,typically 30–50% of other methods.And LIST can directly test the SCC threshold and crack growth rate[54].However,the crack propagation accelerated by LIST may affect the subsequent strict analysis of the SCC fracture surface,and cannot accurately simulate the operating conditions [54,98].
2.3.5.Fracturemechanics(FM)
Due to the occurrence of SCC in corrosive media,it is difficult to detect the initial stage of crack,and later fracture morphology observation cannot accurately predict the main crack [96].Therefore,fracture mechanics technology appeared.This technique can be used to measure the critical stress field intensity factor KISCC(which is the minimum stress intensity required for crack propagation in a particular corrosive environment),stress intensity factor KC(stress intensity factor MPa m1/2)),and stress corrosion crack velocity of metal materials in the application environment.The technology uses prefabricated cracks or design notched specimens,which can shorten the time and improve the reproducibility of the experiment [110].However,prefabricated cracks may change the local stress distribution,and the cracking mechanism of the alloy may even change.And small changes in the corrosion environment will change the KISCCvalue.Most notably,in practical applications,engineering materials will undergo certain plastic deformation at the crack tip before fracture.This method is effective only when the size of the plastic zone is negligible compared with the geometry of the specimen [31,58].
Direct current potential drop (DCPD) method has been widely used.By applying a constant current to the pre-cracked sample,the cross section of the sample will decrease due to the crack propagation,and the resistance of the sample will change,and the changed resistance value will be converted into the crack depth.KISCCis defined as the point where the DCPD curve is obviously nonlinear [111].The curve of crack growth rate and stress intensity factor is also one of the common ways to quantify the SCC sensitivity of alloys.Merson et al.[112] conducted crack propagation tests on notched specimens ZK60 and AZ91 alloys.Both alloys undergo three stages as shown in the Fig.13: rapid crack propagation at KISCC,stable crack propagation and a sharp increase in the final crack propagation rate.And when the two alloys are in the stable SCC propagation stage,the change of the fracture mode of the alloy is related to the crack propagation rate.Winzer et al.[54] tested the SCC behavior of AZ91 in distilled water using DCPD technology,and the KISCCof AZ91 was between 55 and 75 MPa.However,if the principal stress corrosion crack of the alloy cannot be judged,KISCCcannot be measured [111].In view of the tracking and observation of crack propagation process,researchers have also put forward new ideas,using industrial cameras to conduct in-situ stress corrosion detection of metals.At present,it has been reported that the stress corrosion behavior can be analyzedinsituby industrial camera,and the whole process of crack initiation and propagation can be observed,and the accuracy can reach the micrometer level [113,114].Zhao et al.[115] conducted SCCin-situobservation of AZ91 alloy by industrial camera,and usedin-situelectrochemical technology to detect the electrochemical signal changes of samples during SCC process.Through real-time observation and detection,the whole process of SCC evolution was obtained.Therefore,this method can also be used as one of the effective means to detect the crack evolution process.

Fig.13.The crack growth da/dt– K for AZ31 and ZK60 subjected to SCC[112].
The above methods are commonly used to test the SCC sensitivity of Mg alloys.Among them,SSRT is the most commonly used test method because it is the most susceptible to the stress corrosion sensitivity of the test alloy.In addition,SCC fracture of Mg alloy is closely related to hydrogen.The invasion of hydrogen is related to the mechanical or chemical cracking of the surface corrosion film of the alloy.The method of testing SCC may also affect the integrity of the surface corrosion film.Therefore,it is necessary to select a suitable test method to correctly determine the SCC sensitivity of the alloy.
3.SCC research progress of Mg alloys
The standard electrode potential of Mg is low (-2.4 Vvs.standard hydrogen electrode),and the corrosion resistance is poor.Under the combined action of stress and corrosion medium,Mg alloys are prone to SCC failure even if the stress is as low as 50% of the yield stress [96],resulting in immeasurable loss.Therefore,understanding the rules of SCC mechanism and the factors affecting SCC,and proposing measures to improve the SCC resistance of Mg alloys are helpful in reducing unnecessary losses and expand its application fields.The current researches on Mg alloys SCC mainly focuses on the influence of internal microstructure and external environment.This paper mainly introduces alloying,preparation process,surface treatment,corrosion medium,and strain rate,which greatly influence the SCC of Mg alloys.
3.1. Alloying
3.1.1.Commonelements
Generally,Al,Ca,Zn,Mn,Sr,and other elements can affect the SCC sensitivity of Mg alloys owing to different compositions and microstructures [116,117].The addition of Al and Ca forms discontinuously distributedβ-Mg17Al12phase or brittle Al2Ca phase,which will reduce the SCC resistance of the alloy [111].The increase of Zn content will also increase the SCC sensitivity of Mg alloys,such as AZX612(1%Zn),Mg-5Zn,ZK60 (6% Zn),and Mg-Zn-Mn (6% Zn) [118–120].Mn and Sr can effectively enhance the SCC resistance of Mg alloys by controlling their addition.
SCC sensitivity of Mg-Al alloy increases with increasing Al content.When Al content is between 4% and 9%,Mg alloys show high SCC sensitivity [78].Inhomogeneous and discontinuousβ-Mg17Al12phase can be observed in Mg-Al alloy,and the hydrogen overpotential is higher than that of the matrix.As a cathode,the anodic dissolution of the matrix is promoted,leading to the initiation and propagation of SCC cracks [111].In addition,the stress concentration destroyed the surface protective film,and matrix was exposed to the corrosive medium,which was conducive to the diffusion of hydrogen into the matrix and increased the tendency of hydrogen embrittlement [121].When the Mg alloy is subjected to external stress,the stress leads to the formation of a local melting zone in the higher dislocation density area of the alloy surface.Xie et al.found that in the local melting zone of AZ91,pitting preferentially nucleates and expands,producing more corrosion products and covering the alloy surface.With the increase of stress,more high dislocation density regions are formed in the Mg alloy matrix,which will accelerate the nucleation and expansion of pitting corrosion and accelerate the generation of corrosion products.Therefore,the improvement of corrosion sensitivity of AZ91 Mg alloy can be attributed to the effect of growth dislocation/defect density [115].In addition,it shows that the high SCC sensitivity of AZ31 alloy is also related to the existence of deformation twins.Under low strain conditions,twin-sized voids appear in the local area of the alloy,which provides a favorable path for SCC nucleation and expansion [122].The addition of Zn and Ca will increase the hydrogen embrittlement tendency of Mg alloys [29].Prabhu et al.[26] found that Mg-4Zn alloy has high SCC sensitivity in SBF solution.Shi et al.[118] found that Mg-5Zn also had high SCC sensitivity in distilled water,which might be dominated by the combination of HELP,HEDE or DHC mechanisms.The final mechanism mainly depends on the microstructure of the alloy.Yuasa et al.[119] reported that the addition of Ca is conducive to forming Al2Ca phase,which forms the primary battery with the matrix,and deteriorates the SCC resistance of AZX612 alloy.
