High sintering and dielectric performance: The improved (Mg,Zn)3B2O6 ceramics with the help of the DFT calculation
Rui Peng ,Yongheng Lu ,Ling Shi ,Guoling Yu ,Yunming Li ,Gng Wng ,Yunxun Li,b,∗,Hu Su,∗,Huiwu Zhng
aState Key Laboratory of Electronic Thin Films and Integrated Devices,University of Electronic Science and Technology of China,Chengdu 610054,China
b Yangtze Delta Region Institute (Huzhou),University of Electronic Science and Technology of China,Huzhou 313001,China
c China Jiliang University,Hangzhou 310018,China
d Chengdu University of Technology,Chengdu 610059,China
Abstract With the help of the first principle calculation,the solid-state reaction experiment was conducted to investigate the alteration in the sintering and the microwave dielectric properties of Mg3B2O6 ceramic with many Zn2+ substitutions.These properties were characterized using the scanning electron microscopy,network analyzer,X-ray diffraction,Raman spectroscopy,energy-dispersive spectroscopy,and thermomechanical and differential-thermal analyses.The coexistence of Mg3B2O6,Mg2B2O5 and ZnO ceramics could be observed with increasing Zn2+ addition,and the lattice distortion occurred in the Mg2B2O5 and Mg3B2O6 ceramics due to the substitution of Mg2+ with Zn2+.The electron density and the bond property of the MgO6 octahedron changed,and a quantitative method was used to discuss the variation in sintering,substitution and phase formation properties.The densification window was decreased to 1100 °C,and the dielectric properties improved with the formation of a three-phase borate solid solution (dielectric constant=6.73,quality factor=112,000 GHz at 16 GHz (Q=7000),temperature coefficient of resonant frequency=-61.2 ppm °C-1,and relative density=97.0%).
Keywords: DFT;Borate;Solid solutions;Dielectric properties.
1.Introduction
Dielectric ceramics are widely studied recently due to its excellent electrical and mechanical properties,especially in the field of smart home,internet of things,aerospace,and wireless communication [1].The ceramic passive module integration technology is a promising method to minimize the size of electronic equipment and increase the transmission performance of signals,which is the development direction of the electronic component [2–4].In terms of 5 and 6 G communication,materials with relatively high quality factor(Q×f) and low dielectric constant (εr) may be used to realize a fast and high-quality signal transmission and are worth studying in the field of ceramic passive module integration technology [5–7].
Dosler et al.reported that the microwave dielectric performance of the Mg3B2O6(MBO) ceramic sintered at 1350 °C are 7 forεr,108,000 GHz forQ×f,-69 ppm °C-1forτf,97%for relative density[8].The dielectric performance of the MBO meet the requirements for microwave communication,but the densification temperature should be decreased.Wu et al.found the Zn3B2O6(ZBO) material sintered at 925 °C have great dielectric properties (6.7 forεr,58,500 GHz forQ×f,-58 ppm °C-1forτf,and 96% for relative density)[9].Considering the sintering and the microwave properties of the MBO and the ZBO ceramics,the addition of the ZBO ceramic into the MBO ceramic can obtain the following advantages.Firstly,the densification temperature can be decreased without the remarkable deterioration of theQ×fvalue.Secondly,theεrand theτfvalues can be slightly adjusted to the desired direction.
The solid-state reaction method is used to synthesize the(1-x)MBO+xZBO (x=0.00–0.40) composite materials.The sintering and the microwave dielectric performance of it are investigated,and the calculation based on the first principle is conducted to explain the microscopic characteristics theoretically.
2.Experimental
Analytically pure powders (MgO (99%),ZnO (99.5%) and B2O3(99%)) were weighed in accordance with the stoichiometric ratio (B2O3was weighed 10 mol.% in excess for compensation),and Chron Chemicals Co.Ltd (China) is the supplier of raw materials.The prepared materials were premilled(12 h),presintered (900 °C,4 h) and remilled (12 h).Distilled H2O and agate balls were the milling media.The 5 wt.%polyvinyl alcohols and 95 wt.%remilled powders were mixed.The obtained composite material was pressed into cylinders with thickness and diameter of 6 and 12 mm,respectively,and the final sintering temperature was 1050–1200 °C.
