Ameliorating the re/dehydrogenation behaviour of MgH2 by zinc titanate addition
N.A.Ali ,N.A.Szelee ,M.F.Md Din ,M.M.Nsef ,A.A.Jlil ,Hizen Liu ,M.Ismil,c,∗
a Energy Storage Research Group,Faculty of Ocean Engineering Technology and Informatics,Universiti Malaysia Terengganu,21030 Kuala Nerus,Terengganu,Malaysia
b Department of Electrical and Electronic Engineering,Faculty of Engineering,National Defence University of Malaysia,Kem Sungai Besi,Kuala Lumpur,Malaysia
c Center of Hydrogen Energy,Institute of Future Energy,Universiti Teknologi Malaysia,81310 UTM Johor Bahru,Johor,Malaysia
d Department of Chemical and Environmental Engineering,Malaysia Japan International Institute of Technology,Universiti Teknologi Malaysia,Jalan Sultan Yahya Petra,Kuala Lumpur 54100,Malaysia
e Faculty of Chemical and Energy Engineering,Universiti Teknologi Malaysia,81310 UTM Johor Bahru,Johor,Malaysia
fGuangxi Novel Battery Materials Research Center of Engineering Technology,State Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures,School of Physical Science and Technology,Guangxi University,Nanning 530004,China
gState Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures,Nanning 530004,China
Abstract Magnesium hydride (MgH2) is the most feasible and effective solid-state hydrogen storage material,which has excellent reversibility but initiates decomposing at high temperatures and has slow kinetics performance.Here,zinc titanate (Zn2TiO4) synthesised by the solid-state method was used as an additive to lower the initial temperature for dehydrogenation and enhance the re/dehydrogenation behaviour of MgH2.With the presence of Zn2TiO4,the starting temperature for the dehydrogenation of MgH2 was remarkably lowered to around 290 °C–305 °C.In addition,within 300 s,the MgH2–Zn2TiO4 sample absorbed 5.0 wt.% of H2 and 2.2–3.6 wt.% H2 was liberated from the composite sample in 30 min,which is faster by 22–36 times than as-milled MgH2.The activation energy of the MgH2 for the dehydrogenation process was also downshifted to 105.5 kJ/mol with the addition of Zn2TiO4 indicating a decrease of 22% than as-milled MgH2.The superior behaviour of MgH2 was due to the formation of MgZn2,MgO and MgTiO3,which are responsible for ameliorating the re/dehydrogenation behaviour of MgH2.These findings provide a new understanding of the hydrogen storage behaviour of the catalysed-MgH2 system.
Keywords: Hydrogen storage;Solid-state storage;MgH2;Additive;Zn2TiO4.
1.Introduction
Hydrogen has recently drawn global interest as an energy carrier for the future because of its abundant resources and environmentally friendly.The need for safe and effective hydrogen storage is essential for the use of onboard hydrogen applications.Among the several methods of storing hydrogen available (e.g.solid-state,liquid and gaseous),storing hydrogen via solid-state approaches is one of the most superior,efficient and safe.Magnesium hydride (MgH2) is one of the most effective solid-state materials owing to its abundance as well as its light weight,low cost and high hydrogen concentration (7.6 wt.% gravimetric and 110-g/L volumetric) [1–4].Unfortunately,the temperature for the release of hydrogen is high(>400°C),and the desorption kinetics are slowly caused by the high desorption enthalpy (ΔH=76 kJ/mol H2) [5,6].
Multifarious methods have been applied (e.g.alloying,nanosizing,doping with additives and destabilising with other hydrides)to accelerate the re/dehydrogenation kinetics and reduce the operating temperature of MgH2[7–15].Particularly,additive doping has been regarded as the most efficient strategy to tackle the drawbacks of MgH2.The introduction of an additive,such as carbon material,rare metal-based material and transition metal-based material into MgH2,results in the superior performance of MgH2[16–19].Among them,transition metal and their compounds have received special attention because of the distinctive electronic structure that might debilitate the Mg–H bond which then accelerates the dehydrogenation process of MgH2[20].It has been observed that 3d transition metals (e.g.Ni,Cu,Co,Ti and Fe) have the effect of weakening the Mg–H bond because of their distinct electron configurations with the d-band,hence promoting H2molecule dissociation and recombination on the Mg/MgH2surface [21].Moreover,according to first-principle calculations,transition metal additives (Ti,Ni,Fe and Co) can create a change in the Fermi level,which improves MgH2desorption rates [22].Further,Rahwanto et.al [23] showed that the addition of Ni resulted in a high surface area composite with finely dispersed Ni particles on the surface of Mg that led to a significant improvement in the kinetic performance of the MgH2–Ni.The addition of Ti was also found to accelerate the kinetics of MgH2with the ability to absorb 2.7 wt.% H2in less than 7 min [24].
