Shielding and corrosion properties of the Alloy 709 as canister material for spent nuclear fuel dry casks
Zeinab Y. Alsmadi, Mohamed A. Bourham
Department of Nuclear Engineering, North Carolina State University, Raleigh, NC 27695-7909, USA
Keywords:Spent nuclear fuel Dry casks Alloy 79 Shielding properties Corrosion properties
ABSTRACT The shielding and corrosion properties of the Alloy 709 advanced austenitic stainless steel have been investigated as a candidate canister material in spent fuel dry casks. The results revealed that the experimental and computational data of the linear and mass attenuation coefficients of the alloy are in good agreement, in which the attenuation coefficient values decreased with increasing photon energy.Alloy 709 was shown to exhibit the highest linear attenuation coefficient against gamma rays when compared to 304 and 316 stainless steels.On the other hand,Alloy 709 exhibited no considerable weight change over a 69-day period in circulating salt brines corrosion testing, while it showed an exponential increase of corrosion current density with temperature in acidic and basic corrosive solutions during electrochemical polarization corrosion testing. Furthermore, Alloy 709 was the least corroded steel compared to other austenitic stainless steels in both acidic and basic solutions. The optimistic results of the shielding and corrosion properties of Alloy 709 due to its chemical composition,suggest utilizing it as a canister material in spent nuclear fuel dry casks.
1. Introduction
In nuclear industry, the nuclear waste produced is of special attention when compared to other waste streams from other industries,which must be monitored carefully to protect the working personnel and the surrounding environment. The risk of radioactive nuclear waste decreases with time due to decay, and the volume of nuclear waste is much smaller than that of other industries.However, the risk imposed from these wastes does not depend on their volume but their management certainly depends on their volume [1]. The disposal of radiation and heat generating spent nuclear fuel assemblies take place after the spent fuel pools reach their capacity limit of stored assemblies, in which they are then placed in storage systems of dry casks with multilayers of shielding materials to prevent leakage of ionizing radiation to the environment [2-5]. Nevertheless, the disposal of spent fuel in stable and shielded containers is subjected to many obstacles including the integrity of the container itself, the waste and the surrounding environment [6]. Therefore, to prevent transmission of radiation from nuclear waste into the surroundings, radiation shielding properties play an important role in the design and operation of spent nuclear fuel storage systems, in which a lot of studies have been conducted to develop new materials with adequate shielding properties [2-4,7-15]. Additionally, corrosion can occur in the inner of the canister of the dry cask due to self-accelerated corrosion of nuclear waste, in which a severe localized corrosion can form at the interface between the stainless-steel canister and any surroundings. This accelerated corrosion was found by Guo et al.[16], and was attributed to local acidity/alkalinity within a small space and chemistry changes of the solution, which therefore change the corrosion of the waste-form materials and the stainlesssteels canisters. Corrosion properties have been investigated for decades on nuclear waste containers to ensure the effective isolation of radionuclides contained in the waste [1,4,17-21]. One common technique to perform corrosion testing is the electrochemical polarization, in which it depends on measuring the flow of electrons from the oxidation and reduction reactions that lead to corrosion,by applying an external voltage(polarization)to create a non-equilibrium state.In this technique,corrosion is accelerated to determine the corrosion behavior for a long period of time in a short timescale[22-24].Spent fuel dry casks may be constructed of multilayers of shielding materials with different thicknesses to minimize the exposure to nuclear radiation.The first layer is made of stainless steel and represents the dry cask canister followed by a thin coating layer of glass-based material, and then a thick wall of heavy concrete overpack [2-4]. Glass-based materials are known for their good corrosion resistance and thermal stability, due to their content of heavy and transition metal oxides that also play a role in increasing the density [3,25-29]. The outer layer of heavy concrete overpack is widely used in many industries due to its long life and high strength.Also,it exhibits high attenuation coefficients against gamma rays, high density and low thermal conductivity[3,10,25,30]. Furthermore, one important property of both glass coatings and heavy concretes is the ease of production, in which they can be mixed with other additives into different shapes to enhance their shielding properties[3,10,30,31].On the other hand,stainless-steel canisters such as 304 and 316 provide good corrosion resistance, high-temperature strength and economic feasibility [3]. Alsmadi et al. [3] computationally investigated the shielding properties of an advanced austenitic stainless steel known as the Alloy 709 (Fe-25Ni-20Cr), as a candidate canister material in spent fuel dry casks due to its excellent mechanical properties,such as thermal stability,high strength and good creepfatigue properties[32-34].They concluded that Alloy 709 exhibits stronger attenuation,lower half-value layer(HVL)and lower mean free path (MFP) values against gamma rays than other stainless steels, due to its content of Chromium (Cr), Nickel (Ni) and other additives of Niobium (Nb), Boron (B) and Titanium (Ti), which enhances its shielding efficiency and nominate it to become the new stainless-steel canister material in spent fuel dry cask storage systems. However, no experimental studies were conducted so far to study the shielding and corrosion properties of Alloy 709 as a candidate canister material in spent fuel dry casks.
