丝素湿法纺丝中凝固浴对再生丝结构影响机制
陈琴 吴雷蕾 王平

摘要: 为了研究凝固剂种类对再生丝性能的影响,本文分别以十二烷基硫酸钠、去离子水和75%乙醇作为凝固浴,通过再生丝素蛋白湿法纺丝,得到三种再生丝RSF(SDS)、RSF(DI)和RSF(ET)。结果表明,RSF(SDS)具有良好的机械性能,其断裂伸长率达126%,高于20%RSF(DI)和26%RSF(ET)再生丝;傅里叶变换红外光谱分析表明,RSF(SDS)中含有较高比例的β-折叠结构;进一步通过X射线衍射和差示量热法分析显示,RSF(SDS)纤维比RSF(DI)和RSF(ET)纤维具有更多存在于无定形区的β-折叠结构;荧光探针法验证了SDS能够促进丝素蛋白更快速地形成β-折叠结构,乙醇则会诱导丝素蛋白更快速地形成结晶。本文制备了具有优异延伸性的再生丝,可为丝素蛋白湿法纺丝提供一种新思路。
关键词: 丝素蛋白;再生丝;凝固浴;力学性能;聚集態结构;成纤机制
中图分类号: TS101.921;TQ340.64
文献标志码: A
文章编号: 1001-7003(2023)04-0001-09
引用页码:
041101
DOI: 10.3969/j.issn.1001-7003.2023.04.001(篇序)
近年来,蚕丝作为一种天然蛋白质纤维,具有良好的生物相容性、吸湿性和透气性[1],已经广泛应用于生物医疗[2-4]、人体组织工程[5-7]、光学器件[8-11]等热门研究领域。然而,在实际生产、加工和织造过程中,会产生大量废弃物。因此,基于可持续发展的原则,研究人员常从废弃丝织品中提炼获取再生丝素蛋白纤维(Regenerated silk fibroin,RSF),目前主要是通过湿法纺丝法,制备以再生蚕丝蛋白为原料的再生丝素蛋白纤维。再生丝素蛋白纤维具有生物相容性好、可塑性强、可降解性和吸湿性等优点,可应用于人体组织工程[12-13]、生物传感器[14-16]等领域。Ki等[17]以磷酸—甲酸作为溶剂,甲醇作为凝固浴,制备得到了断裂强度和断裂伸长率分别为274 MPa和18%的纤维;Zhou等[18]以水为溶剂,用热的硫酸铵水溶液作为凝固浴进行湿法纺丝,将再生丝进行三倍牵伸后,得到了断裂伸长率为27.7%的再生丝素蛋白纤维;吴惠英等[19]以氯化钙甲酸作为溶剂,以水作为凝固浴,得到了断裂伸长率为23.8%的湿纺再生丝素蛋白纤维。然而,综上所述,由于湿法纺丝所得到的再生丝均表现出较差的断裂伸长率,大幅限制了再生丝的应用。
十二烷基硫酸钠(Sodium dodecyl sulfate,SDS)是一种典型的阴离子表面活性剂,乳化性能较好,具有低皮肤刺激性、高安全性,它能与蛋白质的疏水键发生结合[20-21]。Wu等[22]用SDS作为胶凝剂,加速丝素凝胶化,揭示了SDS诱导快速凝胶化的机理;Li等[23]将SDS添加到丝素水凝胶中,得到了压缩模量为3.0 MPa的水凝胶。因此,上述结果表明,添加适量SDS不仅能够提高水凝胶的力学性能,而且表明其具有诱导丝素蛋白构象演变的功能,在基于湿法纺丝的再生丝重建中具有潜在用途。凝固浴作为湿法纺丝的重要参数,会对再生丝的力学性能产生直接影响[24],但目前未见将表面活性剂作为凝固剂的相关研究。因此,本文以甲酸溶解的再生丝素蛋白为原料,SDS溶液作为凝固浴进行湿法纺丝,对比考察采用去离子水和75%(V/V)乙醇作为凝固浴的再生丝性能,探究SDS对丝素蛋白构象的影响及再生丝的形成机制。
1 实 验
1.1 试 剂
桑蚕丝(江苏鑫缘茧丝绸股份有限公司);无水碳酸钠、无水乙醇、溴化锂、98%甲酸、无水氯化钙、十二烷基硫酸钠均为分析纯(国药集团化学试剂有限公司),硫黄素T(色谱纯,上海阿拉丁生化科技股份有限公司)。
1.2 制备方法
1.2.1 再生丝素蛋白溶液的制备