The First-principle analysis was performed based on the Cambridge Serial Total Energy Package.The pseudopotential of Vanderbilt ultrasoft was taken to treat the ions interplay.The countermeasure of the approximation for the exchange correlation interplay and the atom vibration is the Perdew–Burke–Ernzerhoff standard and the linear response standard.The valence electron configurations were 2p63s2for Mg,3d104s2for Zn and 2s22p4for O.After testing,the energy cutoff,k-point mesh,maximum force,maximum stress,total energy,energy and maximum displacement were 380 eV,3 × 2 × 3,0.05 eV ˚A-1,0.05 GPa,1.0 × 10-6eV per atom,5.0 × 10-5eV per atom and 0.001 ˚A,respectively.The optimised crystal was obtained based on the Broyden–Fletcher–Goldfarb–Shanno standard.A super cell (2 × 1 × 2) with 88 atoms was constructed to investigate the substitution property.The formation energy was calculated using the total energies of the doped (EDo) and the undoped (EUnDo) systems,the enthalpy for the replaced element (pi) and the unit modulus (ki)[10].
The crystal information was confirmed using the X-ray diffraction (DX-2700,Haoyuan Co.) equipped with Cu Kαsource.The Rietveld profile refinement method was used to process all data.The densification level and the composition of sample were determined using scanning electron microscopy (JEOL,JSM-6490LV) and energy-dispersive spectroscopy.The dielectric performance at microwave frequency were obtained using the Agilent N5230A Network Analyzer(300 MHz-20 GHz) and the transmission cavity,and the algorithm is based on the Hakki–Coleman standard [11].The sintering properties were investigated by the thermomechanical (Mettler TMA/SDTA2+,5,10 and 15 K min-1) analyzer(shrinkage characteristic) and the simultaneous thermal (Mettler TGA/DSC3+,5 K min-1) analyzer (chemical characteristic).τfof all samples was calculated using Eq.(2) with the frequency at resonant point,80 °C (fT) and 20 °C (f0) [12].
The bulk density was measured based on the Archimedes way[13].The following mixing rules Eqs.(3)–((5))were used to calculate the theoreticalτf,εrandQ×fvalues [14–16].
va(b)represents the volume ratio of component a(b).τfa(b),εra(b)andQa(b)represent the dielectric performance of componenta(b).Beside,the activation energy (Ea) was calculated using the Arrhenius expression [17].
where R set as 8.3145 J K-1mol-1(gas constant),kset as 5,10 and 15 K min-1(heating rate),Tandzcorrespond to the temperature and the Arrhenius constant.
3.Results and discussion
Fig.1(a–d) manifests the relative density and the dielectric properties of the materials synthesised at increasing sintering temperatures.The variation in density increases whenx=0.00–0.05 and like a parabola whenx=0.10–0.20 andx=0.25–0.40.The peak value is 97.0% when thex=0.15 sample is processed at 1100 °C.The variation trend in theQ×fand theεris close to that in densification level except whenx=0.30.Such phenomenon is because the density is one of the extrinsic dominant factors for the dielectric loss and theεr,and a lower relative density corresponds to a higher dielectric loss and lowerεr.The dielectric properties topped atx=0.15 and 1100 °C,Q×f=112,000 GHz at 16 GHz(Q=7000),εr=6.73.Beside,the measuredτfvalue shows a saddle trend with a turning point whenx=0.10,and theτfis-61.2 ppm °C-1when thex=0.15 specimen is processed at 1100 °C.Hence,the densification level,sintering and dielectric performance and thermal stability of the MBO ceramic can be improved with the moderate substitution of Zn2+to Mg2+.Fig.1(e) shows the summary of various ceramics with relatively lowεrand highQ×f,and the dielectric properties of the ceramics synthesised in this study are excellent[8,9,18–34].