The hydrogen storage properties of MgH2can be also synergized by adding a double catalyst consisting of SiC and Ni.The former improves the kinetics of H2absorption/desorption in MgH2,while the latter acts as a hydrogenation catalyst.The decomposition temperature of the MgH2–SiC–Ni was downshifted to 250 °C and the composite sample was able not only to absorb 5.7 wt.% H2but also to release the same amount of %H2in 8.3 min [25].The improved absorption kinetics of the MgH2–SiC–Ni sample was attributed to the smaller particle size that led to a large contact area between the sample and remarkably increase the absorption rate [25,26].In another study,Chen et al.[27] demonstrated that the addition of transition metal oxide (ZrO2) with carbon significantly improve the hydrogen storage performance of MgH2.One of the key improvements observed in MgH2–ZrO2/C was the downshift of the onset decomposition temperature to 208 °C.The density functional theory (DFT) calculations that was conducted to support the experimental results proved that the addition of ZrO2/C weakened the Mg–H bonding strength and lowered the desorption energy leading to excellent hydrogen storage properties of MgH2at moderate temperature.Moreover,other studies demonstrated that the composite of Mg–Nb2O5–C are beneficial in lowering the desorption temperature of MgH2and also reduced the activation energy [28].In addition,Milanese et al.[29] showed that adding TiO2to the Mg–Ni–C composite resulted in a decrease of the dehydrogenation temperature.They indicated that the addition of TiO2increase the sorption efficiency of the active phases and is responsible for the improved sorption rates.
Ti-based additives,among the transition metal,are significant in accelerating the performance of MgH2[30–32].In particular,Hu et al.[33] found that the Ti compound (K2Ti8O17)could decrease the operating temperature of MgH2to 189 °C and maintain excellent capacity retention (88%) after completing the eight cycles.Another study also indicated that adding K2Ti6O13resulted in the tremendous performance of MgH2because of the positive synergetic function of TiO2,Ti and KMgH3during the heating process [34].At 280 °C,the K2Ti6O13-doped MgH2sample rapidly desorbs 6.7 wt.%H2in 3 min and lower the dehydrogenation activation energy to 105.67 kJ/mol.Thereafter,Shao et al.[35] introduced TiO2into MgH2and resulting in superior performance.At 100 °C,the TiO2-doped MgH2sample rapidly absorbs 4.17 wt.% H2in 30 min and liberates 5.75 wt.% H2in 16.7 min at 300 °C.The significant improvement of the TiO2-doped MgH2sample was ascribed to the ultrafine and uniformly scattered TiO2particle that offers a high number of reactive sites for ab/desorption of MgH2.
Another study has shown that the addition of TiO2nanosheets (NS) can improve the kinetics performance of MgH2[36].The MgH2–TiO2(NS) sample was able to release 1.2 wt.% H2within 300 min at low temperature of 180 °C.This was accompanied by a significant improvement in the absorption kinetics of MgH2–TiO2(NS) that allowed the sample to absorb 6.1 wt.% H2within 10 s at 150 °C.Furthermore,a study conducted by Zhang et al.[37] demonstrated enhanced hydrogen storage performance of MgH2with monodispersed single-crystal-like TiO2wrapped with amorphous carbon (MgH2–TiO2SCNPs/AC).The MgH2–TiO2SCNPs/AC sample attained lower operating temperature and activation energy and exhibited faster kinetics with the ability to be fully rehydrogenated with a reversible capacity of 6.5 wt.% H2within 5 min at 200 °C.A recent work has reported that adding graphene-like TiO2(B) imparted an excellent catalytic effect to the performance of MgH2[38].The MgH2–TiO2(B) sample started to release hydrogen at 200 °C and desorbed 6.88 wt.% H2below 288 °C.The enhanced performance of the MgH2–TiO2(B) sample was ascribed to the reduction of TiO2(B) nanosheets to the metallic Ti nanoparticles and wrinkled Ti2O3during the ball milling and dehydriding processes,resulting in many boundary contacts between MgH2and Ti-based catalysts,which collectively promoted hydrogen diffusion.
Inspired by the above kinds of literature,it is interesting to explore another Ti-based additive,which is Zn2TiO4.Recently,Zn2TiO4has demonstrated promising performance as an lithium-ion battery anode material [39].Apart from that,Zn2TiO4also showed an excellent catalytic effect as an electrode sensor for UV photocatalysts [40].Therefore,it is believed that Zn2TiO4will result in superior performance for the hydrogen storage application.Herein,Zn2TiO4was utilised as an additive to boost the re/dehydrogenation behaviour of MgH2.To date,no study has been conducted on the impact of Zn2TiO4on the re/dehydrogenation behaviour of MgH2.In this study,the Zn2TiO4was synthesised by using solid-state method,and various weight percentages (wt.%) of Zn2TiO4were introduced to MgH2.The specific mechanism of the MgH2–Zn2TiO4system was discussed in depth in this study.
2.Experimental details
Pure MgH2(95%),ZnO (99.9%,<100 nm),anatase TiO2(99.95%,21 nm) and ammonia solution were commercially purchased from Sigma Aldrich.The Zn2TiO4was synthesized by using solid-state method.The ZnO and TiO2were ground together in stochiometric amounts using agate mortar for 15 min.Thereafter,a few drops of ammonia solution were added drop by drop to increase the reaction rate during the synthesis process as reported elsewhere [41,42].The mixture was ground again for another 15 min and then the mixture was subsequently dried for 5 h at 60 °C and annealed at 1000 °C for 3 h.In order to examine the hydrogen storage behaviour of MgH2,various amount of the as-synthesised Zn2TiO4(5,10,15 and 20 wt.%) was then mixed with MgH2via planetary ball mill (NQM-0.4) for 1 h.To minimise oxidation,the sample was prepared in a Mbraun glovebox with Argon gas flowing through it.