将蚕丝按1︰30浴比放入0.05%碳酸钠溶液中,煮沸30 min,取出后用去离子水反复清洗去除杂质和残余离子,重复操作3次后,60 ℃烘干,得到脱胶蚕丝备用。
称取适量脱胶蚕丝,溶解在9.3 mol/L的溴化锂溶液中,质量体积比(M/V)为1︰10,在70 ℃水浴锅中溶解1 h后,装入截留相对分子质量为8 000~14 000的透析袋中,透析数日,直至透析出的溶液电导率小于4 μS/cm时透析结束,溶液质量浓度采用称重法测定,根据下式对丝素蛋白溶液的质量浓度进行计算。
c=M2-M1V×0.001(1)
式中:c为质量浓度,g/L;M1为称量瓶空瓶质量,g;M2为丝素蛋白及称量瓶质量,g;V为加入的丝素蛋白的体积,mL。
1.2.2 纺丝溶液的制备
称取适量无水氯化钙(CaCl2),溶解于甲酸(FA)溶液中,得到质量分数为5% FA-CaCl2溶液。称取4 g脱胶蚕丝于20 mL FA-CaCl2溶液中,室温下以800 r/min的速度搅拌4 h,得到再生丝素蛋白溶液。
1.2.3 再生丝的制备
纺丝液质量浓度为200 g/L,凝固槽长度为0.75 m,凝固浴分别为去离子水(DI)、50 g/L十二烷基硫酸钠(SDS)溶液和75%(V/V)乙醇(ET)溶液,纺丝速度为12 mL/h,纺丝针头内径为0.84 mm,收集时卷绕辊的速度为7.85 cm/s。将纺丝液倒入医用注射器,静置消泡,通过注射泵将注射器中的纺丝液平行挤压到凝固浴中,纺丝原液迅速凝聚成均匀的纤维。最后,将纤维置于恒温恒湿箱中(温度20 ℃±1 ℃,湿度65%±3%)备测。
将RSF汽蒸处理5 min,以1 mm/s的速率将定长10 cm的样品分别拉伸至15、20 cm和30 cm,得到牵伸后的纤维样品,置于恒温恒湿箱中备测。
1.3 测试方法
1.3.1 表面形态
采用SU 1510扫描电子显微镜(日本日立株式会社)观察了湿纺纤维的表观形貌。所有样品经表面喷金处理后,在20 kV的电压下观测。
1.3.2 力学性能
采用PT-1198GTD-C小型拉压力试验机(广东宝大宣力科技有限公司)测试湿纺纤维的力学性能,测试标距20 mm,拉伸速率2 mm/min,温度20 ℃±0.5 ℃,相对湿度50%±5%。每组至少选取5根纤维进行拉伸测试,记录各组样品的应力应变数据,计算杨氏弹性模量、断裂功及断裂伸长率等参数,结果取平均值。
E=ΔσΔε(2)
式中:E为杨氏弹性模量,MPa;σ为应力,MPa;ε为应变,%。
W=l0×A×ε×∫Bp0σ(3)
式中:W为断裂功,J;l0为标距,m;S0为横截面积,m2;ε为应变,%;Bp为断裂点;σ为应力,MPa。
1.3.3 傅里叶变换红外光谱测试
采用Nicolet IS 10傅里叶变换红外光谱仪(美国赛默飞世尔科技(中国)有限公司)分析湿纺纤维的二级结构,光谱范围400~4 000 cm-1,光谱分辨率0.4 cm-1,累计扫描32次,结果取平均值。
1.3.4 X射线衍射测试
采用D2 PHASER X射线衍射仪(德国布鲁克AXS有限公司)测试湿纺纤维的晶体结构,测试条件为:Cu—Ka靶(λ=0.154 18 nm),电压30 kV,电流10 mA,扫描速度3°/s,扫描范围5°~40°。
1.3.5 结晶度表征
将1 g再生丝样品剪成5 mm碎末,以浴比1︰100加入3%的盐酸中,然后置于94 ℃的恒温水浴锅中水解4 h。将残留物过滤、洗涤至中性,烘干、平衡、称重,得到残留物质量,并根据下式对材料结晶度进行计算。
Xc/%=m0-m1m0×100(4)
式中:Xc为结晶度,%;m0为原丝质量,g;m1为盐酸溶解后残留丝质量,g。
1.3.6 差示扫描量热测试
采用Q200差示扫描量热仪(美国TA仪器公司)测定再生丝纤维的热性能,氮气流量40 mL/min,测试范围4~300 ℃,升温速度10 ℃/min。
1.3.7 荧光测试