In this work, the shielding properties of Alloy 709 are investigated experimentally and computationally as a candidate canister material in spent fuel dry casks, using gamma-ray sources at photon energies ranging from 0.302 MeV to 1.332 MeV, and are compared to other canister materials.Also,corrosion properties of Alloy 709 are investigated experimentally involving electrochemical polarization and circulating salt brines with various pHs,to obtain the corrosion current density and weight change of the samples.
2. Materials and methods
2.1. Materials
Alloy 709 advanced austenitic stainless steel is the material investigated in this research. It has the chemical composition shown in Table 1 as received from the manufacturing company,G.O.Carlson heat 58,776-4 (GOC ID: 58,776-4-B1), along with the chemical composition of 304 and 316 stainless steels for comparison [35,36]. Alloy 709 is strengthened by Nb and stabilized by Nitrogen(N)and has a density of 7.85 g/cm3.The history of Alloy 709 started from fabricating the ingot of the alloy by hot-rolling then it was solution-annealed at 1100°C for 75 min, followed by subsequent water-quenching. Two cylindrical samples (A, B) were machined from the as-received plate with their dimensions, volumes and densities presented in Table 2, measured using microbalance RADWAG(Model XA 82/220/2X)and a precision measuring calipers. High-energy gamma-ray sources used in this work are obtained from Spectrum Techniques and are depicted in Table 3,along with their activities and peak photon energies.Table 4 shows the dimensions, volumes and densities of 304 and 316 stainless steels,to compare their shielding and corrosion behavior with Alloy 709.

Table 1 Chemical composition (wt%) of Alloy 709, 304 and 316 stainless steels.

Table 2 Measured dimensions of Alloy 709 samples and their calculated densities and volumes.
Table 3 Gamma-ray sources with their activities and photon energies.

Table 4 Measured dimensions of 304 and 316 stainless steels and their calculated densities and volumes.
2.2. Experimental setup
The experimental shielding and gamma attenuation setup is shown in Fig.1.Integrated ORTEC digibase detector“NaI”(AMETEK 906-3 Scintillation Model 818/2Alfa Spectra, Inc.) is used and it is connected to a computer with MAESTRO multichannel analyzer emulation software [37,38]. Gamma-ray sources are mounted on the bottom base and surrounded by lead collimators to narrow the gamma-ray beam toward the detector. Alloy 709 sample is mounted on the sample holder above the lead collimators between the detector and the sources. The distance between the base and the detector is 22 cm,and the distance between the sample holder and the detector is 11 cm.The stack of sources is about 1.25 cm high and 2.54 cm wide. Shielding attenuation measurements were carried out for 30 min for the background,Alloy 709 shield and no shield,and each measured spectrum is background corrected by subtracting the background counts from the measured counts of shield and no shield. Fig. 2 shows the measured counts of gamma radiation passing through Alloy 709 as a shielding material, and compared to the measured counts when no shielding material is present, using gamma-ray sources of different photon peaks. As shown,the measured counts decrease when Alloy 709 is present as a shield, indicating its high shielding efficiency against gamma radiation. Determination of the attenuation coefficients and othershielding properties of the material depends on measuring the fractional radiation intensity, I, penetrating the shielding material with thickness, x, and knowing the initial radiation intensity, Io.More details are provided later on in Section 3.1.The computational measurements are performed using MicroShield software package[39]. MicroShield software is a comprehensive dose and shielding assessment program that is approved by the US Nuclear regulatory Commission(NRC),and it can be applied to all kind of materials to design shields and determine their gamma radiation attenuation coefficients, half-value layer (HVL), mean free path (MFP) and exposure rate.MicroShield is based on a deterministic code and it is similar to Monte Carlo N-Particle Transport Code(MCNP)and other photon transport packages,regarding the accuracy of the obtained results. Inputs to MicroShield include the shielding materials compositions, the dry cask geometry with source and shields dimensions, source and shields density, and the spent fuel source isotopic composition[3,15,39].