The composition of the samples was identified using Rigaku MiniFlex X-ray diffractometer (XRD) with Cu Kαradiation.For each measurement,the samples were scanned over diffraction angles of 20° <2θ<80° with a speed of 2°/min.The Shimadzu IR Tracer-100 was used to perform the Fourier-transform infrared (FT-IR) measurement between 400 and 2000 cm-1at a resolution of 4 cm-1with a scanning capable of 20 spectra/second,equipped with Attenuated total reflectance (ATR) mode and scanning electron microscopy(SEM;JEOL JSM-6360LA) was employed to investigate the microstructures and morphologies of the samples.Renishaw Raman spectrometer was used to conduct Raman spectra at ambient temperature with a 0.1% power laser (532 nm radiation).The measurement was conducted between 100 to 1000 cm-1with a spectral resolution of <2.5 cm-1in an air atmosphere.For FT-IR and Raman,to minimise the exposure to air and moisture,the sample was placed in microcentrifuge tubes (1.5 mL) during transportation from the glove box.
The hydrogen storage behaviour of MgH2–Zn2TiO4was evaluated using a Sievert-type apparatus from Advanced Materials (pressure–composition–temperature (PCT)).The sample was heated from room temperature to 450 °C to determine the starting dehydrogenation temperature.The dehydrogenation and rehydrogenation kinetics experiments were performed at 300 and 320 °C (1 atm H2pressure) and 250 and 320 °C (33 atm H2pressure),respectively.The differential scanning calorimetry (DSC) was tested using TGA/DSC 1,Mettler Toledo in the temperature range from room temperature to 550 °C at varied rates (15 to 30 °C/min).For comparison,all of the characterizations were also performed on as-milled MgH2.
3.Results and discussions
3.1. Synthesis of Zn2TiO4
The crystallographic structure of the Zn2TiO4was examined by using XRD as demonstrated in Fig.1(a).The XRD spectra of the as-synthesised Zn2TiO4were perfectly indexed with the Zn2TiO4phase (JCPDS 13-536),confirming the high purity of Zn2TiO4.The diffraction peaks of 2θat 29.7°,35.0°,36.6°,42.6°,52.9°,56.4°,61.9°,70.3°,73.1°,74.2°and 78.0°are ascribed to the (220),(311),(222),(400),(422),(511),(440),(620),(533),(622) and (444) planes of Zn2TiO4.The mean crystallite size of the Zn2TiO4was determined to be 26.7 nm using the Scherrer equation as in Eq.1 [43]:
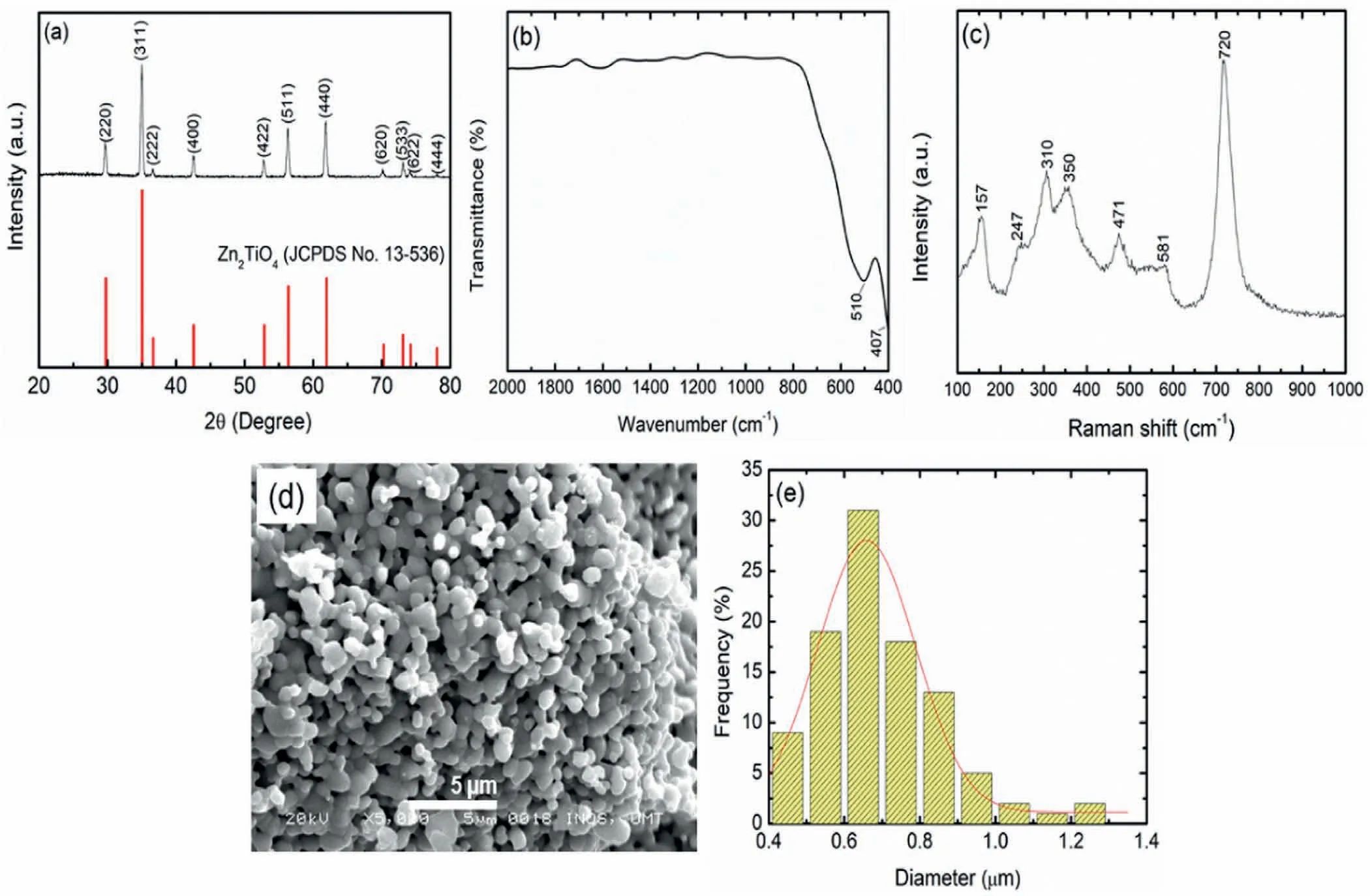
Fig.1.The (a) XRD pattern,(b) FT-IR profile,(c) Raman profile,(d) micrograph and (e) particle size distributions of the Zn2TiO4.