采用Hitachi F-4600荧光光谱仪(日本日立株式会社)测试丝素蛋白水溶液的荧光性能。硫黄素T(ThT):激发波长Ex为420 nm,狭缝为5 nm,发射波长范围430~700 nm,狭缝为5 nm,电压700 V。取30 mg/mL的丝素溶液1 mL于离心管中,加入7 μL摩尔浓度为300 mmol/L SDS溶液,使SDS摩尔浓度为0.2 mmol/L,最后加入200 μL ThT溶液,用去离子水定容至10 mL,ThT的最终摩尔浓度为20 μmol/L。样品室温静置2 h,测定溶液的荧光光谱。每个样品重复测试3次,结果取平均值。
2 结果与分析
2.1 再生丝形貌分析
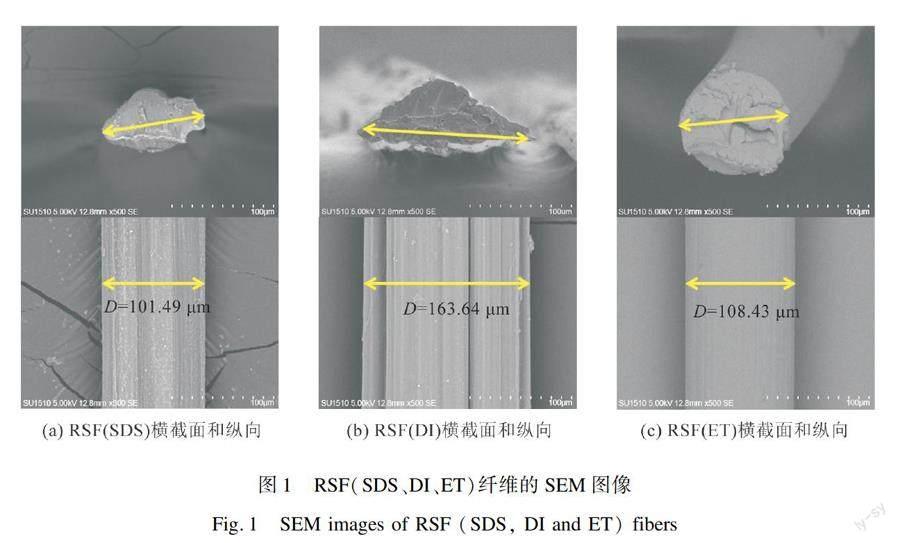
为了比较RSF的表观形貌差异,本文采用扫描电镜对三种RSF进行表征,如图1所示。从图1(a)可以看出,RSF(SDS)纤维截面近椭圆形,纤维表面有不规则的沟槽。这是因为纤维经过凝固浴时,通过拉伸作用使纤维表面迅速脱水和取向,從而形成沟壑。从图1(b)可以看出,RSF(DI)纤维截面近似三角形,且边缘有大量锯齿,纤维表面有更明显的沟壑。这是因为以水为凝固浴时,纤维的凝固速度较慢,会
逐渐沉积到凝固浴槽的底部,进而在外力作用下呈现出不规整的形貌。从图1(c)可以看出,RSF(ET)纤维截面近圆形,表面光滑平整,说明在乙醇中丝素蛋白凝固速度很快,呈现各向同速的特点。
综上,丝素蛋白在三种凝固浴中凝固速度不同,其中在乙醇中最快,去离子水中最慢,SDS溶液速度适中。另外,对三种RSF纤维的纤度进行分析,结果表明RSF(DI)最粗为11.61 tex,RSF(SDS)适中为9.48 tex,RSF(ET)最细为8.44 tex。
2.2 再生丝力学性能分析
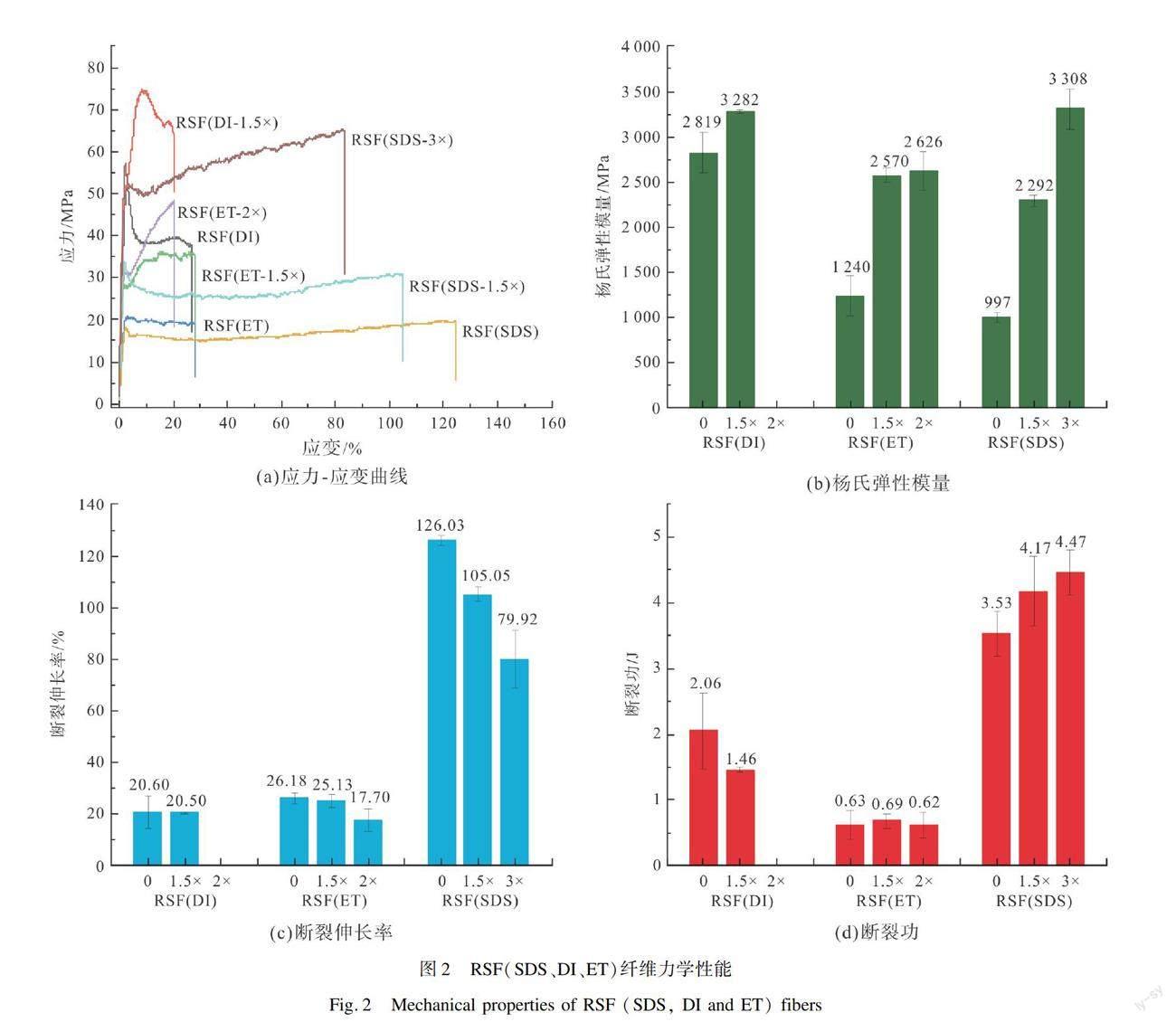
通过拉伸实验评价三种RSF(SDS、DI和ET)及不同牵伸倍数样品的力学性能。在进行牵伸后处理时,RSF(DI)最多能牵伸到原长的1.5倍,记为RSF(DI-1.5×),RSF(ET)可以牵伸到原长的1.5和2倍,记为RSF(ET-1.5×)和RSF(ET-2×),RSF(SDS)可以牵伸到原长的1.5倍和3倍,记为RSF(SDS-1.5×)和RSF(SDS-3×)。纺丝液在进入凝固浴时会发生溶剂交换时,基于凝固速率的不同,所形成初生纤维的结晶程度有差异,因此三组样品可承受的牵伸倍数有所差异。
对样品进行力学测试,获得结果如图2所示。结果表明,RSF(DI)表现为硬而强,模量为2 819 MPa,且有较高的屈服应力51 MPa和20%的断裂伸长率。经过牵伸后,RSF(DI-1.5×)模量为3 282 MPa,增加了16%,屈服应力为74 MPa。这是因为RSF(DI)成纤过程缓慢,纤维较粗,易形成较大尺寸的结晶区,因此,当出现外力拉伸时,表现出较高断裂强度和弹性模量,但不具有延伸性。对于RSF(ET)而言,其整体呈现为软而弱,模量为1 239 MPa,屈服应力为20 MPa,断裂伸长
率为26%。经牵伸后,RSF(ET-1.5×)模量提高为2 570 MPa,屈服应力稍提高为27 MPa,断裂伸长率为25.13%没有明显下降;RSF(ET-2×)则表现为硬而脆,模量为2 626 MPa,屈服应力为31 MPa,且断裂伸长率降低为17.70%,表现出脆性。这是由于RSF(ET)成纤快,纤维细,纤维质地均匀,能够承受一定程度的牵伸作用,但有所限制。相比于其他两种再生丝纤维,RSF(SDS)表现为软而韧,模量为997 MPa,具有很好的延性(断裂伸长率达到126%),屈服应力较低,约为18 MPa。经牵伸后,RSF(SDS-1.5×)表现为硬而韧,模量为2 292 MPa,增加了130%,屈服应力提高到33 MPa,断裂伸长率有所降低但是仍然较高为105%;RSF(SDS-3×)表现为硬而韧,模量为3 307 MPa,增加了231%,屈服应力提高至57 MPa,断裂伸长率(79%)有所降低。RSF(SDS)成纤速度适中,纤维粗细也适中,因此表现出优异的延伸性。经过蒸汽加湿之后,能够在外力牵伸的作用下使丝素分子链发生排列取向而趋于更稳定的结构,因而明显提高了断裂强度和弹性模量。整体看来,RSF(SDS)组样品断裂伸长和断裂应力均较高,表现出较好的韧性。