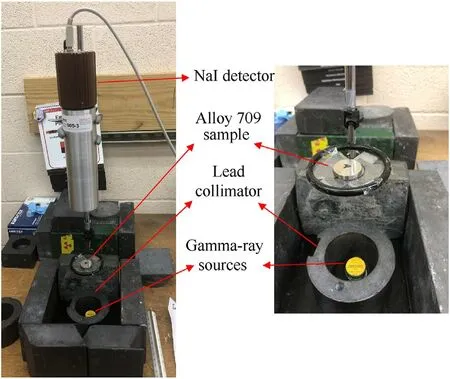
Fig. 1. Experimental shielding attenuation setup for measuring gamma-ray attenuation coefficients of Alloy 709 using high-energy gamma-ray sources.
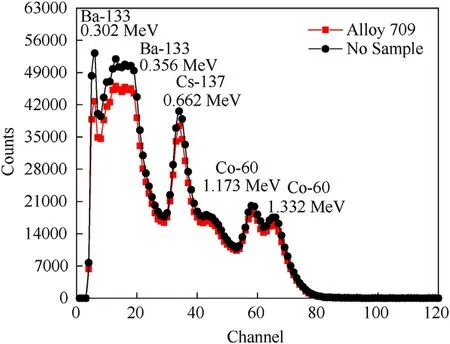
Fig.2. Measured counts of gamma radiation passing through Alloy 709 as a shield and compared to the measured counts without a shield using gamma-ray sources.
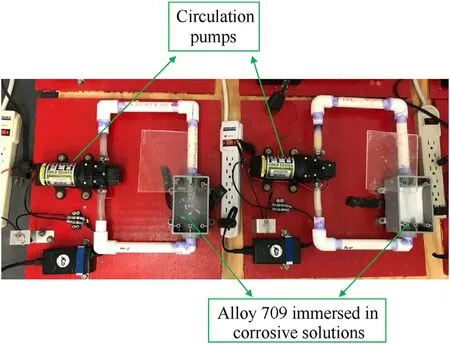
Fig.3. Experimental setup of circulating salt brines corrosion testing of various pHs to obtain the weight change of Alloy 709 samples.
On the other hand, corrosion tests were carried out in two experimental setups. The first setup is the prolonged exposure in circulating salt brines of various pHs, in which the sample is immersed in circulating corrosive solutions for a period of time(Fig.3).The pumps can circulate the solution at 1 gallon per minute.To obtain the weight change of the sample due to corrosion, the mass of the sample is measured at certain time intervals. The compositions of the two corrosive solutions used for sample A and sample B of Alloy 709 are shown in Table 5.The chosen circulating solutions resemble ground water compositions that are present in Yucca Mountain Nuclear Waste Repository [24,40].