Typically,Mn can reduce the content of impurities such as Fe in Mg alloys [123,124].The addition of Mn is conducive to reducing the number of Mg17Al12phases,alleviating the galvanic corrosion betweenβphase and matrix,and improving the SCC threshold (90% of yield strength),thereby improving the SCC resistance [14,58].In addition,Mn and Sr can also refine grains to improve the SCC resistance.Yu et al.[25] added appropriate amount of Sr (≤0.2%) in Mg-4Zn alloy,and found that the microstructure of Mg-4Zn was refined and more uniform,and the surface protective film was denser,which improved the SCC resistance of the alloy.However,excessive Sr induces the discontinuous distribution of the second phase along grain boundaries,which deteriorates the SCC resistance[31,32].It is worth noting that too much addition of Sr into Mg alloy is harmful to cell proliferation [125].Fig.14 shows the SCC performance of Mg alloys with various common alloying elements.Effective alloying can purify Mg alloys and reduce the number of second phases (as cathode phases).It can also effectively reduce local corrosion and hydrogen by refining grains,which enhances the composition and compactness of the surface film,thereby improving SCC sensitivity.

Fig.14.SCC properties of Mg alloys with different alloying elements (a) SSRT stress-strain curves at strain rate of 10-7 s-1 for (left) AZ31 and AZ80 alloys in air and (right) in NaCl solution [126];(b) stress versus strain curves for the ZK60 alloy at a strain rate of 1 × 10–6 s-1 under different conditions[80];(c) apparent stress-strain curves of the specimens tested in different environments.1: in Glycerol,2: in 0.6 M NaCl,3 in 0.6 M NaCl+10-2 M NaHAsO4,4: in 0.6 M NaCl held at-1.9 VSCE,5 in 0.6 M NaCl+10-2 M NaHAsO4 held at-1.9 VSCE,6 in 0.6 M NaCl held at-2.4 VSCE and 7: in 0.6 M NaCl+10-2 M NaHAsO4 held at-2.4 VSCE [124];(d) tensile stress–strain curves of the as-cast ZK40xSr alloys tested in air and m-SBF solution at 37 ± 1 °C [33];(e) fracture cross-section images of Mg-4Zn-xSr alloys in Hank’s solution [25];(f) stress-strain curves of the as-received and solution-treated(a) AZX612 and (b) AZ61 alloys in air and 0.01 M NaCl solution [119].
3.1.2.Rareearthelements
Studies have shown that the appropriate addition of rare earth elements is beneficial to improving the SCC resistance of Mg alloys[127–129].In fact,the volume fraction,distribution,and cathodic reduction rate of the second phase containing rare earth show different impacts on different Mg alloys.Generally,the corrosion film of pure Mg is soluble and cannot protect the matrix from corrosion.However,a denser and more stable surface protective film is easily to be formed on the surface when some rare earth elements are added,which limits the penetration of hydrogen into the matrix [88].When the corrosion potential of rare earth containing phase is lower than that of the matrix,it can act as micro anode to protect Mg matrix [127,130].However,this phase may form corrosion pits in these areas after preferential corrosion which would cause stress concentration and promote hydrogen entry into the alloy,resulting in crack initiation[131].Therefore,when the rare earth containing phase acts as micro anode,it may deleterious to SCC resistance of Mg alloys.Nevertheless,some kinds of second phase containing rare earth will also act as a cathode to accelerate the corrosion of matrix.Brittle rare earth phases will also act as crack initiators,increasing corrosion and accelerating the SCC process.The common rare earth elements in Mg alloys are Gd,Nd,Ce,Y,and others,which have different influences on SCC behavior of Mg alloys [130–140].
Padekar et al.[129] compared the SCC resistance of EV31A and AZ91E,and found that EV31A containing Nd and Gd had higher SCC resistance.The addition of Nd and Gd is conducive to the formation of a stronger and stable protective film,and improves the SCC threshold.The addition of Nd and Gd obviously inhibits the formation of Mg17Al12phase in Mg alloys,reduces the accumulation of hydrogen inβphase,decreases the formation of local corrosion pits.Finally,it is beneficial to improve the protection performance of the surface protective film,thus showing a good anti-SCC performance [131–135].Nevertheless,Elkaiam et al.[34] found that the addition of 3% Nd in Mg-Zn alloy has no effect on the surface protective film,so it has no effect on the SCC behavior of the alloy itself.The final determinant depends on the ability of the alloy to influence the microstructure.Table 1 lists the differences in SCC resistance of Mg alloys due to different addition elements.It is beneficial to reduce SCC sensitivity only by selecting the appropriate alloying elements.It can also be seen from the table that the fractures of different Mg alloys in air and corrosive media are not the same.This is mainly due to the mechanical overload fracture caused by slow tensile of Mg alloys in air.When the alloy in corrosive medium,SCC cracks are generated and the fracture is accelerated under the action of stress.In addition,the fracture of Mg alloy in corrosive medium is mainly TGSCC,which is also related to the important role of hydrogen embrittlement.
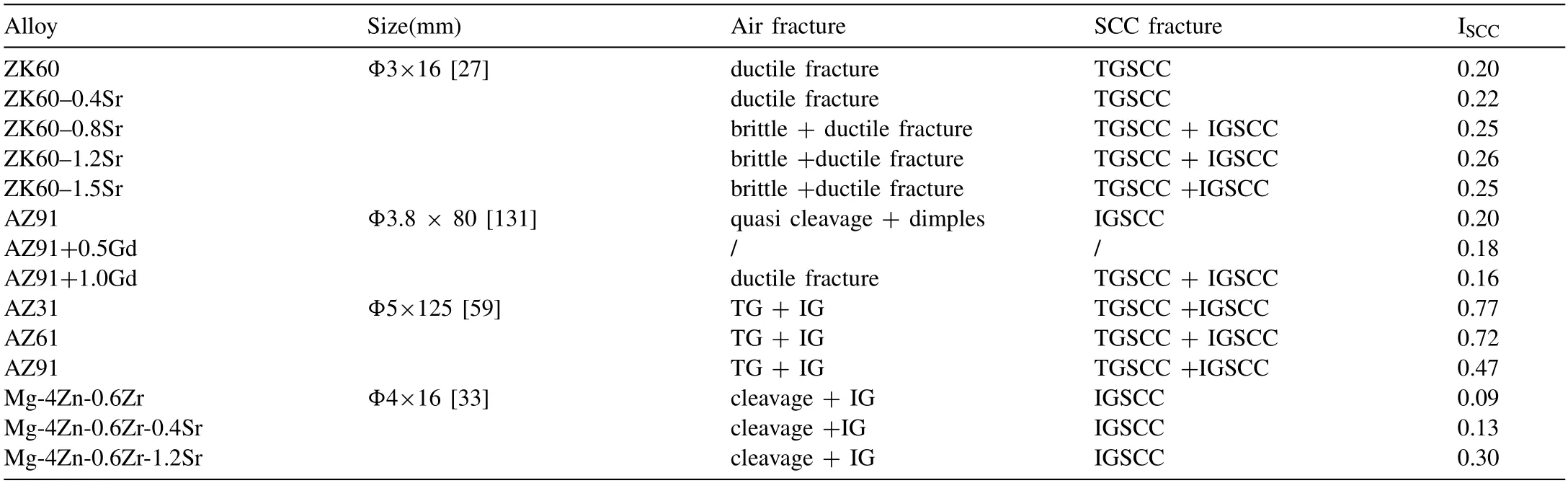
Table 1Comparison of SCC properties of different alloys.