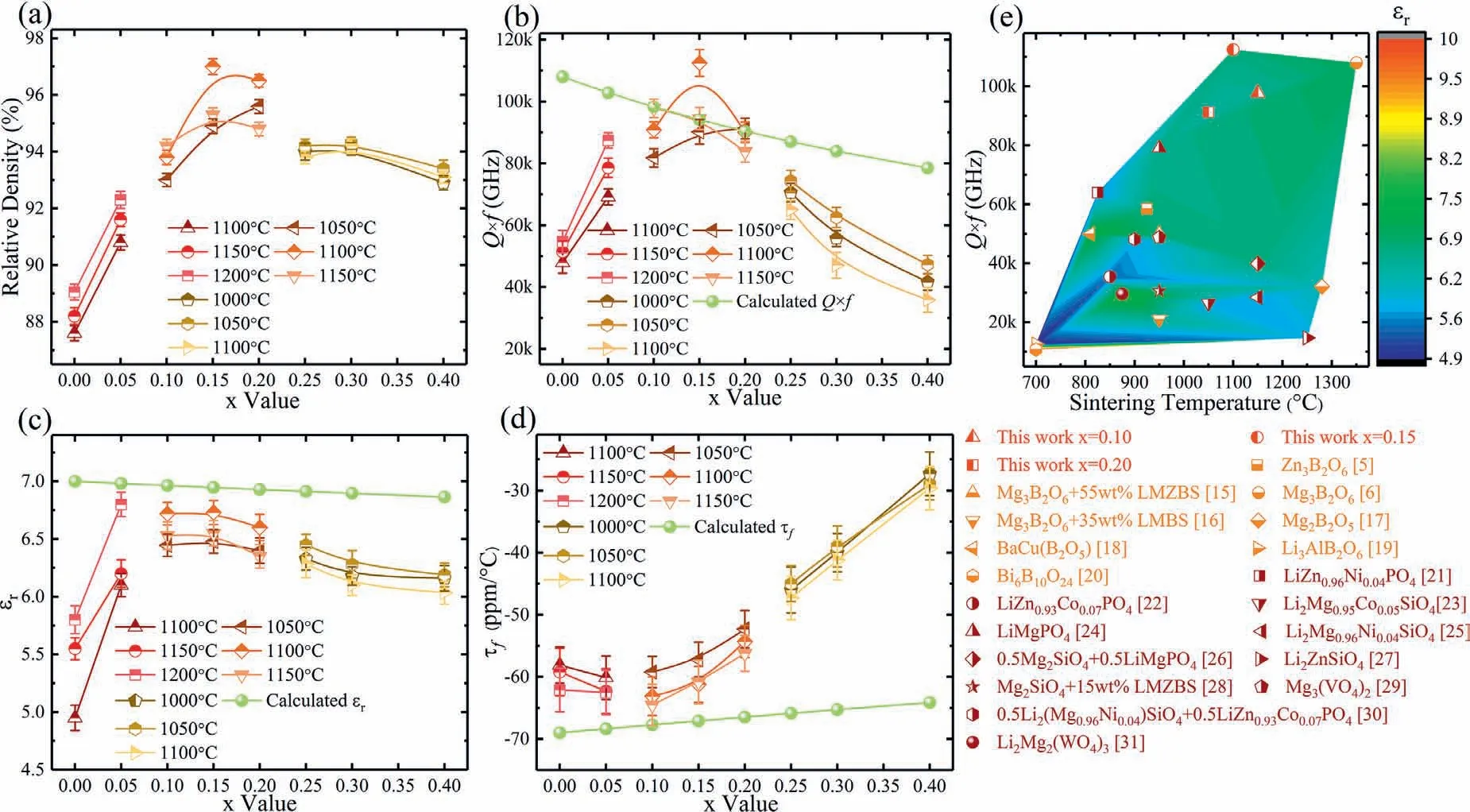
Fig.1.Relative density (a) and dielectric values (b-d) of the (1-x)MBO+xZBO (x=0.00–0.40) ceramics sintered at 1100–1200 °C (x=0.00–0.05),1050–1150 °C (x=0.10–0.20) and 1000–1100 °C (x=0.25–0.40).Comparison of the sintering and the dielectric performance of different ceramics (e).