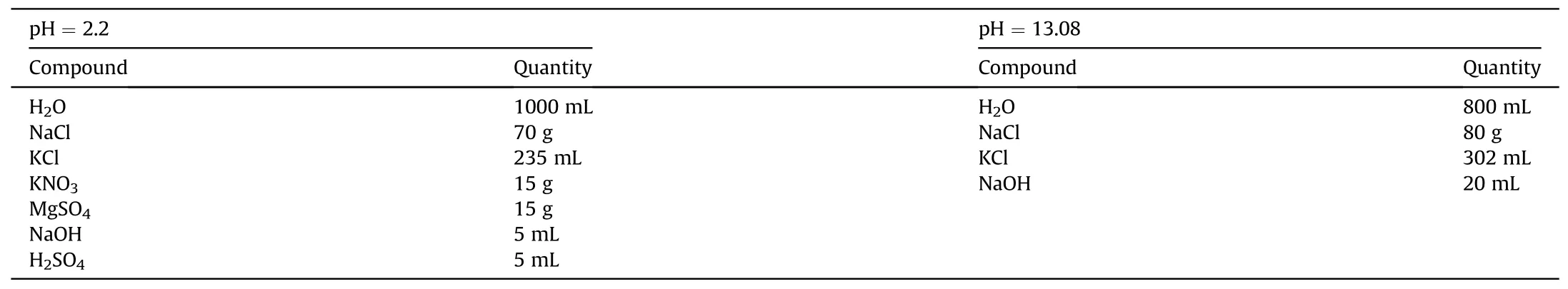
Table 5 Compositions of the corrosive solutions used for extended corrosion testing.
The second corrosion testing setup is the electrochemical polarization performed using the MULTIPORT™corrosion cell kit and Interface 1000 potentiostat from Gamry Instruments [41], and connected to DC105 Electrochem software to apply and measure the voltage and current. The experimental setup consists of three electrodes, a graphite counter electrode to balance the reactions occurring at the surface of the working electrode by acting as an electron sink,a standard Ag/AgCl reference electrode that is placed in the corrosion cell to prevent salt bridge problems,and a working electrode that is the sample itself (Fig. 4) [24]. In electrochemical polarization testing, Alloy 709 sample is mounted inside a sample holder conductive to testing disks in which the exposed surface area of the sample is 3.14 cm2.Alloy 709 samples are immersed in the testing corrosive solutions for 15-24 h before testing to allow the open circuit potential to stabilize.The corrosive solutions used are 1 M NaCl of pH=8.22 and 1 M NaCl with H2SO4of pH=2.2,to simulate the environmental conditions where spent fuel dry casks are located near coastal areas. Corrosion testing is performed at high temperatures by placing the corrosion test cell in a water bath with controlled heating(20°C,40°C,60°C and 80°C),to simulate the temperature of the dry cask canister due to decay heat(Fig.4).As a result, corrosion rate (corrosion current) is determined by measuring the cyclic potentiodynamic polarization curves that contain low and high overpotential regions [24,41-43].

Fig.4. Experimental setup of electrochemical polarization corrosion testing performed on Alloy 709 at various temperatures.
3. Results and discussion
3.1. Shielding properties
One of the most important shielding properties to consider is the linear attenuation coefficient, μ, defined as the probability of interaction between gamma rays and the shielding material per unit path length,in which it is determined based on the fractional radiation intensity,I,the source initial intensity,Ioand the shielding material thickness, x, as given [3,11]:
Moreover, the mass attenuation coefficient, μ/ρ, describes the interaction probability per unit mass and it is expressed in cm2/g[3,11,25].In Table 6,the calculation of experimental linear and mass attenuation coefficients of Alloy 709 is presented at each photon energy. Each photon energy corresponds to a certain channel number and the initial radiation intensity corresponds to the measured counts when no shield is present, while the fractional radiation intensity corresponds to the measured counts when Alloy 709 is present as a shield. Fig. 5 shows the linear attenuation coefficient of Alloy 709 obtained as a function of photon energy while Fig. 6 shows the mass attenuation coefficient. As shown, the experimental and computational data of the linear and mass attenuation coefficients of Alloy 709 appear in good agreement,in which the attenuation coefficient values decrease with increasing photon energy. The computational results appear within the uncertainty of the experimental values except at 0.662 MeV. The difference between experimental and computational results can be attributed to the inhomogeneity within Alloy 709 sample or slight compositional differences. Also, some factors such as geometry deviation, experimental alignments and variation of experimental conditions can result in such differences that are not taken into consideration in the computational calculations.Both experimental and computational observations align with what is observed in Alsmadi et al. [3] where Alloy 709 exhibited a decrease in the attenuation efficiency with increasing gamma-ray energy,due to its high content of Cr (19.930%), Ni (24.980%) and other additives of Nb, B and Ti.