It is generally believed that SCC is subjected to external stress.Soza´nska et al.[136] also found cracks in the microstructure of WE54 alloy in SSRT experiment.However,these crack initiations are attributed to hydrogen and tensile stress (external stress).However,recent studies have found that stress also exists in the internal microstructure of Mg alloys,which may accelerate SCC.In Mg-3Gd-1Zn-0.4Zr alloy(GZ31K),Zhang et al.[137] observed the changes of vacancies and voids produced by the dissolution ofα-Mg matrix and the volume of porous Mg oxide/ hydroxide formed during the corrosion process,forming stress in the interior.Stacking faults (SFs) rich in Gd and Zn atoms deformed and dissolved under stress.Fig.15 shows the cracks observed in the microstructure of GZ31K alloy.It can be seen that rare earth elements not only affect the outer protective film,but also may produce internal stress or change the microstructure to affect the SCC behavior of Mg alloys.
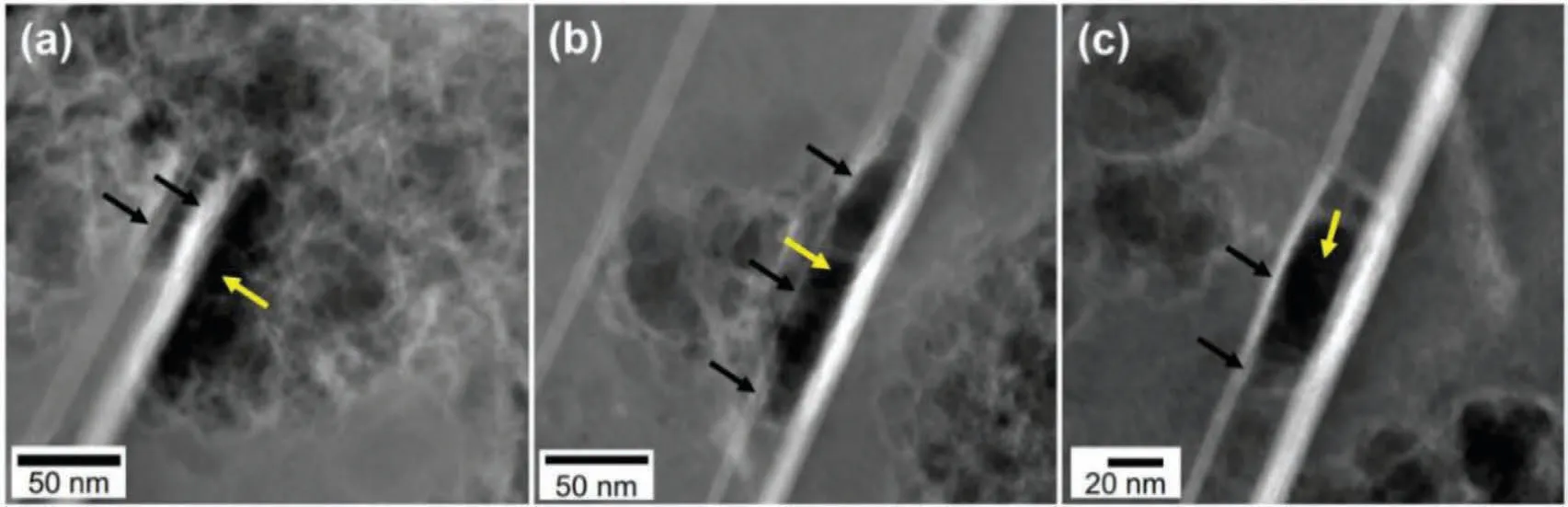
Fig.15.STEM images of GZ31K alloy after immersion in 0.1 M NaCl solution for 1 min.The yellow arrows in a,b and c point to the dissolution of the dominant α-Mg matrix at the edge of the solute-rich stacking faults.The black arrows point to distortion or cracks [137].
Y element can accelerate the corrosion rate of Mg-Y alloys and form corrosion pits,which is bad for SCC resistance[138–140].There are precipitates formed by Y,Nd and Mg in WE54 alloy,which accelerates the corrosion pits in the matrix,facilitates hydrogen permeation and weakens the SCC resistance [136].Manivannan et al.[140] found that the addition of Ce induced the formation of brittle Al4Ce phase in AZ91 alloy,which increased the hydrogen embrittlement tendency of AZ91 alloy and improved the SCC sensitivity of the alloy.And with the increase of Ce content,the ultimate tensile strength (UTS) of AZ91 decreases obviously.
As shown in Fig.16(a),the existence of different second phase affects the SCC sensitivity of Mg alloys and the development of SCC [90].In general,SCC cracks in Mg-Al alloys are first initiated inβphase.In Fig.16(d),by alloying treatment,the number ofβphase in AZ91 is reduced,the distribution is more uniform,and the SCC resistance of the alloy is improved by about 37%.The ZE41,QE22,and EV31A alloys in Fig.16(b) show IGSCC+TGSCC in 0.5 wt.% NaCl solution.The cracks of the three alloys are related to the intergranular micro-galvanic corrosion of the second phase[63].In Fig.16(c) and (e),as the alloy content changes,the volume fraction and distribution of the second phase change,which in turn affects the galvanic corrosion and stress concentration of the adjacent matrix.An excessive and continuous networklike second phase can even be a potential SCC crack source.

Fig.16.Microstructure and SCC fracture morphology of Mg alloys with different alloying: (a) microstructure and corresponding fracture cracks of AZ91 and AM30 alloys [90];(b) (1) fracture surfaces of ZE41 in 0.5 wt.% NaCl-predominant intergranular and isolated transgranular (arrows) cracking,(2) fracture surfaces of QE22 in 0.5 wt.% NaCl-intergranular and transgranular (arrow) cracking,and (3) fracture surfaces of EV31A in 0.5 wt.% NaCl-intergranular and transgranular (arrow) cracking [63];(c) the crack propagation paths of the Mg-4Zn-xSr alloys (1) and (5) Mg-4Zn,(2) and (6) Mg-4Zn-0.1Sr,(3) and(7) Mg-4Zn-0.2Sr,(4) and (8) Mg-4Zn-0.4Sr [32];(d) microstructure and slow rate stress-strain curves of AZ91 and AZ91ErCe alloys [141];(e) the BSE images and corresponding EDS analysis of the as-cast ZK40xSr alloys: (1) and (4) Mg-4Zn-0.6Zr-0.4Sr,(2) and (5) Mg-4Zn-0.6Zr-1.2Sr,(3) and (6)Mg-4Zn-0.6Zr-1.6Sr [33].
The addition of alloying elements mainly affects the internal microstructure.The generated brittle phase will be beneficial to SCC,and the change of microstructure will also change the diffusion of internal hydrogen and the corrosion of the substrate surface.The uniform and continuous microstructure is beneficial to reduce local corrosion and internal hydrogen diffusion,so as to improve the SCC resistance of the alloy.Moreover,the second phase of rare earth has higher stability in the corrosive medium and plays a more protective role in the corrosion barrier of the matrix.In particular,the continuous distribution of the second phase can also effectively inhibit galvanic corrosion and slow down local corrosion [139].With the application of Mg alloys in biomedical field,the selection of alloying elements tends to be multi-functional,such as strong antibacterial,biosafe,and biocompatible.