蚕丝是一种半结晶性的天然高分子材料,纤维中的β-折叠纳米晶体结构嵌入富含甘氨酸的无定形结构(非结晶区域)中,这常被认为是蚕丝内部分子网络的物理交联点,是影响蚕丝物理性能的主要因素[25-26]。已有研究表明,丝蛋白纤维中二级结构结晶区/非晶区之间的氢键作用决定了纤维的力学性能,其中,丝纤维间的二级结构是影响丝纤维力学性能的关键因素[27-28]。因此考虑进一步对RSF的二级结构进行表征,探索力学性能与蛋白结构的关系。
2.3 再生丝聚集态结构分析
利用傅里叶变换红外光谱、X射线衍射技术等对三种RSF纤维进行结构表征,探究凝固浴对再生丝纤维力学性能的影响,如图3所示。从图3(a)可以看出,四组纤维样品在1 230 cm-1(酰胺Ⅲ区,归属于Silk Ⅰ构象,包括α-螺旋结构和无规则卷曲结构)[29],1 513 cm-1(酰胺Ⅱ区,归属于β-折叠)和1 619 cm-1(酰胺Ⅰ区,归属于β-折叠)[30]处出现了3个主要吸收峰,说明丝纤维中无规卷曲、α-螺旋和β-折叠3种构象并存。将酰胺Ⅰ带(1 700~1 600 cm-1)细分为10个子峰,通过各子峰的面积分析出聚集态结构在三种RSF纤维中的不同[31]。图3(b)为RSF(SDS)纤维在酰胺Ⅰ区的红外分峰拟合曲线,拟合曲线的相似度达0.999及以上。桑蚕丝和三种RSF(SDS、DI、ET)纤维的二级构象含量如图3(c)所示。桑蚕丝中的β-折叠含量为47.7%,低于三种再生丝中的β-折叠含量,这是由于桑蚕丝含有结晶区和非晶区,表现为总的β-折叠比例较少。而再生丝是从凝固浴中失水后成纤,在凝固时需要快速完成从无规卷曲和α-螺旋转变为疏水的β-折叠进而析出,表现为相对较高的β-折叠含量。
利用X射线衍射技术和酸解法表征了三种RSF纤维的结晶情况。图3(d)为三种RSF纤维及桑蚕丝的XRD谱图。丝素蛋白β-折叠主衍射峰为9.1°、18.9°、20.7°和24.0°[32],无规卷曲和α-螺旋的主衍射峰分别在12.2°、19.7°、24.7°和28.2°[32-34]。四种样品在上述特征峰的位置都出现了对应了衍射峰,只是强度有所不同,这说明RSF与桑蚕丝具有相似的结晶情况。桑蚕丝在20.7°附近的结晶峰最为尖锐,而三种RSF纤维在此处的结晶峰表现为相对平滑的馒头峰,这说明RSF纤维的结晶程度均不如桑蚕丝高。
根据酸解法测试,主要得到的是蚕丝纤维中不溶性的结晶区,测得三种RSF纤维的结晶度如表1所示,相比于桑蚕丝的结晶度(~55%)[35],三种RSF纤维的结晶度都很低,这与XRD谱图的峰型结果基本吻合。蚕丝纤维的结晶区与不溶性相关,结晶区的形成与β-折叠相关[36]。如果纤维中所有β-折叠均参与结晶区形成,根据红外分峰拟合的结果可以推测结晶度大小为RSF(SDS)>RSF(DI)>RSF(ET),但是实际上三种RSF纤维结晶度的结果为RSF(ET)>RSF(SDS)>RSF(DI),由此推断RSF纤维中的β-折叠结构并未全部参与结晶区的形成。
2.4 再生丝热性能分析
为了进一步说明RSF纤维性能与蛋白结构的关系,本文利用差示扫描量热技术对RSF纤维进行表征。如图4所示,在140~155 ℃有一个热吸收峰,由于样品的无定形区的紧密