Fig. 5. Experimental and computational values of linear attenuation coefficient of Alloy 709 as function of photon energy.
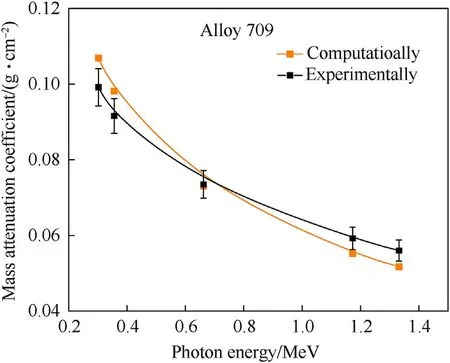
Fig.6. Experimental and computational values of mass attenuation coefficient of Alloy 709 as function of photon energy.
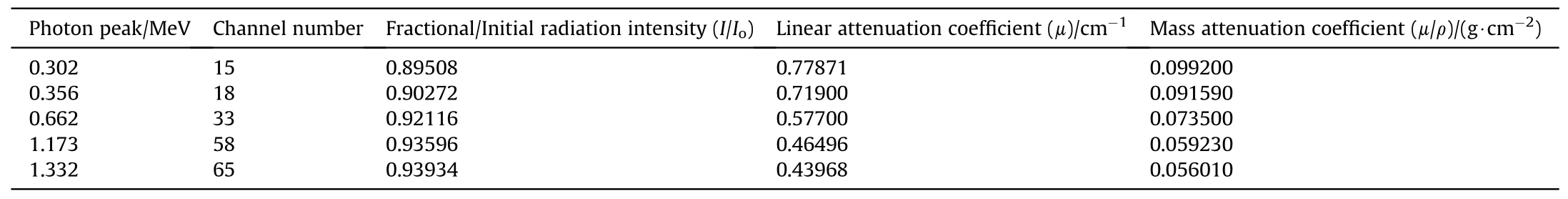
Table 6 Calculation details of experimental attenuation coefficients of Alloy 709 at each photon energy.
Moreover, the half-value layer (HVL) describes the thickness of shielding material (Alloy 709) at which radiation intensity is reduced by one half and it is expressed as ln2/μ, while the meanfree path (MFP) describes the average distance traveled by gamma rays in shielding material before interacting and it is expressed as 1/μ[3].Therefore,the shielding efficiency increases as HVL and MFP values decrease.Fig.7 and Fig.8 show the variations of the HVL and MFP values of Alloy 709 as function of photon energy,obtained experimentally and computationally.As shown,the HVL and MFP values obtained using MicroShield are not significantly different from the experimental measurements,especially at 0.662 MeV. The shielding efficiency of Alloy 709 decreases with increasing photon energy since HVL and MFP values keep on increasing. This behavior is attributed to the linear attenuation coefficient shown in Fig. 5 which shows the opposite trend experimentally and computationally.
The experimental gamma-ray attenuation of Alloy 709 as a proposed canister material in spent fuel dry casks is compared to other stainless steels(304 and 316)(Fig.9).As shown in Fig.9 and Table 7, all stainless steels exhibit reduction in their attenuation values with increasing photon energy, with Alloy 709 lying on top of them and exhibiting the highest linear attenuation coefficient values and thus, suggesting Alloy 709 as a candidate structural material for spent fuel dry casks.
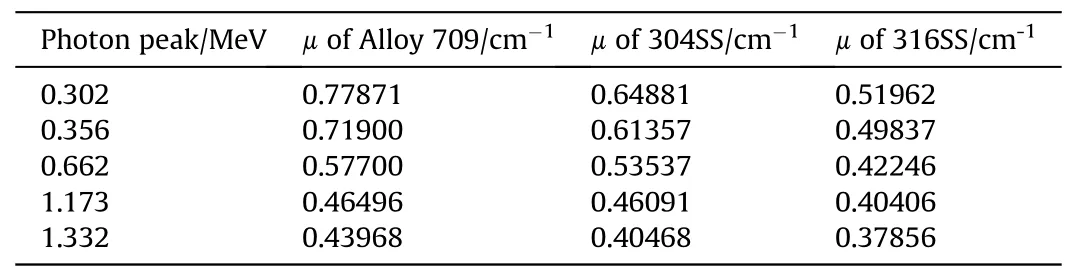
Table 7 Comparison of the experimental values of linear attenuation coefficient of Alloy 709 and other stainless steels at different photon energy.