3.2. Preparation process
Preparation processes directly affect the microstructure(grain size,distribution,and shape characteristics of the second phase) and internal stress distribution of Mg alloys [142].In general,finer grains mean more grain boundaries and thus result in more stable protective film on the surface of Mg alloys which is good for SCC resistance [143].In addition,uniform and fine grains are beneficial to improve corrosion resistance and stress corrosion resistance [131,144].The second phase as the cathode becomes small and uniformly distributed after extrusion,which can inhibit the local corrosion of Mg alloys.The second phase are conducive to form a more reliable dense uniform film in corrosion media,which is conducive to reducing the matrix SCC initiation zone [139].Nevertheless,the residual stress in the machining process will reduce the critical threshold stress of SCC and accelerate the initiation and propagation of SCC cracks[145,146].Heat treatment can effectively reduce or even eliminate residual tensile stress[14].The main preparation processes of Mg alloys include forming,plastic deformation,and heat treatment [147–157].
Compared with conventional casting alloys,rapid solidification (RS) can significantly make the microstructure more uniform and finer,and thus improve the SCC resistance of Mg alloys [147].The passivation film of Mg alloys prepared by RS is more stable and can be passivated rapidly after being destroyed by corrosive medium [145].Moreover,RS can improve the ductility of Mg alloys and inhibit the expansion and brittle fracture of SCC.Hakimi et al.[146] found that the SCC resistance of EW62 prepared by RS was modified mainly due to the following reasons: one is that the increase of Nd2O3content in the external oxide improves the stability of protective film,and the other is that more uniform and finer microstructure caused by RS restricts the formation and penetration of hydrogen.However,different from the traditional theory,Argade et al.[148] found that the ultrafine grained AZ31 alloy obtained by friction stir processing showed high SCC sensitivity under 3.5 wt.% NaCl condition.It is likely to be related to the acceleration of hydrogen diffusion.Ultrafine grain means that there are finer and more grain boundaries.If the hydrogen concentration at these locations exceeds a critical value,these locations are likely to become crack initiation sites,thereby accelerating the hydrogen embrittlement process [55].Therefore,grain refinement is not necessarily beneficial to improve SCC resistance.The grain size is refined and the number of grain boundaries increases.Theoretically,the grain boundaries can also be used as hydrogen traps to capture free hydrogen ions.Moreover,the micro-couple corrosion along the grain boundaries may also increase[27],which increases the possibility of hydrogen embrittlement of the alloy.
The processing technology can also affect the SCC sensitivity by changing the stability of the passivation film on the alloy surface and the diffusion rate of hydrogen in the matrix.It has been reported [35] that the SCC resistance of AZ31 alloy is effectively improved by cryogenic machining,and its SCC sensitivity indices IUTSand Iεare reduced by about 23%and 11%,respectively.This is owing to the cryogenic machining broadens the nanocrystalline layer and promotes the accelerated formation of oxide on the passivated surface.More importantly,the cryogenic machining sample is not prone to local corrosion and reduces the SCC sensitivity.Peron et al.[36] found that the main reason for the increase of SCC resistance of AZ31 alloy after equal channel angular extrusion(ECAP) was that the decrease of grain size after ECAP made the formation of oxide on the passive surface faster and had stronger protection for the matrix.Nevertheless,Xie et al.[149] pointed out that ECAP processed AZ61 alloy showed more severe SCC sensitivity.Although the grains were refined after ECAP,which could delay the crack propagation to a certain extent,severe plastic deformation occurred after repeated ECAP not only produces more polycrystalline defects and accelerates the diffusion of internal hydrogen,but also increases the number ofβphases.Hence it provides a path for SCC expansion and deteriorates the SCC resistance of AZ61 alloy.It can be seen from the above that appropriate processing technology can slow down the SCC process by refining the grain,impeding internal hydrogen diffusion and enhancing the protection and stability of the protective film.
The SCC behavior of the internal residual stress field in the material also plays a very important role,especially the residual stress generated near the free surface or crack tip.Residual stress mainly exists inside the metal material,especially near the surface,which is due to the introduction of external mechanical stress in the manufacturing process (hot rolling,cold drawing,welding,etc.).Other researchers have found that the corrosion product film formed by the alloy in the corrosion solution may also introduce residual stress [150].The formation of the corrosion product film includes the inward diffusion of anions and the outward migration of cations.When the cation moves faster,a large number of cation vacancies will be generated on the metal side,and the film side will hinder the contraction of the metal layer,resulting in tensile stress at the internal interface.On the contrary,the film grows to the metal side.At this time,the volume of the film is larger than that of the oxide metal.Due to the lattice expansion of the surface layer,the metal matrix will hinder the growth of the film and form compressive stress[151,152].The existence of compressive stress can hinder the formation and propagation of SCC cracks,so it is beneficial to alleviate the SCC sensitivity of the alloy.For the internal residual stress of the metal,it can be effectively eliminated by solid solution,aging,or annealing heat treatment.The residual stress in MA5 alloy was eliminated by annealing,and the SCC resistance was improved with the increase of annealing temperature [14].And heat treatment can also control the size and distribution of microstructure,which obstructs the hydrogen evolution process of the cathode,thus weakening the hydrogen embrittlement effect [153–155].Wang et al.[155] carried out T4 treatment of Mg-Zn-Y-Zr alloy at 400 °C for 2 h,and found that its SCC resistance was significantly increased compared with the forged sample,because T4 treatment could effectively reduce the combined effect of anodic dissolution and hydrogen embrittlement,alleviate local corrosion phenomenon,and reduce local hydrogen concentration.They also found that the cracking mode of T4 sample is TGSCC but that of the forged was TGSCC+IGSCC.Tsao et al.[122] found that after artificial aging at 260 °C for 4 h,the corrosion potential of AZ31 alloy increased,the breakdown potential decreased.Hence,the pitting tendency decreased,effectively improving the corrosion resistance and reducing the SCC sensitivity.Zhang et al.[156] also found that the internal stress of GZ51K alloy was released after aging treatment,which improved the corrosion resistance of the alloy.Generally,improved corrosion resistance helps to reduce SCC initiation.However,Argade et al.[157] carried out isothermal aging treatment on WE43 alloy at 210 °C,and found that SCC sensitivity from high to low was peak aging (48 h) >underaging (15 h) >overaging(144 h).The fracture of the sample is IGSCC,which is due to that the precipitation phase after aging treatment accelerates the anodic dissolution of grain boundaries and reduces the bonding strength of WE43,providing a path for SCC crack propagation.In order to better compare the effects of different processing techniques and grain size on the SCC sensitivity of Mg alloys,we summarized the SCC performance results of some Mg alloys,as shown in Table 2.we can also find that grain refinement is not necessarily beneficial to reduce the SCC of the alloy.This is mainly because although grain refinement reduces grain size,it may also introduce highenergy crystal defects such as dislocations and high-angle grain boundaries [14].Hydrogen tends to be trapped by these defects,which accumulates in the matrix to form high pressure and induces crack initiation [52].At the same time,it is worth noting that grain refinement also has a contradictory effect on the corrosion resistance of Mg alloys.The fine grain boundary can act as a barrier to hinder corrosion or a crystal defect to accelerate the diffusion rate of hydrogen [158].

Table 2ISCC comparison of Mg alloys with different grain sizes.