whereas
L=mean size of the crystallite
K=Scherer constant
λ=X-ray wavelength
β=full width at half maximum (FWHM)
θ=diffraction angle
Fig.1(b) shows the FT-IR spectra of Zn2TiO4which present the standard bands of Zn2TiO4,that occur at 407 and 510 cm-1and correlate to the Ti–O and Zn–O bond,respectively [44].Additional characterisation performed by Raman spectroscopy as in Fig.1(c) showed the typical peaks of Zn2TiO4.The peaks at 247,310,350,471 and 720 cm-1are assigned to the spinel structure of Zn2TiO4[45].The Raman mode at 581 cm-1was attributed to the order–disorder effect of Zn and Ti ions while the peaks at 157 cm-1were due to the laser-induced plasma effect [46,47].The morphology of the as-synthesised Zn2TiO4,which consists of spherical particle that seems to be generally uniform,is shown in Fig.1(d).The mean particle size of the Zn2TiO4evaluated by the Image J software as represented in the histogram (Fig.1(e)) was 0.65 μm.
3.2. Hydrogen storage behaviour of MgH2–Zn2TiO4 system
As depicted in Fig.2(a),the influence of Zn2TiO4on the hydrogen storage behaviour of MgH2was scrutinised by the TPD(temperature–programmed–desorption).The initial dehydrogenation temperature for the commercial MgH2happened at around 405°C and released 7.2 wt.%H2.Subsequently,the initial dehydrogenation temperature of MgH2was decreased to 340 °C after 1 h of milling suggesting that the milling procedure contributed to the reduction of the dehydrogenation temperature of MgH2.The amount of hydrogen released from the as-milled MgH2was 7.0 wt.%.Furthermore,the initial temperature for the dehydrogenation of MgH2was lowered significantly by adding different weight percentages of Zn2TiO4.The MgH2+5 wt.% Zn2TiO4sample commenced desorbing hydrogen at 305 °C,releasing a 6.9 wt.% of H2.Then,by varying the amount of Zn2TiO4to 10,15,and 20 wt.%,the initial dehydrogenation temperature was reduced to roughly 290 °C.The amounts of hydrogen released for the 10,15,and 20 wt.%-doped MgH2samples was 6.8,6.4 and 6.1 wt.%,respectively.This finding suggests that an increased amount of additive lowers the initial dehydrogenation temperature even further.Moreover,the results demonstrated that the addition of Zn2TiO4is promising for the downshift of the initial dehydrogenation temperature of MgH2.Table 1 shows a comparison between the hydrogen storage performance of the MgH2+Zn2TiO4system in the present study and composites obtained by the same method with other additives and reported in literature.
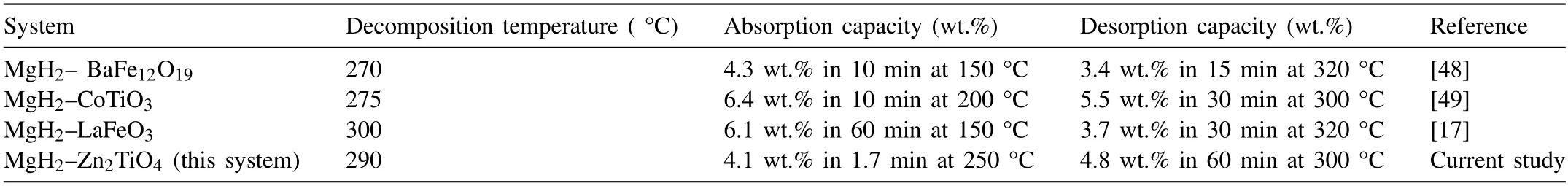
Table 1Comparison of the hydrogen storage performance of MgH2+Zn2TiO4 sample with other additives synthesized via the solid-state method.