程度有差异[37],结合水的蒸发温度因此有略微的差别。在278 ℃附近的热吸收峰,为丝素蛋白降解的特征峰[1]。在160~200 ℃,随着温度的升高,丝素大分子链段运动加剧,分子内部的结合水进一步得到释放,分子链重新组合,无规卷曲结构逐渐转变成低结晶度的β-折叠[32,38]。由图4可知,三种RSF纤维发生构象转变的温度比为RSF(SDS)>RSF(DI)>RSF(ET),反映出三种RSF纤维中二级构象比例的不同。同时,结果表明由于RSF(SDS)纤维中大量β-折叠的存在,使得进一步形成β-折叠的构象转变中需要更高的起始温度,从而使RSF(SDS)有更好的热稳定性。
2.5 SDS与丝素作用机理分析
为了验证SDS对丝素蛋白构象的影响,本文采用荧光探针法阐明SDS对于RSF纤维的成型影响。ThT能与蛋白质二级结构中的β-片层(β-折叠结构的基础)发生高度特异性的结合[39],当激发波波长为485 nm时会发射出强烈的荧光,同时,荧光强度随着β-片层数量的增加而增强,因此,荧光探针能够表征蛋白质的二级结构[40]。本文将ThT加入SF溶液、含SDS的SF溶液和含ET的SF溶液中,分析了時间对溶液荧光强度的影响,如图5所示。
荧光分析法中,荧光强度一般与入射光强度和溶液浓度(β-片层结构的比例)呈线性关系[41]。图5显示的是三种溶液中,丝素蛋白聚集体在485 nm处的荧光强度。当入射光强度一致时,0~30 h内有SDS和ET的SF溶液的荧光强度均比纯SF溶液的高,这说明含SDS和ET时,在相同的时间内丝素蛋白能生成更多的β-片层。在30~80 h内,三种溶液的荧光强度均呈现下降趋势,这是由于大量β-片层的存在,使丝素蛋白发生成核—生长[26],进而以沉淀的形式从溶液中析出,导致溶液中可检测到的β-片层含量减少,表现为荧光强度的降低。此外,在测试过程中,能够明确观察到丝素蛋白溶液从澄清状态逐渐变浑浊,直至出现沉淀。因此,荧光测试结果表明,乙醇和SDS均能够促进丝素蛋白生成β-片层,进而形成β-折叠,以促进丝素蛋白的凝固。相对而言,乙醇能够在更短的时间内促进丝素蛋白快速形成β-折叠。
根据以上分析,本文推测RSF纤维成型的作用机理为:SDS是一种典型的小分子阴离子表面活性剂,其疏水长链与蛋白质的疏水区结合,诱导丝素蛋白生成β-折叠结构。部分β-折叠能够进一步堆叠形成结晶区,剩余部分的β-折叠则留在无定形区相互连接,最后形成类似渔网状的结构[26]。在外力牵伸作用下,β-折叠结构进一步重排[42],使RSF(SDS)成为韧性材料。而乙醇会诱导丝素蛋白迅速结晶,使得内部丝素蛋白分子结构以杂乱无序的状态稳定下来[43],从而表现为脆性。去离子水的作用机理与乙醇类似,不过由于凝固速度相对较慢,凝固时最外层最先以无序状态稳定下来。在外力的牵引作用下,由于内部还没完全凝固,因此能够承受一定的变形,后续内部脱水凝固体积收缩,表面就会呈现大量沟壑。这与SEM的结果一致。
3 结 论
本文采用湿法纺丝制备了丝素蛋白再生丝纤维(RSF),研究了SDS凝固浴对RSF力学性能的影响,分析了RSF的聚集态结构,并探究了SDS促进RSF成纤的机理,从而得出以下结论。
1) 以去离子水、乙醇和SDS作为凝固浴均能获得RSF,其中RSF(SDS)及牵伸样品具有较优的延伸性,RSF(SDS)斷裂伸长率达126%。
2) 相比于桑蚕丝,三种体系获得的RSF纤维结晶度均较低,且β-折叠含量与结晶区比例不完全一致。其中,RSF(SDS)纤维具有较高的β-折叠含量(60.4%),但β-折叠结构并未完全形成结晶区。此外,相比于未添加SDS的SF溶液,SDS能诱导丝素蛋白溶液产生更多β-折叠,且无定形区β-折叠结构的存在使RSF(SDS)具有更好的牵伸性。
3) 成纤过程中,SDS的疏水链段与蛋白质疏水区结合,可快速诱导丝素蛋白形成大量β-折叠。其中,部分β-折叠进一步形成结晶区,剩余的则β-折叠分散在无定形区,在外力作用下发生进一步重排,使RSF(SDS)纤维表现出韧性材料的特征。
参考文献:
[1]向仲怀. 蚕丝生物学[M]. 北京: 中国林业出版社, 2005.