Therefore,we need to consider that the SCC process is not a single reference factor.Complex SCC behavior requires comprehensive consideration of all possible influencing factors.Before choosing and setting the right process,we need to consider the difference between the material itself and the application environment.However,there is little research on the effect of heat treatment on the SCC resistance of Mg alloys,and there is a lack of experimental basis.
From the above factors,we can find that the SCC behavior of Mg alloys is closely related to its corrosion resistance.Generally,the better the corrosion resistance of Mg alloys,the lower the pitting degree.The reduction of the number of pitting pits is beneficial to reduce the stress concentration at the bottom of the pitting pits.The microstructure has a significant effect on the corrosion properties of Mg alloys.Song et al.[160] have reported that the second phase has a dual role: When the content of second phases is high,it can play a useful role in corrosion protection;When the content is low,it will accelerate the corrosion of the matrix.Zhao et al.[161] and Ambat et al.[162] also proved that most of the continuously distributed second phase can protect the matrix.However,the second phase particles reduce the cohesion between the particles by introducing more hydrogen around the particles.Therefore,cracks are also more likely to reinitiate[108].
Fig.17 shows the microstructure and SCC fracture changes of Mg alloys after heat treatment.It can be seen from Fig.17(a) that the MgZn2phase inside the Mg-Zn-Y-Zr alloy is completely dissolved and the grain structure is coarsened after T4 treatment.By dissolving the MgZn2phase which acts as the cathode,the galvanic corrosion of the alloy is reduced and the SCC sensitivity coefficient is improved by 9%.In addition,after T4 treatment,the dissolution of the second phase is beneficial to reduce the number of corrosion pits on the surface of the alloy and delay the initiation of cracks.As shown in Fig.17(b) and (d),the number of second phases of the alloy decreases,and the SCC cracks distributed along the second phase also decrease.However,after T5 treatment,the precipitates in the alloy precipitate,and these locations are likely to provide a strong path for SCC,so SCC accelerates expansion.Combining Figs.16 and 17,we can find that due to the low standard electrode potential of Mg,the second phase usually acts as a micro cathode to accelerate the galvanic corrosion of adjacent substrates,thereby accelerating the initiation of SCC.However,reducing the second phases by heat treatment or improving the distribution of the second phases by alloying can effectively help Mg alloys reduce the stress concentration of galvanic corrosion and localized corrosion pits,thus effectively slowing or even inhibiting the initiation of SCC.Moreover,due to the neurotoxicity of Al,more researches on biomedical Mg alloys will be biased towards Al-free Mg alloys in the future,so it is necessary to focus on improving the SCC resistance of Al-free Mg alloys.
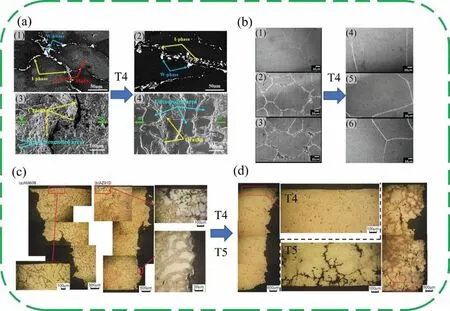
Fig.17.Microstructure and SCC Fracture of Mg alloys after heat treatment: (a) microstructure of Mg-6.7%Zn-1.3%Y-0.6%Zr alloy,(1) as-forged,(2) T4 sample,and secondary electron images of sample surfaces before and after T4 treatment [155];(b) microstructures of samples (1) Mg-2Sn,(2) Mg-5Sn,(3)Mg-8Sn,and corresponding samples after T4 treatment [103];(c) microstructures adjacent to the fracture surfaces of AM60B and AZ91D alloy ingots after SCC test [163];(d) microstructures adjacent to the fracture surfaces of (a) solution-treated and (b) peak-aged AZ91D alloy ingot after SCC test [163].
3.3. Surface treatment
Although appropriate preparation process and alloying can effectively regulate the microstructure of Mg alloys and improve their SCC resistance,the improvement effect is relatively limited [164].Surface treatment has become one of the more direct and effective methods to improve the SCC and corrosion resistance of Mg alloys [165].In general,surface treatment can be divided into physical and chemical treatment.The physical surface treatment mainly adopts laser shock peening (LSP) and surface mechanical wear treatment(SMAT) [166,167].Plastic deformation can change the corrosion resistance of Mg alloys and promote the formation of nanocrystalline layer on its surface [168].In addition,plastic deformation will also introduce residual stress which affects the SCC resistance of Mg matrix[38].Chemical surface treatment can be divided into conversion and deposition coating according to its formation mechanism [169–171].
3.3.1.MAOcoating
MAO,also known as plasma electrolytic oxidation (PEO),is developed on the basis of traditional anodic oxidation[172].MAO coating has high stability,not easy to fall off,and has a stronger protective effect on the substrate.However,the ceramic coating cannot avoid some defects such as micropores and microcracks [173,174].
Under stress,Cl ions have strong penetration ability to penetrate these defects and invade the Mg matrix,which provides favorable conditions for the occurrence of SCC.However,it was also thought that corrosion products would occur immediately after the corrosive medium entered these pores[39,175].After the corrosion products covered these micropores,the subsequent chloride ion intrusion was hindered,and the stress concentration and crack formation were reduced.He et al.[176] believed that when the MAO coating was immersed in a 3.5 wt.% NaCl solution,the corrosion products would eventually cover the pores.The contact between the substrate and the corrosion medium disappeared,reducing the possibility of stress concentration and crack initiation.
However,if there are numerous micropores in the MAO coating and the corrosion products cannot be coated in time,Cl-can use these micropores to quickly invade the matrix,resulting in corrosion reactions,forming corrosion pits on the surface of the matrix,causing stress concentration,and providing a favorable way for SCC [71].Therefore,porosity and formation rate of corrosion products will affect the SCC resistance of MAO coating.Single MAO coating can only slow down the SCC process to a certain extent,and cannot completely eliminate the SCC sensitivity of Mg alloys [177,178].
3.3.2.LSPprocessing
LSP is a non-contact surface modification technology[179].The shock wave generated by laser makes the substrate surface plastic deformation,and changes the microstructure and stress distribution of the substrate surface [180].LSP can also refine grains,increase the number of grain boundaries,hinder the propagation path of SCC,and enhance the interfacial adhesion [181].Moreover,grain refinement can improve the yield strength of Mg alloys,thereby increasing the SCC threshold [182].In addition,the residual compressive stress introduced by plastic deformation can effectively offset the tensile stress of external load,which is conducive to reducing the driving force of SCC crack propagation [74].
LSP is one of the most effective surface modification techniques to improve the SCC resistance of Mg alloys.Ge et al.[38] found that LSP treatment could induce the nano-crystallization of AZ31B surface.Nanolayers can effectively reduce pitting and SCC paths.Compared with the sample without LSP treatment,the corrosion current density of AZ31B alloy after LSP treatment decreased by 85.4%,and the ISCCdecreased from 0.61 to 0.32.Li et al.[183] proved that LSP could produce high residual compressive stress on the surface while obtaining fine microstructure,which reduced the SCC sensitivity of AZ31 alloy from 0.8 to 0.69.Fig.18 shows SCC test results of AZ31B with or without LSP coating [164].