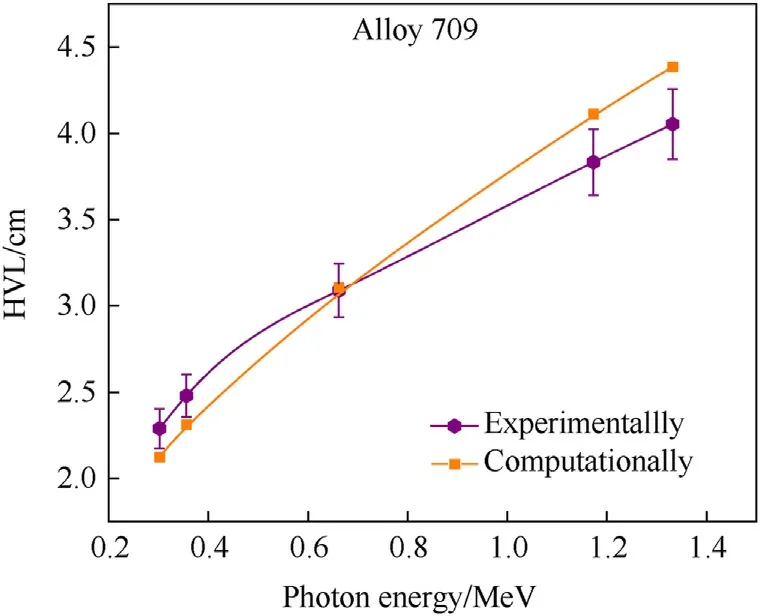
Fig. 7. Experimental and computational variations of the half-value layer (HVL) of Alloy 709 as function of photon energy.
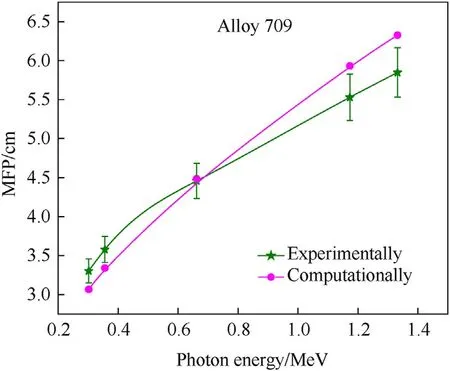
Fig. 8. Experimental and computational variations of the Mean free path (MFP) of Alloy 709 as function of photon energy.
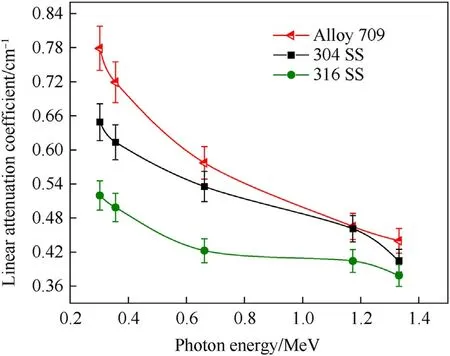
Fig. 9. Experimental comparison of the linear attenuation coefficient between Alloy 709 and other austenitic stainless steels as function of photon energy.
3.2. Corrosion behavior
The first setup of corrosion testing in which two samples of Alloy 709 are immersed in acidic(pH=2.2)and basic(pH=13.08)circulating corrosive solutions, showed no considerable weight change over a 69-day period (Fig. 10) and thus, showing good corrosion resistance of Alloy 709.Fig.11 shows a comparison of the weight change of Alloy 709 and other stainless steels, such as 304 and 316 from the work of Fusco[24],immersed in acidic and basic corrosive solutions at different pHs. As shown, the weight of 304 and 316 stainless steels also remains unchanged over a 20-day period in acidic solutions and a 55-day period in basic solutions.The exposure of Alloy 709 to acidic solution for a longer period of time than 304 and 316 stainless steels with no significant weight change,confirms its proven good corrosion resistance in aggressive acidic corrosive solutions due to its chemical composition.The 69-day period of exposure to corrosive solutions provides a reasonable preliminary indication of the expected extrapolated corrosion behavior of longer periods.