Then,the different weight percentage of Zn2TiO4was milled together with MgH2and their corresponding absorption kinetics curve is displayed in Fig.2(b) to assess the catalytic performance of the Zn2TiO4on the absorption performance of MgH2.Based on the figure,in 100 s,the as-milled MgH2absorbed 3.3 wt.% H2while in the same time duration,the MgH2+5 wt.% Zn2TiO4composite rapidly absorbed 4.4 wt.% H2and the 10,15 and 20 wt.%-doped MgH2sample absorbed 4.1 wt.%,4.0 wt.% and 3.6 wt.% H2,respectively.All the doped samples present better absorption performance than as-milled MgH2.Considering the previous study of the MgH2with the addition of a TiO2sample [35],one can observe that MgH2–TiO2sample absorbed 4.17 wt.% H2in 30 min compared to the same amount in 1.7 min for MgH2–Zn2TiO4sample in the present study.This confirms the presence of acceleration absorption kinetics in the MgH2–Zn2TiO4sample because of the addition of Zn2TiO4.As depicted in Fig.2(c),further study was done on hydrogen desorption at 300 °C which was recorded for 60 min.From the figure,MgH2doping with Zn2TiO4presents the highest amount of hydrogen desorbed.The amount of hydrogen desorbed within the first 30 min for the as-milled MgH2sample was 0.1 wt.%.The amount of hydrogen desorbed was significantly high by doping with a various amount of Zn2TiO4.The amount of hydrogen desorbed for the 5,10,15 and 20 wt.% Zn2TiO4-doped MgH2in 30 min was 2.2,2.5,3.6 and 3.0 wt.%H2,respectively,which is 22–36 times more rapidly than as-milled MgH2.Even after 1 h,the as-milled MgH2was unable to reach the same high capacity as the MgH2–Zn2TiO4sample.The MgH2–Zn2TiO4sample desorbed 3.8,4.4,4.8 and 4.5 wt.% H2in 60 min for the 5,10,15 and 20 wt.% Zn2TiO4,respectively,but the as-milled MgH2desorbed only 0.3 wt.%H2.Consequently,the addition of the Zn2TiO4additive (even in a low amount)results in a large improvement in the desorption kinetics of MgH2.For further analysis,the MgH2+10 wt.% Zn2TiO4sample was selected due to the low dehydrogenation temperature with high hydrogen content and faster absorption/desorption kinetics.
A long-life cycle is a great challenge for hydrogen storage applications.Hence,the cycling performance of the MgH2+10 wt.% Zn2TiO4sample was studied.Temperature of 320°C were applied in the cycling study of the MgH2+10 wt.% Zn2TiO4sample.Fig.3(a) and 3(b) presents the cycling performance of the MgH2+10 wt.%Zn2TiO4sample over 10 cycles.For the absorption kinetics,in the 10thcycle,6.6 wt.%H2capacity was maintained which showed a small degradation compared to the 1stcycle (7.0 wt.%).For the desorption kinetics,the capacity for the 1stcycle was 4.6 wt.% H2.Surprisingly,after the 10 cycles,4.5 wt.% H2was maintained indicating a remarkable stability where the MgH2+10 wt.%Zn2TiO4sample retained 97.8% of the original capacity.The cycling performance indicates that the addition of Zn2TiO4is highly beneficial for maintaining the superior cyclability behaviour of MgH2.Further,the XRD analysis that was carried out on the MgH2+10 wt.% Zn2TiO4samples after completing the 10thcycle depicted in Fig.3(c) to ascertain the reason for the decreased hydrogen storage capacity.For the dehydrogenated state (Fig.3(c)(i)),the peaks that were detected were that of Mg coupled with the additional peaks of MgO,MgTiO3and MgZn2.The peaks of MgO,MgTiO3and MgZn2remain unchanged for the rehydrogenated state(Fig.3(c)(ii)).The presence of Mg suggests a complete dehydrogenation of MgH2.The formation of MgO,MgTiO3and MgZn2is believed to reduce the capacity of the MgH2+10 wt.% Zn2TiO4samples.These findings are in line with the previous study that reported reduced hydrogen capacity due to the formation of active species during cycles [50].