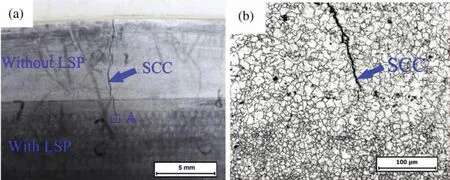
Fig.18.SCC test results of AZ31B with or without LSP coating (a) a photograph of SCC specimen;and (b) the high magnification OM micrograph of the rectangular region A in (a) [164].
It can be seen in the figure that after soaking in 0.1 wt.%NaOH solution for 500 h at room temperature,there is no crack in the area treated by LSP,and SCC mainly occurs in the area not treated by LSP.It indicates that LSP can effectively hinder the SCC process of AZ31B alloy in corrosive medium.
Therefore,internally,LSP slows down corrosion by refining the structure.Externally,LSP can form a nanocrystalline layer and introduce compressive stress to effectively slow down the SCC process.
3.3.3.Atomiclayerdeposition(ALD)coating
ALD is based on chemical vapor deposition.At low temperature,the material is adsorbed on the substrate surface in the form of single atomic film [184].And each reaction will only precipitate a single layer of atomic layer.Repeating the process can increase the coating thickness,thus ALD can provide dense,uniform,pore-free,and thickness-controlled films[185].Previous studies [186,187] have proved that ALD can effectively improve the corrosion resistance of AZ31 alloy.Recently,some studies have explored the influence of ALD coating on SCC sensitivity of AZ31 alloy in SBF solution[40].They found that the ALD coating could effectively prevent hydrogen intrusion and reduce pitting corrosion,and it had excellent mechanical properties,which could resist high strain without fracture.In addition,the longer protection time of the substrate by the coating reduced the SCC sensitivity of AZ31 alloy.Therefore,ALD coating is expected to improve the SCC behavior of biomedical implanted Mg alloys in human body.
3.3.4.Compositecoating
Single surface modification technology still has defects such as micro pores,micro cracks,insufficient adhesion,and poor stability.Table 3 lists the mechanical properties of some Mg alloys after coating.It can also be seen that the tensile strength and elongation of the alloys in the solution are improved after coating,but the improvement of the composite coating is more significant.Therefore,it is necessary to focus on the influence mechanism of composite coating on SCC.In addition,surface modification technology is commonly used in biomedical field.If the composite coating technology can be developed,it can not only improve the SCC resistance of Mg alloys,but also improve the biocompatibility of implants and accelerate tissue healing by adding bioactive coatings,which can effectively improve the application range of Mg alloys in the biomedical field [188–191].
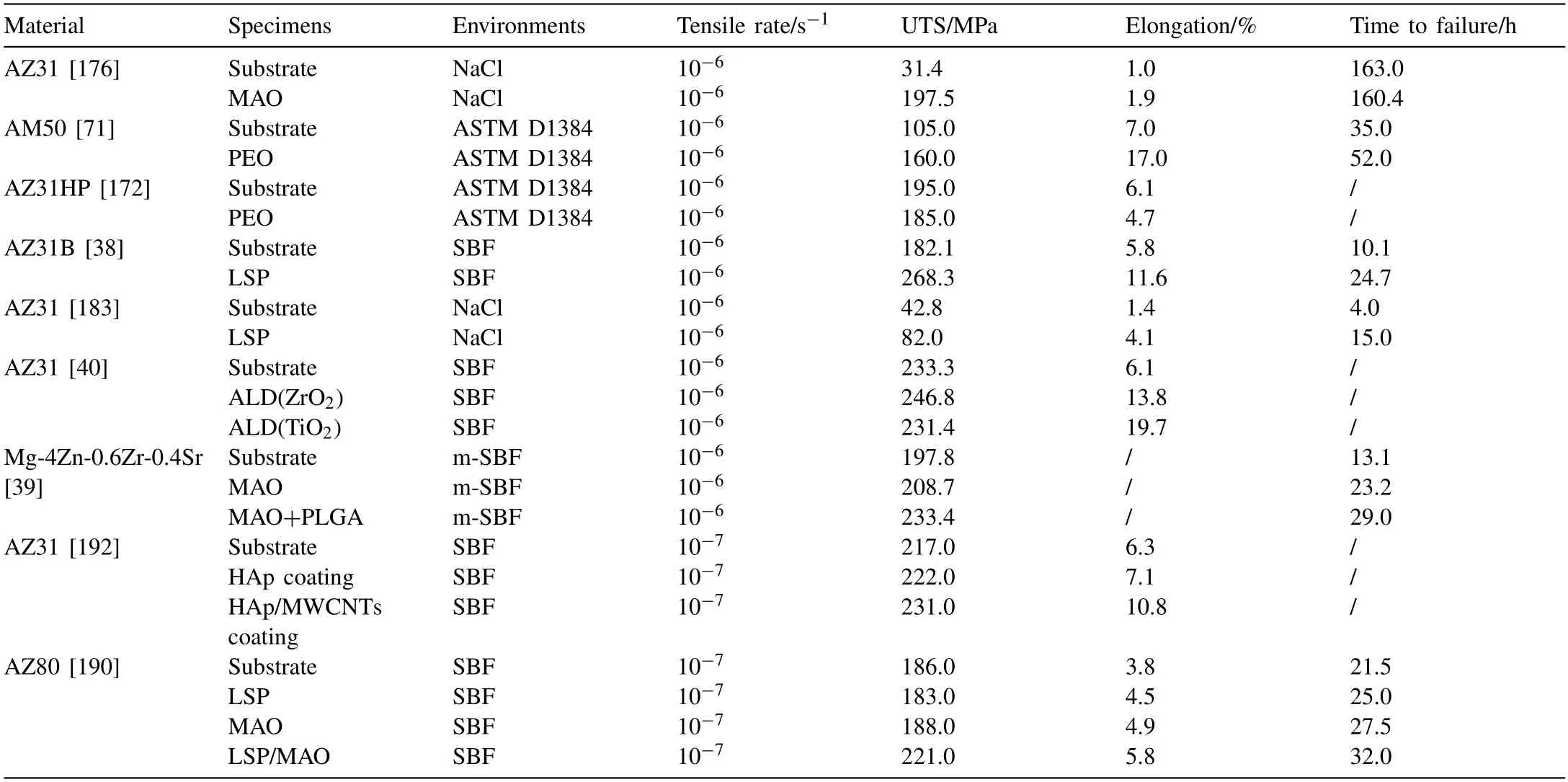
Table 3Changes of SCC performance parameters of Mg alloys after different surface treatments.
Xiong et al.[190] found that LSP/MAO composite coatings prepared on AZ80 surface were divided into external loose layer,central dense layer,and internal nanocrystalline layer.The existence of nanocrystalline layer can increase the number of grain boundaries and the density of surface protective film.In addition,the nanocrystalline layer has the selfhealing effect and reduces the adsorption capacity of chloride ions[191].The surface and cross-sectional SEM of MAO and LSP/MAO samples are shown in Fig.19[188].The composite coating shows lower porosity and higher thickness.The corrosion resistance of AZ80 alloy is improved,the pitting tendency is reduced,and the SCC process is delayed.LSP/MAO composite coating can also effectively improve the mechanical integrity,but it is still necessary to study the mechanical properties attenuation and SCC behaviorinvivo.He et al.[41] also prepared a layer of LSP/MAO coating on the surface of AZ80 alloy.It was found that the LSP layer mainly provided residual compressive stress,reduced dislocation slip,and delayed crack formation,while the MAO coating mainly hindered the corrosion of chloride ion in corrosive medium.Moreover,the porosity of the composite coating was reduced by an order of magnitude compared with that of the single MAO coating.The composite coating can also be modified by adding hydroxyapatite to enhance biocompatibility,but furtherinvivostudies are needed [192].