The second setup of corrosion testing is the electrochemical polarization, performed at elevated temperatures using the MULTIPORT™corrosion cell kit and Interface 1000 potentiostat from Gamry Instruments [41], where uniform corrosion rates are determined by measuring the cyclic potentiodynamic polarization curves from DC electrochemical measurements that control the voltage and measure the current. Corrosion rate, Kcorr, can be calculated as described below[24,41]:
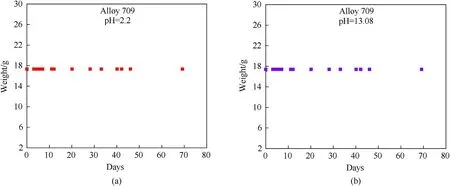
Fig.10. Measured weight change of Alloy 709 over time using circulating salt brines in two different corrosive solutions: (a) pH = 2.2; (b) pH = 13.08.
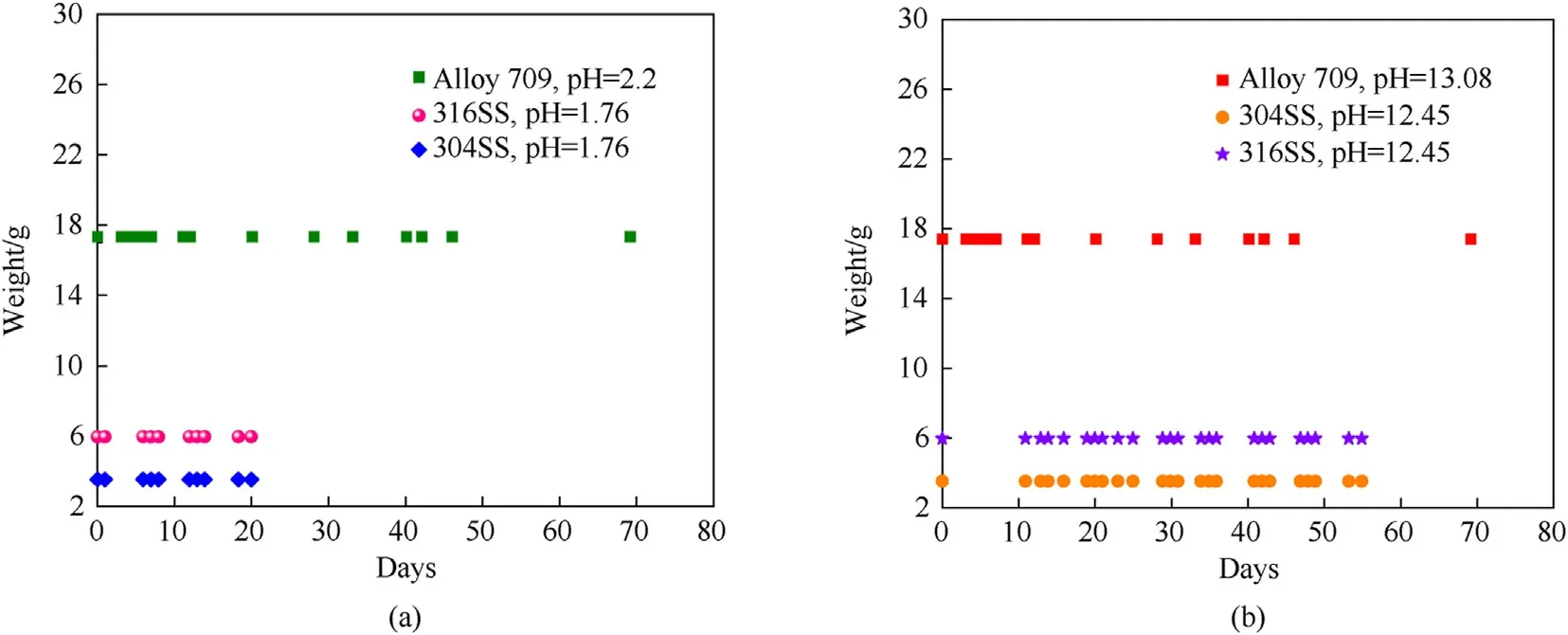
Fig.11. Comparison of the measured weight over time of Alloy 709 using circulating salt brines in acidic(a)and basic(b)corrosive solutions of different pHs,and compared to 304 and 316 stainless steels [22].