XIANG Zhonghuai. Biology of Silk[M]. Beijing: China Forestry Publishing House, 2005.
[2]SHI Z F, ZHENG F M, ZHOU Z T, et al. Silk-enabled conformal multifunctional bioelectronics for investigation of spatiotemporal epileptiform activities and multimodal neural encoding/decoding[J]. Advanced Science, 2019, 6(9): 1801617.
[3]GOGURLA N, KIM Y, CHO S, et al. Multifunctional and ultrathin electronic tattoo for on-skin diagnostic and therapeutic applications[J]. Advanced Materials, 2021, 33(24): 2008308.
[4]WANG L, XU B, NONG Y L, et al. Laccase-mediated construction of flexible double-network hydrogels based on silk fibroin and tyramine-modified hyaluronic acid[J]. International Journal of Biological Macromolecules, 2020, 160: 795-805.
[5]姚梦竹. 载药碳纳米管复合骨组织工程支架的制备及其与骨髓间充质干细胞的相容性评价[D]. 杭州: 浙江大学, 2016.
YAO Mengzhu. Construction a Composite Bone Scaffold with Drug-loaded CNT and Its Compatibility with Bone Marrow Mesenchymal Stem Cells[D]. Hangzhou: Zhejiang University, 2016.
[6]ZOU S Z, WANG X R, FAN S N, et al. Electrospun regenerated Antheraea pernyi silk fibroin scaffolds with improved pore size, mechanical properties and cytocompatibility using mesh collectors[J]. Journal of Materials Chemistry B, 2021, 9(27): 5514-5527.
[7]WU X L, ZHOU M L, JIANG F, et al. Marginal sealing around integral bilayer scaffolds for repairing osteochondral defects based on photocurable silk hydrogels[J]. Bioactive Materials, 2021, 6(11): 3976-3986.
[8]LEE W, ZHOU Z T, CHEN X Z, et al. A rewritable optical storage medium of silk proteins using near-field nano-optics[J]. Nature Nanotechnology, 2020, 15(11): 941-947.
[9]TOFFANIN S, KIM S, CAVALLINI S, et al. Low-threshold blue lasing from silk fibroin thin films[J]. Applied Physics Letters, 2012, 101(9): 091110.
[10]JUNG H, IN C, CHOI H, et al. Electromagnetically induced transparency analogue by self-complementary terahertz meta-atom[J]. Advanced Optical Materials, 2016, 4(4): 627-633.
[11]HONG M S, CHOI G M, KIM J, et al. Biomimetic chitin: Silk hybrids: An optically transparent structural platform for wearable devices and advanced electronics[J]. Advanced Functional Materials, 2017, 28(24): 1705480.
[12]吳慧英. 再生丝素纤维的制备及其在人工韧带中的应用[D]. 苏州: 苏州大学, 2015.
WU Huiying. Preparation of Regenerated Silk Fibroin Fiber and Its Application on Artificial Ligament[D]. Suzhou: Soochow University, 2015.
[13]LIU M W, ZHANG Y J, LIU K Y, et al. Biomimicking antibacterial opto-electro sensing sutures made of regenerated silk proteins[J]. Advanced Materials, 2021, 33(1): 2004733.
[14]ALI B A, ALLAM N K. Silkworms as a factory of functional wearable energy storage fabrics[J]. Scientific Reports, 2019, 9(1): 12649.
[15]ZHANG S, ZHOU Z T, ZHONG J J, et al. Body-integrated, enzyme-triggered degradable, silk-based mechanical sensors for customized health/fitness monitoring and in situ treatment[J]. Advanced Science, 2020, 7(13): 1903802.
[16]JIA T J, WANG Y, DOU Y Y, et al. Moisture sensitive smart yarns and textiles from self-balanced silk fiber muscles[J]. Advanced Functional Materials, 2019, 29(18): 1808241.
[17]KI C S, LEE K H, BAEK D H, et al. Dissolution and wet spinning of silk fibroin using phosphoric acid/formic acid mixture solvent system[J]. Journal of Applied Polymer Science, 2007, 105(3): 1605-1610.