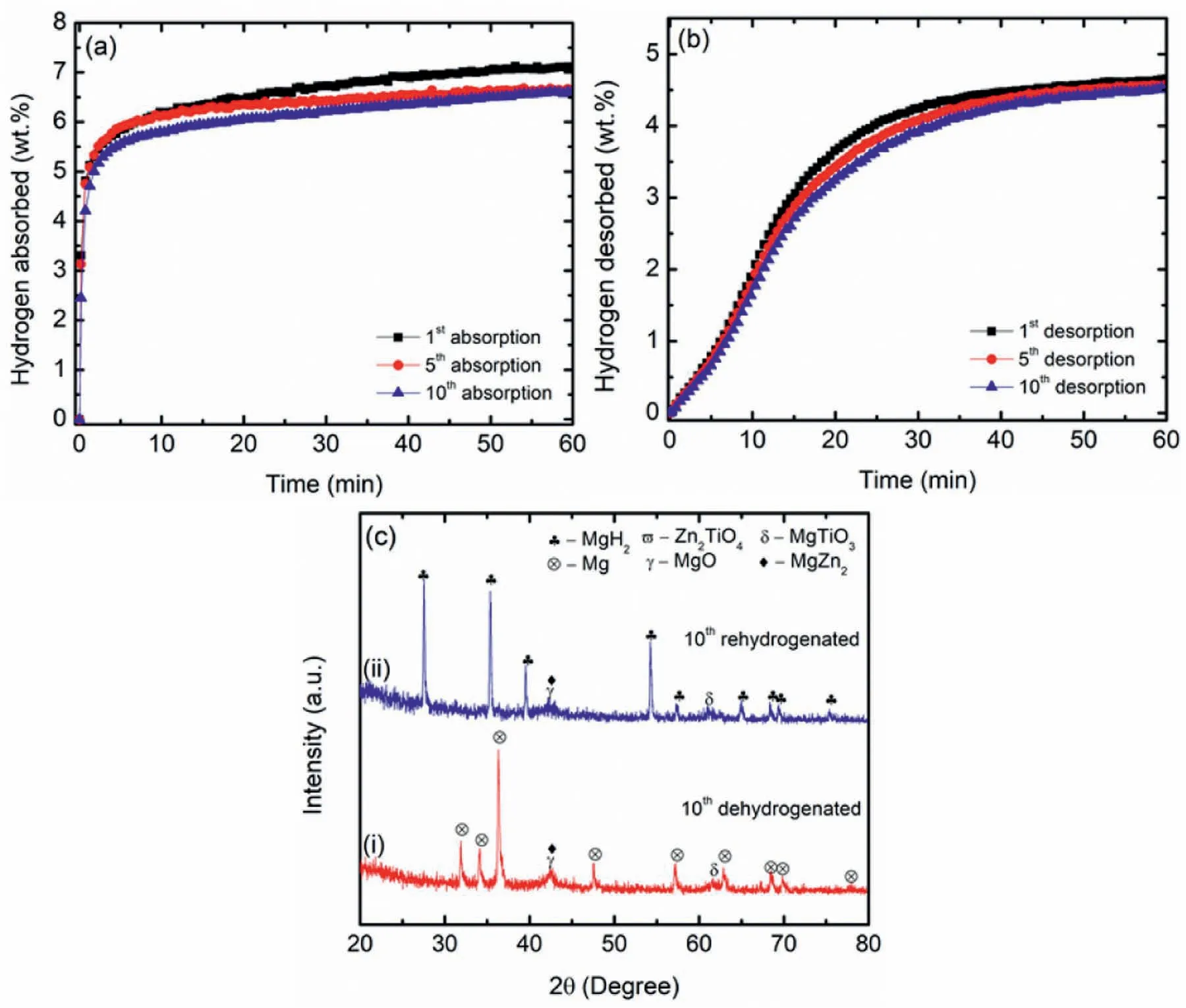
Fig.3.The (a) rehydrogenation cycling at 320 °C under 33 atm H2 pressure,(b) dehydrogenation cycling at 320 °C under 1 atm H2 pressure and (c) XRD patterns of the (i) dehydrogenated and (ii) rehydrogenated sample of MgH2+10 wt.% Zn2TiO4 samples after completing the 10th cycle.
Then,the DSC experiment was used to comprehensively evaluate the dehydrogenation behaviour of the MgH2+Zn2TiO4sample.Fig.4 shows the DSC profile of asmilled MgH2and MgH2+10 wt.% Zn2TiO4sample (heating rate: 25 °C/min).As indicated in the figure,the endothermic peak of as-milled MgH2appeared at 430 °C,while the endothermic peaks of MgH2+10 wt.% Zn2TiO4appeared at 390 °C.The endothermic peaks of the MgH2+10 wt.%Zn2TiO4sample was lower by 40 °C than undoped MgH2indicating an enhancement of the dehydrogenation behaviour of MgH2with the inclusion of Zn2TiO4.The lower endothermic peak of the doped sample represents the positive effects of Zn2TiO4and benefits the dehydrogenation behaviour of MgH2.It is important to note that the onset dehydrogenation temperatures obtained from DSC are higher than the curves determined by the PCT.The disparities could be attributed to the differences in heating rates and measuring atmospheres between PCT and DSC.The DSC was conducted at a heating rate of 25 °C/min with 50 mL/min Argon flow while the TPD experiment was conducted at a heating rate of 5 °C/min under vacuum.Similar outcomes were reported in previous studies [51–53].

Fig.4.DSC trace of as-milled MgH2 and MgH2+10 wt.% Zn2TiO4 at 25 °C/min.
Furthermore,to measure the enhancement of the dehydrogenation behaviour of MgH2,the dehydrogenation activation energy (EA) was evaluated by using Kissinger analysis as in Eq.(2) [54,55]:
whereas,
β=heating rate used in the DSC measurements
Tp=temperature of the endothermic curve
R=gas constant
A=linear constant
As shown in Figs.5(a) and 5(b),DSC experiments were performed at different heating rates to calculateEA.Thereafter,the activation energy was calculated using the Kissinger plot of ln [β/Tp2] vs 1000/Tpas depicted in Fig.5(c).As in Fig.5(c),a satisfactory linear relationship between ln[β/Tp2]and 1000/Tpwas established for the MgH2+10 wt.%Zn2TiO4sample and as-milled MgH2.The calculated activation energy for the dehydrogenation process for the as-milled MgH2was 135 kJ/mol.The activation energy was lessened to 105.5 kJ/mol corresponding to a decrease of 22%to as-milled MgH2when Zn2TiO4was added.This greatly reduces activation energy may be due to the particle sizes that have been reduced,which then minimises the distance for hydrogen diffusion and also broaden the specific surface area,favouring hydrogen transport and surface reaction [56].These factors contribute to a significant decrease in operating temperatures.

Fig.5.DSC curve of (a) as-milled MgH2,(b) MgH2+10 wt.% Zn2TiO4 and (c) Kissinger plot of as-milled MgH2 and MgH2+10 wt.% Zn2TiO4.
In terms of thermodynamic properties,the DSC curves were analysed using the STARe software to calculate the enthalpy (ΔHdec) of the decomposition of MgH2.The enthalpy of the MgH2decomposition was determined from the integrated peak area.The hydrogen desorption enthalpy for the as-milled MgH2was found to be 75.7 kJ/mol H2which is matching the theoretical value (76 kJ/mol H2) reported in a previous study [57].The enthalpy of the MgH2–Zn2TiO4was similar to that of milled MgH2.These outcomes agree with previous studies that reported that despite of enhancement in kinetic performance of MgH2by the addition of the catalyst,there was no impact on the thermodynamic of MgH2[58,59].