The crystallinity of the samples is confirmed using XRD patterns (Fig.2 [a–c]).A composite material is formed with MBO (JCPDS#38–1475) and Mg2B2O5(JCPDS#15–0537)whenx=0.00–0.10,and a third phase,i.e.the ZnO phase(JCPDS#36–1451),has emerged whenx≥0.15.With increasingxvalue,the intensities of peak for Mg2B2O5and ZnO strengthen,and that of MBO weakens.As illustrated in Fig.2(b–c),the(121),(211) and(131) peaks of MBO and the(0–21) peak of Mg2B2O5move to a low angle,demonstrating that the crystal volumes of the MBO and the Mg2B2O5increase with the addition of Zn2+.Given that the radius of magnesium ion (0.72 ˚A) is smaller than that of zinc ion(0.74 ˚A),the increasing Zn2+concentration enhances the substitution of Zn2+to the Mg2+of MBO and Mg2B2O5,which can explain the movement of peaks [35].Beside,all raw data are refined with acceptable Fullprof parameter,and results are presented in Fig.2(e–j) and Table 1.Thea–cvalues of MBO and Mg2B2O5manifest an increasing trend,and all the angle parameters of MBO remain unchanged.Theαand theγvalues of Mg2B2O5present a vibration trend,and theβvalues decreases monotonously with increasingxvalue.The cell volumes of MBO and Mg2B2O5increase too,which is in accordance with the peak movement of the XRD patterns.In the component fraction of composite materials,the fraction value of MBO decreases with increasing Zn2+addition,and those of Mg2B2O5and ZnO increase.Notably,the measured component ratio of MBO is larger than the designed component ratio of MBO whenx=0.10–0.15,whereas the designed component ratio surpasses the component ratio whenx=0.00–0.05 andx=0.20–0.40.The substitution of Zn2+to Mg2+of MBO and the formation of Mg2B2O5are responsible for the discrepancy whenx≤0.10.Moreover,the emergence of ZnO should be accounted too for the discrepancy whenx≥0.15.Considering the refinement result,the increasing relative density of the samples sintered at temperature lower than 1350 °C can be ascribed to the decreased intrinsic sintering temperature due to the lattice distortion (substitution of Zn2+to Mg2+in the crystal of MBO and Mg2B2O5).The Mg2B2O5ceramic has the following dielectric performance:εr,6.2;Q×f,32 × 100 GHz;andτf,-18 ppm °C-1,and the densification temperature of it is 1250 °C (lower than 1350 °C) [20].Therefore,the formation of Mg2B2O5is also responsible for the density improvement whenx=0.00–0.15.The deterioration of density is attributed to the relatively low sintering temperature (x=0.25,0.30) and the oversintering phenomenon (x=0.40).Apart from the variation in relative density,the difference between the measured and the inferredεr/τfis ascribed to the formation of Mg2B2O5with relatively smallerεr(6.2) and largerτf(-18 ppm °C-1) contrasted with that of MBO.Hence,the synthesized ceramic has a relatively lowerεrand higherτfcompared with the calculated values.Beside,the divergence between the measured and the inferredQ×fvalues can be due to many factors,such as the lattice distortion due to Zn2+to Mg2+substitution (MBO and Mg2B2O5),the formation of Mg2B2O5phase and the improvement of the relative density.In addition,the appearance of ZnO is accounted for the variation in the sintering and the dielectric performance of the(1-x)MBO+xZBO composite materials.