[18]ZHOU G Q, SHAO Z Z, KNIGHT D P. et al. Silk fibers extruded artificially from aqueous solutions of regenerated Bombyx mori silk fibroin are tougher than their natural counterparts[J]. Advanced Materials, 2009, 21(3): 366-370.
[19]吴惠英, 左保齐. 氯化钙—甲酸溶解体系再生丝素长丝的制备及其性能[J]. 纺织学报, 2016, 37(2): 1-6.
WU Huiying, ZUO Baoqi. Preparation and properties of regenerated silk fibroin filament using CaCl2-ormid acid system[J]. Journal of Textile Research, 2016, 37(2): 1-6.
[20]余晶梅. 荧光探针法和疏水相互作用层析法分析蛋白表面疏水性[D]. 杭州: 浙江大学, 2014.
YU Jingmei. Measurement of Protein Surface Hydrophobicity by Fluorescence Probe Method and Hydrophobic Interaction Chromatography[D]. Hangzhou: Zhejiang University, 2014.
[21]左锦静, 姚永志. 荧光法研究表面活性剂SDS与蛋白质的相互作用[J]. 轻工科技, 2018, 34(3): 16-17.
ZUO Jinjing, YAO Yongzhi. Study on the interaction between surfactant SDS and protein by fluorescence method[J]. Light Industry Science and Technology, 2018, 34(3): 16-17.
[22]WU X L, HOU J, LI M Z, et al. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin[J]. Acta Biomaterialia, 2012, 8(6): 2185-2192.
[23]LI Z, ZHENG Z K, YANG Y H, et al. Robust protein hydrogels from silkworm silk[J]. ACS Sustainable Chemistry & Engineering, 2016, 4(3): 1500-1506.
[24]YAN J P, ZHOU G Q, KNIGH D P. Wet-spinning of regenerated silk fiber from aqueous silk fibroin solution: Discussion of spinning parameters[J]. Biomacromolecules, 2010, 11(1): 1-5.
[25]NOVA A, KETEN S, PUGNO N M, et al. Molecular and nanostructural mechanisms of deformation, strength and toughness of spider silk fibrils[J]. Nano Letters, 2010, 10(7): 2626-2634.
[26]SCHOR M, BOLHUIS P G. The self-assembly mechanism of fibril-forming silk-based block copolymers[J]. Physical Chemistry Chemical Physics, 2011, 13(22): 10457-10467.
[27]KETEN S, BUEHLER M J. Nanostructure and molecular mechanics of spider dragline silk protein assemblies[J]. Journal of the Royal Society Interface, 2010, 7(53): 1709-1721.
[28]KETEN S, XU Z P, IHLE B, et al. Nanoconfinement controls stiffness, strength and mechanical toughness of beta-sheet crystals in silk[J]. Nature Materials, 2010, 9(4): 359-367.
[29]LING S J, QI Z M, KNIGHT D P, et al. Synchrotron FTIR microspectroscopy of single natural silk fibers[J]. Biomacromolecules, 2011, 12(9): 3344-3349.
[30]LU Y H, LIN H, CHEN Y Y, et al. Structure and performance of Bombyx mori silk modified with nano-TiO2 and chitosan[J]. Fibers and Polymers, 2007, 8(1): 1-6.
[31]HU X, KAPLAN D, CEBE P. Determining beta-sheet crystallinity in fibrous proteins by thermal analysis and infrared spectroscopy[J]. Macromolecules, 2006, 39(18): 6161-6170.
[32]DRUMMY L F, PHILLIPS D M, STONE M O, et al. Thermally induced alpha-helix to beta-sheet transition in regenerated silk fibers and films[J]. Biomacromolecules, 2005, 6(6): 3328-3333.
[33]LU Q, WANG X L, LU S Z, et al. Nanofibrous architecture of silk fibroin scaffolds prepared with a mild self-assembly process[J]. Biomaterials, 2011, 32(4): 1059-1067.
[34]MAGOSHI J, NAKAMURA S. Studies on physical properties and structure of silk: Glass transition and crystallization of silk fibroin[J]. Journal of Applied Polymer Science, 1975, 19(4): 1013-1015.
[35]TRABBIC K A, YAGER P. Comparative structural characterization of naturally-and synthetically-spun fibers of Bombyx mori fibroin[J]. Macromolecules, 1998, 31(2): 462-471.
[36]MADURGA R, GAN-CALVO A M, PLAZA G R, et al. Straining flow spinning: Production of regenerated silk fibers under a wide range of mild coagulating chemistries[J]. Green Chemistry, 2017, 19(14): 3380-3389.
[37]潘志娟, 陳宇岳, 盛家镛, 等. 蜘蛛丝的热性能研究[J]. 丝绸, 2002(10): 13-16.
PAN Zhijuan, CHEN Yuyue, SHENG Jiayong, et al. Study on thermal properties of spider silk[J]. Journal of Silk, 2002(10): 13-16.
[38]MAGOSHI J, MAGOSHI Y, BECKER M A, et al. Crystallization of silk fibroin from solution[J]. Thermochimica Acta, 2000, 352: 165-169.
[39]BIANCALANA M, KOIDE S. Molecular mechanism of Thioflavin-T binding to amyloid fibrils[J]. Biochimica et Biophysica Acta-Proteins and Proteomics, 2010, 1804(7): 1405-1412.
[40]王建南, 陆长德, 白伦. Thioflavine T荧光探针法研究丝素结晶区典型肽段自聚集的β-片层结构[J]. 化学学报, 2007(2): 111-115.