Fig.6 presents the SEM images of the commercial MgH2,as-milled MgH2and MgH2+10 wt.% Zn2TiO4sample.As can be seen in Fig.6(a),the commercial MgH2consists of a large particle with a smooth surface whereas,after the 1 h of the ball milling(Fig.6(b)),the as-milled MgH2presents a defect structure with a reduction of the particle size.Moreover,some agglomerations with non-uniform particle sizes were observed in as-milled MgH2.These findings are in line with the previously reported in the literature [60],but the particle size was significantly reduced and become more homogenous after 10 wt.% Zn2TiO4was added (Fig.6(c)).Smaller and more homogenous particle size is vital in lowering the dehydrogenation temperature and improving the re/dehydrogenation kinetic characteristics of the MgH2-catalysed system [49].

Fig.6.The SEM profiles of (a) commercial MgH2,(b) as-milled MgH2 and (c) MgH2+10 wt.% Zn2TiO4.
Fig.7 depicts the particle size distribution of commercial MgH2,as-milled MgH2,and MgH2+10 wt.%Zn2TiO4samples quantified using Image J software.The average particle size of the commercial MgH2was calculated to be ∼65 μm.Upon milling for 1 h,the average particle size of the as-milled MgH2was ∼0.5 μm,which was significantly reduced compared to commercial MgH2.The average particle size was significantly reduced to ∼0.3 μm with the presence of Zn2TiO4.This demonstrates that the ball milling techniques and the inclusion of the Zn2TiO4are beneficial in reducing the particle size of the MgH2+10 wt.% Zn2TiO4sample.The diffusion of H atoms to MgH2boundaries would be slower with larger particle sizes and fewer grain boundaries [61,62].Hence,smaller particle sizes could reduce the length of hydrogen diffusion,thus accelerating the hydrogen storage behaviour of MgH2.

Fig.7.Histogram of the particle size distribution of (a) commercial MgH2,(b) as-milled MgH2 and (c) MgH2+10 wt.% Zn2TiO4.
The phase composition of the sample was then characterised using the XRD method,as shown in Fig.8.The characterisation was performed in different states: a) after ball milling for 1 h,b) dehydrogenated state and c) rehydrogenated state.Referring to Fig.8(a),MgH2peaks dominated the XRD patterns with several peaks of Zn2TiO4.There was no new peak recorded,demonstrating that there was no chemical reaction throughout the ball milling process.For the dehydrogenated state (Fig.8(b)),all MgH2peaks vanished and Mg peaks formed presenting the complete dehydrogenation of MgH2.Additional peaks of MgO,MgTiO3and MgZn2appear showing that a chemical reaction occurred during the heating process.For the rehydrogenated state,similar peaks are obtained with the exception of the Mg peaks that are supplanted by the MgH2peaks (Fig.8(c)).
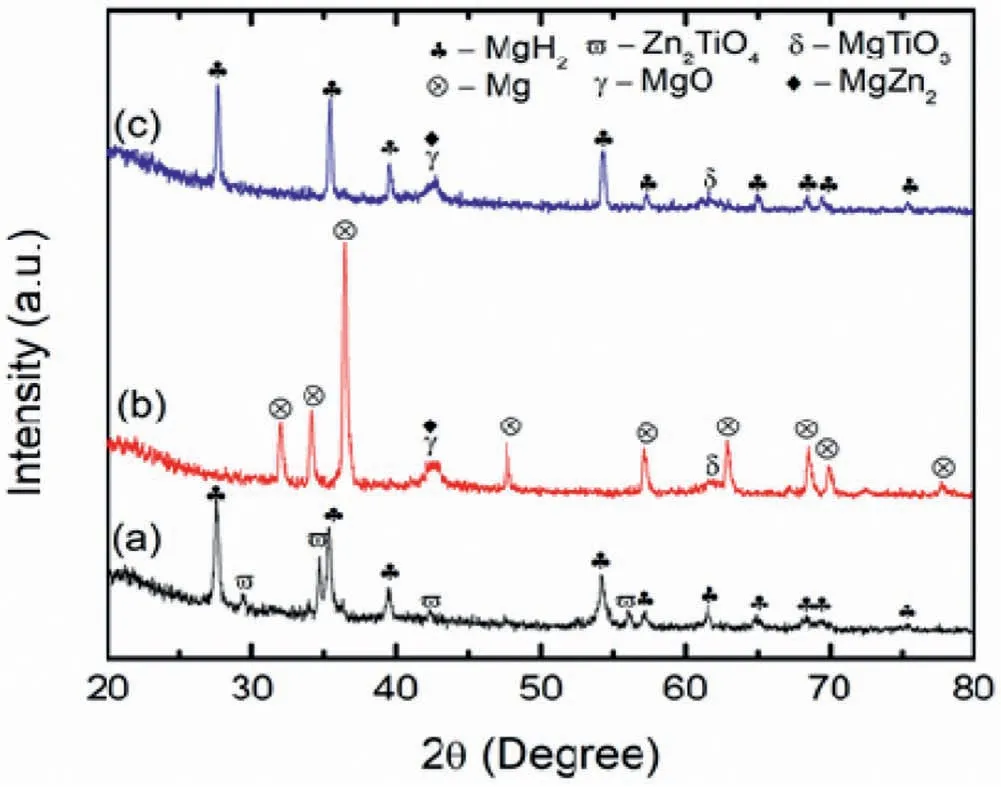
Fig.8.The XRD pattern of the (a) as-milled,(b) dehydrogenated state and(c) rehydrogenated state of MgH2+10 wt.% Zn2TiO4.