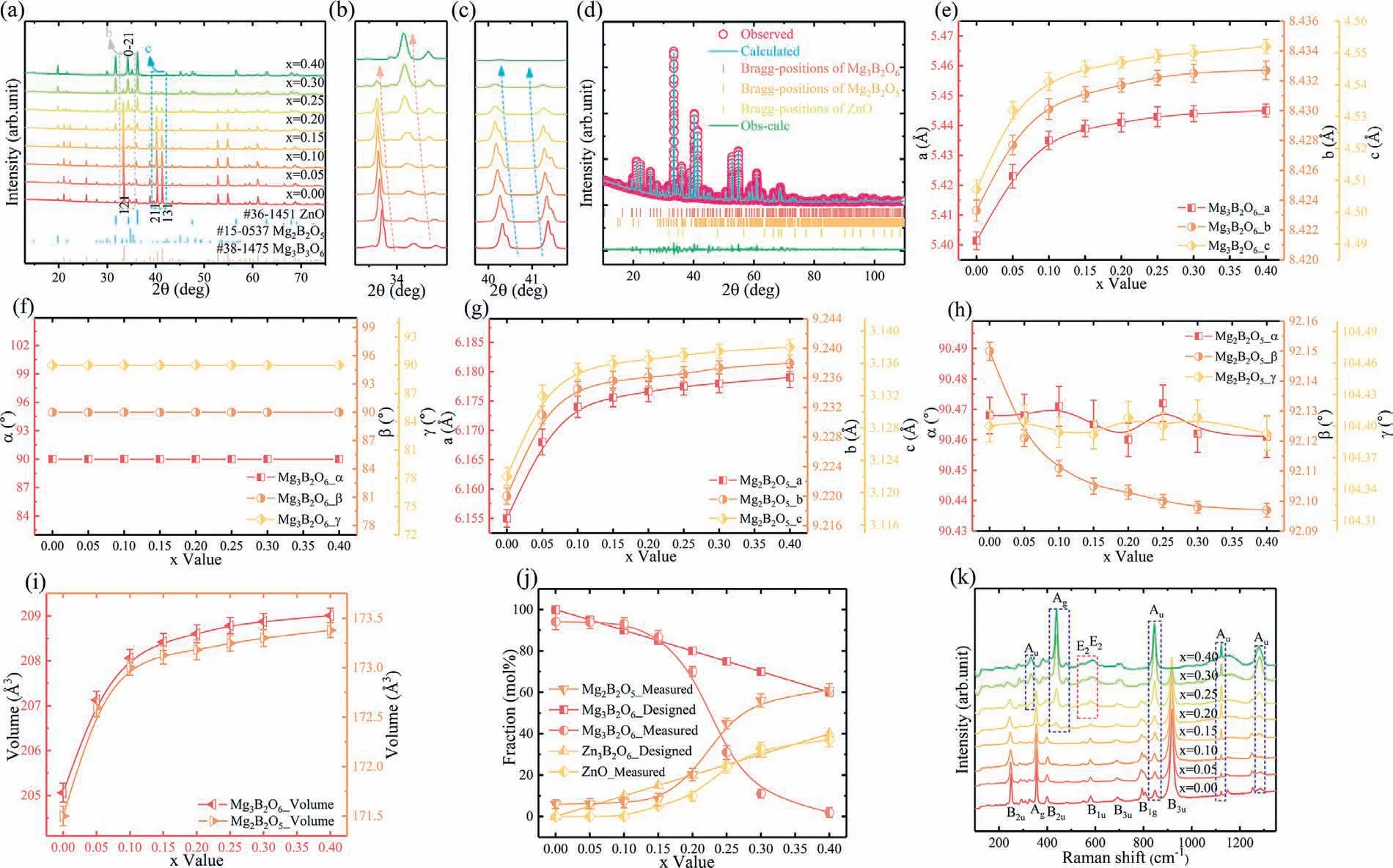
Fig.2.XRD data of the (1-x)MBO+xZBO (x=0.00–0.40) materials obtained at 1200 °C (x=0.00–0.05),1100 °C (x=0.10–0.20) and 1050 °C(x=0.25–0.40) (a-c).Refinement profiles of the 0.85MBO+0.15ZBO material obtained at 1100 °C (d).Lattice parameters of the MBO and the Mg2B2O5 ceramics (e-j).Raman spectra of the (1-x)MBO+xZBO (x=0.00–0.40) materials (k) (For interpretation of the references to color in this figure,the reader is referred to the web version of this article).
The vibration property of all samples is confirmed using Raman spectroscopy,and the result is presented in Fig.2(k).The vibration modes are obtained using the Bilbao Crystallographic system and the DFT calculation.The vibration modes of MBO,Mg2B2O5and ZnO materials are as follows.
R and IR represent the Raman and the Infrared modes,respectively.For the MBO and the Mg2B2O5ceramics,the dividing lines between the external and the internal modes are observed at 700 and 740 cm-1,respectively.The vibration status at the band lower than the dividing line are the rotational and the translational modes of MgO6.The bending and the stretching vibration modes of BO3are corresponding to the other half vibration status.With the enhanced substitution,the peak intensity of MBO weakens,and those of Mg2B2O5(blue circled) and ZnO (pink circled) strengthen.Hence,the Raman spectra can verify the result of phase formation.
The microstructure of samples processed at 1050–1200 °C is analysed using SEM(Fig.3(a–h)).The image is distributed with small grains for thex=0.00 sample,and the addition of Zn2+results in the growth of grains (Fig.3 (b–d)).A compact microstructure is obtained whenx=0.15,and increasing thexvalue from 0.25 to 0.30 leads to decreased grain size.Beside,the melting grain boundary and trapped pores have appeared in thex=0.40 sample.The SEM images and the variation in relative density agree well.The EDS mapping images of thex=0.15–0.20 samples are shown in Fig.3(i–r),and the distribution of O,Zn and Mg are uneven in general (too light to be detected for B).The distribution of the O–and the Mg-rich areas are the same,and the Zn-rich area is also an O-deficient area.In addition,the proportion of different elements manifested in the mapping images is in accordance with the designed value.Notably,three phases are present in the backscatter images (Fig.3(i,n)),and the EDS point detection is performed to check the composition of these phases in thex=0.15 sample (Fig.3(s–v)).The atom ratios of Zn:Mg:O are 3.65:33.27:63.08,3.74:28.27:67.99 and 42.59:0.00:57.41 for spots 1,2 and 3,respectively.Considering that the atom ratios of Zn:Mg:O are 0.00:33.33:66.67,0.00:28.57:71.43 and 50:0.00:50 for MBO,Mg2B2O5and ZnO,respectively,the phases of spots 1,2 and 3 should be MBO,Mg2B2O5and ZnO,respectively.The existence of Zn for spots 1 and 2 should be attributed to the substitution of Zn2+to Mg2+.Therefore,the EDS result is consistent with the phase formation and the lattice distortion from the XRD results.