Fig.19.SEM morphologies of surface and cross-section of MAO sample (a,c) and LSP/MAO sample (b,d);three-dimensional laser scanning morphologies of MAO sample (e) and LSP/MAO sample (f);stress-strain curves (g),and potentiodynamic polarization curves of BM,LSP,MAO,and LSP/MAO samples tested in vitro environment (h) [188].
Chen et al.[39] prepared MAO+PLGA composite coating on Mg-4Zn-0.6Zr-0.4Sr alloy.The MAO coating has micro pores and micro cracks,and the m-SBF solution is easy to penetrate into the matrix and shows serious local corrosion.However,PLGA coating can seal these pores,avoid the contact between the external solution and the substrate,and reduce the environmental impact on the substrate.Moreover,the combination of composite coating and substrate is stronger.SSRT and constant load tensile test showed that the UTS and elongation of the composite coating were significantly higher than those of the single MAO coating,and the SCC resistance was also significantly higher than that of other samples.Therefore,the composite coating can make up for the limited improvement of MAO coating,and maintain a certain integrity of mechanical properties during service.
Compared with a single coating,the composite coating exhibit more obvious improvement on the SCC resistance of Mg alloys.Although the composite coating does not affect the microstructure of the alloy,the composite coating can effectively reduce or even isolate the solution invasion,and the composite coating effectively reduces the porosity to hinder local corrosion,thereby reducing the stress concentration effect caused by corrosion pits.The SCC process will be effectively slowed down.In the biomedical field,it is necessary to consider not only the impact of SCC,but also whether the substrate and coating are harmless to the human body and whether the implants can maintain mechanical integrity before healing the tissues.Nevertheless,as can be seen from Table 3,most of the studied alloys are Al-containing alloys which is toxicity and not suitable to be used as biomedical implants.Therefore,in future,more advanced coatings can be developed on novel biomedical Mg alloys by making full use of the multifunctionality of composite coatings to expand the application range of Mg alloys in the biomedical field.
3.4. Corrosion media
In general,Mg alloys also have high SCC sensitivity in distilled water.Especially in the environmental medium containing Cl-,the corrosion rate is accelerated,and many pitting pits are formed on the surface of the substrate,which is easy to cause stress concentration[67].Hydrogen produced by corrosion is also easy to penetrate into the matrix,increasing the sensitivity of SCC [193,194].Therefore,the SCC of Mg alloys in corrosive medium is usually dominated by hydrogen embrittlement.TGSCC is the most common form [195].The surface protective film in neutral or alkaline environment has good stability and high passivation rate,which can effectively hinder the expansion of SCC and improve the SCC resistance[141].Therefore,it is of great significance to study the variation of SCC sensitivity of Mg alloys in different corrosive media for its application fields.
Chen et al.[194] tested SCC of AZ91 alloy in 0.5 M MgCl2solution by constant load test.It was found that hydrogen embrittlement was the dominant factor of SCC,and all samples showed TGSCC failure.The SCC threshold is only 1/3 of the yield strength,and the SCC threshold of AZ91 alloy decreases with the increase of MgCl2solution concentration.He et al.[195]pointed out that the pH value of corrosion medium also affected the SCC behavior of AZ31 alloy.In the solution containing Cl-,pitting corrosion is easily formed on the surface of the alloy due to the small radius of Cl-and strong penetration.With the decrease of pH,hydrogen embrittlement increased.All these are conducive to the formation and propagation of SCC crack in AZ31 alloy.Therefore,AZ31 alloy has high SCC sensitivity at low pH.Under alkaline condition,the protective film on AZ31 surface is stabler,which protects the substrate from the influence of corrosion environment and improves the SCC resistance.Catar et al.[59] also found that with the increase of pH,the UTS and elongation of AZ31,AZ61,and AZ91 alloys were increased,which was beneficial to improve the SCC resistance of the alloys.Fig.20 shows the variation of UTS and elongation of AZ31,AZ61,and AZ91 alloy in different pH solutions.

Fig.20.The UTS (a) and elongation change (b) of AZ31,AZ61,and AZ91 alloys in acidic (pH=2),neutral (3.5% NaCl,pH=Neutral),and basic (pH=11)solutions [59].
It can be concluded from the existing literature that SCC is inevitable for Mg alloys in most solutions,which is due to hydrogen embrittlement and surface pitting.Therefore,the SCC sensitivity can be improved by reducing the pitting of Mg alloys,enhancing the compactness and uniformity of corrosion product protective film,and reducing hydrogen intrusion.
3.5. Strain rate
Different strain rates will affect the contact time between Mg alloys and corrosive medium,thereby affecting the sensitivity of SCC [196].With the decrease of strain rate,the contact time between matrix and medium increases,which is conducive to the invasion of hydrogen ions and the formation of cracks[197,198].Therefore,the lower the strain rate is,the higher the SCC sensitivity is.However,some researchers believe that in a strong passivation solution containing K2CrO4,the growth rate of the corrosion film is fast enough to protect the bare metal surface at a sufficiently low strain rate,and the integrity of the corrosion film can hinder the invasion of hydrogen [199,200],so that SCC does not occur in alloys.The effect of low strain rate on the SCC of Mg alloy is attributed to the balance between the corrosion medium and the repassivation of the corrosion layer at the crack tip and the mechanical film rupture.But in fact,this conclusion mainly depends on the effect of strong passivation solution medium environment,rather than the characteristics of Mg alloy itself[14,55].Therefore,in different rate tests,the properties of the alloy are also quite different.It can be seen from Table 4 that in common systems (distilled water,SBF,3.5% NaCl,and 0.1 M Na2SO4),when the strain rate of Mg alloys is low enough,the failure strain of Mg alloys cannot even exceed 10%.The YS and UTS of Mg alloys also decrease with the reduction of strain rate.The repassivation effect at the crack tip is almost negligible.Generally,hydrogen embrittlement is the main reason for SCC of Mg alloys at different strain rates.