WANG Jiannan, LU Changde, BAI Lun. Study on β-lamellar structure formed by self-assembly of fibroin crystal typical peptides using Thioflavine T fluorescence probe[J]. Acta Chimica Sinica, 2007(2): 111-115.
[41]DU H N, TANG L, LUO X Y, et al. A peptide motif consisting of glycine, alanine, and valine is required for the fibrillization and cytotoxicity of human alpha-synuclein[J]. Biochemistry, 2003, 42(29): 8870-8878.
[42]NING D, YANG Z, LIU X Y, et al. Structural origin of the strain-hardening of spider silk[J]. Advanced Functional Materials, 2011, 21(4): 772-778.
[43]张鸿昊. 超强再生丝素蛋白纤维及其形成机理[D]. 厦门: 厦门大学, 2018.
ZHANG Honghao. Ultra-strong Regenerative Silk Fibroin Protein Fiber and Formation Mechanism[D]. Xiamen: Xiamen University, 2018.
Influence mechanism of coagulation bath on the structure of regenerated silk inwet spinning of silk fibroin
CHEN Qin, WU Leilei, WANG Ping
(College of Textile Science and Engineering, Jiangnan University, Wuxi 214122, China)
Abstract:
As a natural protein fiber with good biocompatibility, silk has a lot of wastes in production, processing, weaving and after-use. Based on the principle of sustainable development, silk fibroin protein can be recycled by chemical method. After recycling, silk fibroin solution can be obtained and recycled silk fibroin materials can be prepared for reuse.
Wet spinning is the first to be developed and applied in industrial production because of its uniqueness. As the process has been optimized in industrial production for a long time, wet spinning has become a relatively mature spinning method in all aspects. The choice of coagulating bath plays a key role in the solidification of spinning fluid in the coagulating basin, especially in the mechanical properties of RSF fibers. Sodium dodecyl sulfate (SDS) is a typical anionic surfactant with good emulsification performance, low skin irritation, high safety, and can interact with protein. Based on this, the study intends to establish a wet spinning process using SDS as the solidifying agent.
In the paper, SDS was used as the coagulation bath to improve the ductility of regenerated silk fibroin fibers. First of all, the silk was degummed through degumming and other pretreatment, and then the degummed silk obtained after drying was dried for later use. After that, the degummed silk was dissolved with the formic acid-calcium chloride solution to obtain the spinning stock solution. In this way, deionized water, 75% ethanol solution and 50 g/L SDS solution were selected as the coagulation bath for wet spinning, and three kinds of RSF fibers were obtained. In the end, the mechanical properties and aggregation structure of three RSF fibers were analyzed. Subsequently, the mechanism of SDS promoting fibrogenesis was verified by the fluorescence probe method.
The morphology of the three kinds of RSF fibers was characterized by scanning electron microscope. Deionized water was used as the coagulation bath to obtain the most surface folds and the largest diameter of recycled silk. The recycled silk with ethanol as the coagulation bath has a roughly round cross section, smooth surface and the smallest diameter. When SDS is used as the coagulation bath, the cross section of fiber is approximately elliptic, with a few furrows on the surface and moderate thickness. Tensile tests were conducted to the three kinds of RSF fiber samples. The results show that when SDS is used as the coagulation bath, RSF shows the characteristics of elastic materials, and its fracture strain is 126%, which is much higher than that of the two kinds of regenerated fibers using deionized water (20%) and 75% ethanol solution (26%) as the coagulation bath.
In order to explore the effect of the coagulation bath on the aggregation state of recycled silk, Fourier transform infrared spectroscopy (FTIR) was first used to analyze the secondary structure. The contents of three RSF secondary structures were obtained by the peak separation of amide Ⅰ region, and specifically, the recycled silk with SDS as the coagulation bath had the highest β-folding content. The content of β-folding of recycled silk in the coagulating bath with 75% ethanol was the lowest. In order to further explain the relationship between the mechanical properties and protein structure, X-ray diffraction was used to characterize the crystallization of the three kinds of RSF fibers, and it can be seen that the crystallinity degrees of the regenerated fibers are all low. In order to further explain the relationship between β-folding and the crystallization region, differential scanning calorimetry analysis was carried out for the three kinds of RSF, which confirmed the presence of β-folding in large quantities in RSF (SDS) fiber samples. The effect of SDS on silk fibroin protein was characterized by fluorescence probe. The results show that the surface of silk fibroin protein can produce more β-folds at the same time in the presence of SDS.
In this study, SDS was used as the coagulating bath for wet spinning, anionic surfactant was innovatively introduced as the composition of coagulating bath, and a recycled silk material with high extensibility was obtained, providing a new research idea for silk fibroin protein wet spinning.
In this paper, only the influence of the coagulation bath on the properties of recycled silk has been investigated, and the influence of post-treatment on the properties of recycled silk has not been involved. RSF (SDS) fibers, with excellent fracture elongation, can be treated to further improve the properties of recycled silk fibers.
Key words:
silk fibroin; regenerated silk; coagulation bath; mechanical properties; aggregation structure; fiber forming mechanism
收稿日期:
2022-06-16;
修回日期:
2023-02-23
基金項目:
江苏省六大人才高峰高层次人才项目(XCL133)
作者简介:
陈琴(1998),女,硕士研究生,研究方向为再生丝素材料。通信作者:王平,教授,pwang@jiangnan.edu.cn。