The sintering property of the composite ceramic is confirmed through TG,DSC and TMA(Fig.4(a–c)).Three stages of weight loss,i.e.12% for 100–300 °C,3% for 300–600 °C and 1% for 600–1150 °C,are observed in the TG pattern.The decomposition of H3BO3from B2O3and H2O and the removal of hydration H2O are responsible for the weight loss of the first stage,which corresponds to the endothermic peak at around 200 °C observed in the DSC.The ratio of the weight loss and the intensity of the endothermic peak in the first stage decreases with increasingxvalue,manifesting that the decomposition amount of H3BO3has declined.Hence,the addition of ZnO can restrain the formation of H3BO3.The second stage is ascribed to the decomposition furtherly of H3BO3and the preliminary phase emergence of the composite ceramics,and no evident peak is observed in the DSC patterns at the same temperature range.The weight loss in the third stage is weak and attributed to the phase formation furtherly of the composite ceramics.Notably,the intensity of the exothermic peak in the blue dotted box shows an increasing trend,which is attributed to the emergence of Mg2B2O5.The intensity of the exothermic peak in the orange dotted box shows a decreasing trend,which is ascribed to the formation of MBO.The variation in such peak intensity can verify the variation in the phase fraction in the XRD patterns.The evaporation of B may be responsible for the endothermic peak near 980 °C.Beside,the exothermic peaks near 650 °C moves to a relatively low temperature.The temperature of the shrinkage onset moves to a low temperature with increasing Zn2+addition,which is similar to the peak movement of DSC.Therefore,increasing Zn2+addition can result in the formation of a heterophase,and the densification window can be lowered.In addition,the sintering property is further investigated using theEaat 3,6 and 9% shrinkage values,and the plots of lnkagainst1/Tare shown in Fig.4(d–i).As shown in Fig.4(j),theEapresents a declining trend,and theEavalue of thex=0.15 sample is 476 ± 66 kJ mol-1.Therefore,the addition of Zn2+can decrease theEavalue and the densification temperature of the composite ceramics.These conclusions are the same as the discussion regarding the relative density.
The crystal variation is discussed with the help of DFT calculation,and the schematic of the ion substitution is presented in Fig.5.Two sites are available in the MBO and Mg2B2O5for Mg2+to occupy,and the lattice parameters arePnmn(No.58),a=5.4014 ˚A,b=8.4233 ˚A,c=4.5071 ˚A andα=β=γ=90° for MBO andP1(_) (No.2),a=6.187 ˚A,b=9.219 ˚A,c=3.119 ˚A,α=90.4°,β=92.13° andγ=104.32°for Mg2B2O5.In this study,each site with Mg2+is changed into Zn2+successively,and the microstructure properties,including electron distribution,bond length and population around the octahedron,are discussed(Figs.5(b,d)–7).The variation in ion position,bond length and population are observed in the MgO6octahedron for MBO and Mg2B2O5(Length/Population-B/A indicates the bond length/population before/after ion substitution,and Length-E indicates the bond length from the XRD data).The bond length and population of the MgO6octahedron for MBO and Mg2B2O5increase after the substitution,indicating that the covalency of the cation and the O-ion bond is strengthened.This variation is responsible for improved sintering properties.The increasing bond length and population is driven by the diversity of the ionic polarizability and the ionic radius between Zn2+(2.04 ˚A3,0.74 ˚A) and Mg2+(1.32 ˚A3,0.72 ˚A),and the different extranuclear electron pairing status of the Zn2+(3d10)and Mg2+(2p6) should be accounted [35,36].The variation tendency in the Length-E is consistent with that of calculation,manifesting that the substitution process involves every Mg2+site and verifying the XRD results.Beside,the bonding property of the MgO6octahedron can be further investigated using the electron density distribution of the (001) face around the Mg2+.The electron density near the cation of the octahedron is strengthened after substitution compared with the undoped plane,and the electron interplay between the cation and the O is enhanced.The larger polarizability of Zn2+compared with that of Mg2+may be responsible for such variation,and the symmetry of the electron distribution near the cation is preserved for MBO and Mg2B2O5.The variation in bond parameters is the quantification of the change for the electron density distribution.Considering the driven factor for the intrinsic loss,the modification of bond properties and electron distribution results from the Zn2+substitution may have an important influence on the improvement of theQ×fvalue[37].