The XRD measurement for the 20 wt.% Zn2TiO4-doped MgH2sample was performed as shown in Fig.9 to confirm the reaction that happens during the milling process and after re/dehydrogenation process.After milling for 1 h (Fig.9(a)),the only peaks available are the peaks of MgH2and Zn2TiO4.For the dehydrogenated state (Fig.9(b)),the peaks available are the peaks of Mg indicating the complete dehydrogenation of MgH2with the additional peaks of MgO,MgTiO3and MgZn2.The peaks of MgO,MgTiO3and MgZn2remain unaltered for the rehydrogenated state (Fig.9(c)),and the Mg peaks are supplanted by the MgH2peaks.The presence of MgH2peaks indicates the reversibility of Mg,which is one of the most significant indicators for hydrogen storage.Based on this XRD analysis,a probable reaction that took place during the heating process is best summarised as follows:

Fig.9.The XRD pattern of the (a) as-milled,(b) dehydrogenated state and(c) rehydrogenated state of MgH2+20 wt.% Zn2TiO4.
Referring to the XRD analysis,the enhanced kinetics of MgH2doped with Zn2TiO4are benefited by the formation of new active species of MgO,MgTiO3and MgZn2.These active species function as an active channel at the MgH2matrix’s surface,providing a rapid pathway for the H atom to diffuse throughout the re/dehydrogenation processes [16,63].For instance,Ares-Fernandez and Aguey-Zinsou [64] found that MgO can act as a control process agent,reducing and preventing MgH2agglomeration by attaining an ideal breakage rate.In addition,the presence of MgTiO3performs superior kinetics performance of MgH2with the ability to absorb 5.0 wt.% H2in 500 s [65].Other research also indicates that the presence of MgZn2can assist hydrogen diffusion and promotes structural defects (such as lattice dislocations and grain boundaries) [66,67].Moreover,the in situ formation of MgZn2benefited the hydrogen storage performance of the Mg95Sn3Zn2alloy [68].According to another study,Mg0.97Zn0.03is converted into MgH2and intermetallic MgZn2compounds during the early phases of the hydrogenation process [69].During dehydrogenation,the generated MgZn2nanoparticles were converted to an Mg(Zn) solid solution,which was distributed homogenously in the MgH2matrix.This intriguing reversible phase change of Mg(Zn) benefits substantially from the decrease in the thermodynamic stability of MgH2[70].As a consequence,the catalytic effects of MgZn2,MgTiO3and MgO could collaborate to further boost the re/dehydrogenation kinetics of MgH2as well as lower the dehydrogenation temperature.However,to assess the overall impact and mechanism of the MgH2–Zn2TiO4system,more characterization employing transmission electron microscopy and X-ray photoelectron spectroscopy is required.
4.Conclusions
The inclusion of solid-state synthesized Zn2TiO4benefited the hydrogen storage behaviour of MgH2.By adding different wt.% of Zn2TiO4,the initial dehydrogenation temperature of MgH2was downshifted to around 290 °C–305 °C.The kinetics behaviour of the MgH2was also boosted with the capability to absorb 5.0 wt.% H2by the MgH2–Zn2TiO4sample at 250 °C within 300 s and liberates 2.2–3.6 wt.% H2from the composite sample at 300 °C within 30 min.The dehydrogenation activation energy of the MgH2–Zn2TiO4system was also reduced by 22% than undoped MgH2.The excellent hydrogen storage behaviour of the MgH2–Zn2TiO4system is corresponding to the synergistic effect of MgO,MgTiO3and MgZn2that formed in situ during the heating process that is beneficial in ameliorating the re/dehydrogenation behaviour of MgH2.From the result,it can be deduced that the solidstate synthesized Zn2TiO4is a promising additive to boost the hydrogen storage behaviour of MgH2.These findings may be advantageous modifications to the MgH2system.
Acknowledgment
The authors acknowledged Universiti Malaysia Terengganu(UMT) for the funding provided by Golden Goose Research Grant (GGRG) VOT 55190.N.A.Ali and N.A.Sazelee are thankful UMT for the SIPP and BUMT scholarship.M.Ismail,M.M.Nasef and A.A.Jalil also thank the Prominent Visiting Researcher Scheme awarded by The Department of Deputy Vice Chancellor (Research &Innovation),Universiti Teknologi Malaysia.
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Inhibiting effect of I-phase formation on the plastic instability of the duplex structured Mg-8Li-6Zn-1.2Y (in wt.%) alloy
- PEO coating on Mg-Ag alloy: The incorporation and release of Ag species
- Underlying mechanisms of variation in yield asymmetry and strain hardening behavior of extruded pure Mg with Gd addition
- Edge crack damage analysis of AZ31 magnesium alloy hot-rolled plate improved by vertical roll pre-rolling
- High sintering and dielectric performance: The improved (Mg,Zn)3B2O6 ceramics with the help of the DFT calculation
- Corrosion behavior of magnesium in aqueous sulfate-containing electrolytes