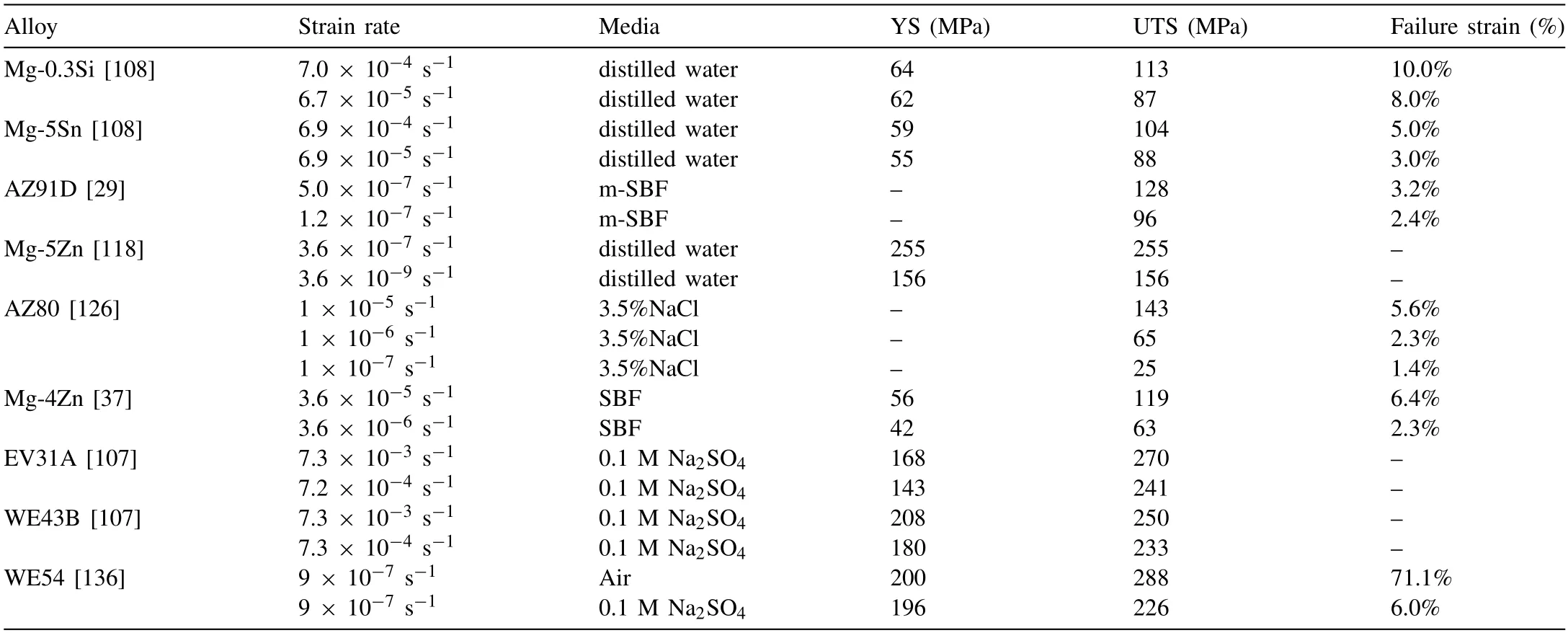
Table 4SCC parameters of Mg alloys at different strain rates.
Srinivasan et al.[198] found that the SCC of AZ61 alloy was more serious at lower strain rates.Dubey et al.[126] also found that the SCC sensitivity of AZ80 increased as the strain rate decreased from 10-5s-1to 10-7s-1.This is because the degree of hydrogen permeation under low strain rate is much higher than that under high strain rate,which accelerates the rupture of surface protective film and expands the local corrosion area.Fig.21 shows the SCC of Mg-4Zn alloy at fast strain rate and slow strain rate [37].At the slow strain rate,the contact time between H atoms in the corrosive medium and the surface film increases,and the local corrosion on the surface intensifies,which is conducive to crack initiation and propagation.In fact,the effect of strain rate on Mg alloy SCC is related to the alloy surface film.Due to the corrosion of the corrosive medium,the surface film of the Mg alloy is cracked.When the repassivation rate of the film cannot protect the bare metal quickly enough,continuous pitting will occur and cracks will begin to propagate under the promotion of external stress and hydrogen.Therefore,increasing the repassivation rate of the surface film is also one of the effective methods to improve the SCC resistance.
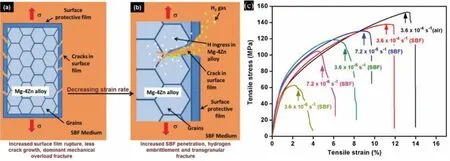
Fig.21.Biomedical Mg-4Zn alloy: (a) Schematic representation of multi-crack formation mechanism at high strain rates;(b) Hydrogen intrusion and embrittlement at lower strain rates;(c) The stress-strain curve of Mg-4Zn alloy at 3.6 × 10-4 ∼3.6 × 10-6 s-1 rate [37].
4.Summary
Mg alloys are the most promising lightweight material and can be used in automotive industry,aerospace,biomedicine,and other fields.However,the biggest threat to its application limitation is its poor corrosion resistance,especially its high sensitivity to SCC.Most of the current studies focus on the mechanism and influencing factors of SCC in engineering applications,including alloying,preparation process,surface treatment,corrosion medium,and strain rate.From the above factors,we can know that for Mg alloys,adding proper amount of appropriate alloying elements can improve SCC sensitivity.In addition,rapid solidification preparation,cryogenic machining,and solution treatment are useful to reduce local corrosion and improve the SCC resistance.Surface treatment is also a direct and effective method to improve the SCC susceptibility.Nevertheless,there is no recognized effective method to solve the sensitivity of SCC completely.Therefore,further research is needed to improve the SCC resistance of Mg alloys and make them widely used.
(1) Alloying and preparation process are common methods to improve the intrinsic SCC resistance of Mg alloys,but the improvement effect is limited.Novel Mg alloys and processes have been developed mainly to enhance their strength and corrosion resistance recently.Hence more attention should be paid to SCC if these Mg alloys are used under corrosive and stress environments.
(2) The effect of composite coating on the SCC resistance of Mg alloys is more significant as compared to that of single coating.As the most direct and effective method,the SCC mechanism of the composite coating should be well illustrated,and more multi-functional characteristics of the composite coating are desirable.
(3) Currently,the SCC test method of biomedical Mg alloys shortens the experimental period,but ignores the role of corrosion in long-term service.Therefore,we need to consider the synergistic effect of strain and corrosion in order to better match the practical application.We can regulate the corrosion process of the alloy by adjusting the solution pH in time or regulating the self-corrosion potential of the sample.Moreover,SCC is an extremely complex dynamic process,which is affected by many factors at all times during service.Consequently,more dynamic experiments are expected to explore the SCC process of Mg alloys,which helps to better understand SCC and effectively prevent SCC in advance.
(4) Most studies are limited to the corrosion resistance of Mg alloysinvitroand degradabilityinvivofor biomedical applications,but only a few studies focus on the SCC resistance.Hence more researches should be carried out on SCC behavior of biodegradable Mg alloys.In addition,invitroexperiments are difficult to fully simulate the complex internal environment and human regulation system.Consequently,more animalinvivoexperiments are necessary in order to obtain more accurate SCC experimental results and better understand the SCC mechanisminvivofor biomedical applications.
Declaration of competing interest
There is no conflict of interest,and all the fund projects are acknowledged in this manuscript.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (52071175) and the Key Research &Development Plan (Social Development) of Jiangsu Province(BE2020702).
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Ameliorating the re/dehydrogenation behaviour of MgH2 by zinc titanate addition
- Inhibiting effect of I-phase formation on the plastic instability of the duplex structured Mg-8Li-6Zn-1.2Y (in wt.%) alloy
- PEO coating on Mg-Ag alloy: The incorporation and release of Ag species
- Underlying mechanisms of variation in yield asymmetry and strain hardening behavior of extruded pure Mg with Gd addition
- Edge crack damage analysis of AZ31 magnesium alloy hot-rolled plate improved by vertical roll pre-rolling
- High sintering and dielectric performance: The improved (Mg,Zn)3B2O6 ceramics with the help of the DFT calculation