Fig.5.Cell structure of MBO (a).Schematic of the MgO6 octahedron before and after the Zn2+ substitution (b).Crystal structure of Mg2B2O5 (c).Schematic of the MgO6 octahedron before and after the Zn2+ substitution (d).
The formation enthalpy of each substituted system is obtained to research the substitution property of the MBO and the Mg2B2O5ceramics.The chemical potentials of Mg,Zn,MBO and Mg2B2O5are-973.5126,-1710.5005,-5712.6593 and-4298.7629 eV,respectively(Fig.6(e)).Notably,all formation energies are positive,and the formation energies for the Mg1 and the Mg2 site substitutions of MBO and Mg2B2O5are the same in general.Hence,the occupied possibility of the Mg1 site and the Mg2 site is equal in the MBO and the Mg2B2O5ceramics.In addition,the designed and measured energies per mole of the composite ceramics(-7912.8141 eV for ZBO and-2147.1981 eV for ZnO) are shown in Fig.6(f).These values present a monotonous declining trend,and the designed value is smaller than the measured value whenx=0.05–0.40.Therefore,the increasing Zn2+concentration has a more important influence than the energy of the system on the component ratio discrepancy.All explanations on energy can verify the XRD refinement result.
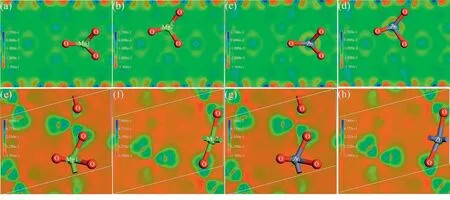
Fig.7.Electron distribution map of the face with two kinds of Mg2+ before and after the Zn2+ substitution for MBO (a-d) and Mg2B2O5 (e-h).
4.Conclusion
With the help of the first principle calculation,the solidstate reaction experiment was conducted to investigate the alteration in the sintering and the microwave dielectric properties of Mg3B2O6ceramic with many Zn2+substitutions.The coexistence of Mg2B2O5,MBO and ZnO ceramics can be observed with increasing Zn2+addition,and the lattice distortion occurs in the Mg2B2O5and the MBO ceramics due to the substitution of Zn2+to Mg2+.The electron density and the bond property of the MgO6octahedron have changed,and a quantitative method is used to discuss the variations in sintering,substitution and phase formation properties.The densification window is decreased to 1100 °C,and the dielectric properties are improved with the formation of a three-phase borate solid solution (εr=6.73,Q×f=112,000 GHz at 16 GHz (Q=7000),τf=-61.2 ppm °C-1,relative density=97.0%).The substitution of Zn2+to Mg2+is effective in improving the sintering and the dielectric performance of the MBO ceramic at microwave frequency.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.61771104 and 62071106),Jiangxi Innovative Talent Program,and Sichuan Science and Technology Program (Grant No.2021JDTD0026).
 Journal of Magnesium and Alloys2023年6期
Journal of Magnesium and Alloys2023年6期
- Journal of Magnesium and Alloys的其它文章
- Ameliorating the re/dehydrogenation behaviour of MgH2 by zinc titanate addition
- Inhibiting effect of I-phase formation on the plastic instability of the duplex structured Mg-8Li-6Zn-1.2Y (in wt.%) alloy
- PEO coating on Mg-Ag alloy: The incorporation and release of Ag species
- Underlying mechanisms of variation in yield asymmetry and strain hardening behavior of extruded pure Mg with Gd addition
- Edge crack damage analysis of AZ31 magnesium alloy hot-rolled plate improved by vertical roll pre-rolling
- Corrosion behavior of magnesium in aqueous sulfate-containing electrolytes