where k is a constant and equals 3272 mm/(A-cm-year),EW is the equivalent weight of the electrode in grams (g), ρ is the mass density(g/cm3),Aeis the electrode area exposed to corrosion(cm2)and Icorris the corrosion current in ampere(A) which is expressed as following [24,41]:
where βaand βcare the anodic and cathodic Tafel slopes(V/decade)and RPis the polarization resistance. The Tafel slopes equal 2.3RT/αnF in which R is the universal gas constant (8.3144598 J/(K∙mol)-1), T is the absolute temperature (K), α is the charge transfer coefficient, n is the number of electrons involved in the reactions and F is Faraday's constant (9.6485E+04C/mol). Cyclic potentiodynamic polarization curves are measured to determine the corrosion rate or corrosion current, in which these curves include low and high overpotential regions and can provide corrosion rates without additional testing. According to ASTM standard G61-86[43],the voltage scan rate in this study is 0.6 V/h(0.1667 mV/s).Detailed analysis of calculating the corrosion current is depicted in Fig.12,in which anodic and cathodic Tafel slopes(βa,βc) can be determined from extrapolating the anodic and cathodic current curves,while polarization resistance,RP,is the slope of the straight line in the cyclic potentiodynamic polarization curves.
The calculation of corrosion rate requires an appropriate equivalent weight and mass density of the material subjected to corrosion, which both parameters require determining the maximum depth from which the ions in the material could reach the surface and get released into the corrosive solution.Therefore,the calculation of corrosion current is more representative of corrosion behavior than corrosion rate since no material properties are included.Fig.13 and Table 8 show the corrosion current density of Alloy 709 in acidic (pH = 2.2) and basic (pH = 8.22) corrosive solutions at various temperatures, in which it increases exponentially with temperature in both solutions. Corrosion current has been shown to follow an Arrhenius relationship with temperature[24,44-46]:
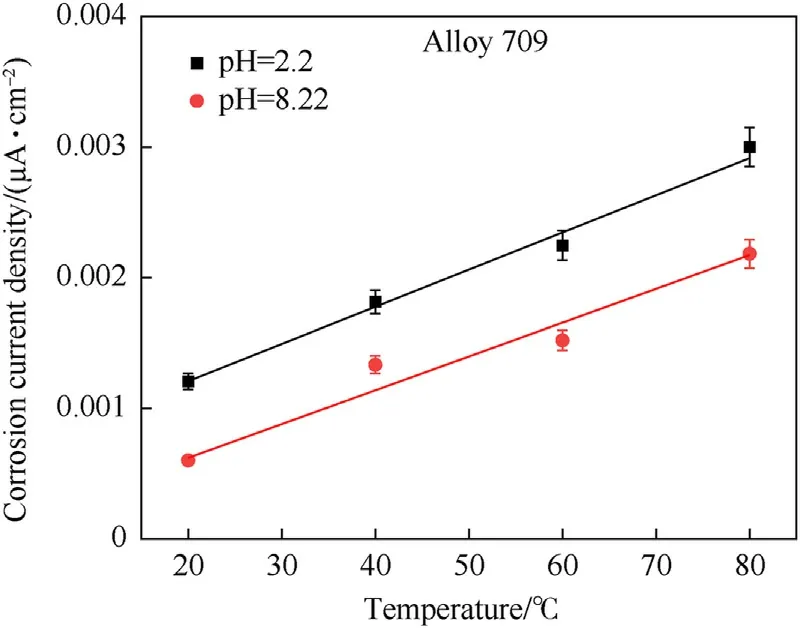
Fig. 13. Corrosion current density of Alloy 709 in acidic (pH = 2.2): (a) and basic(pH = 8.22) (b) corrosive solutions, using electrochemical polarization testing at various temperatures.
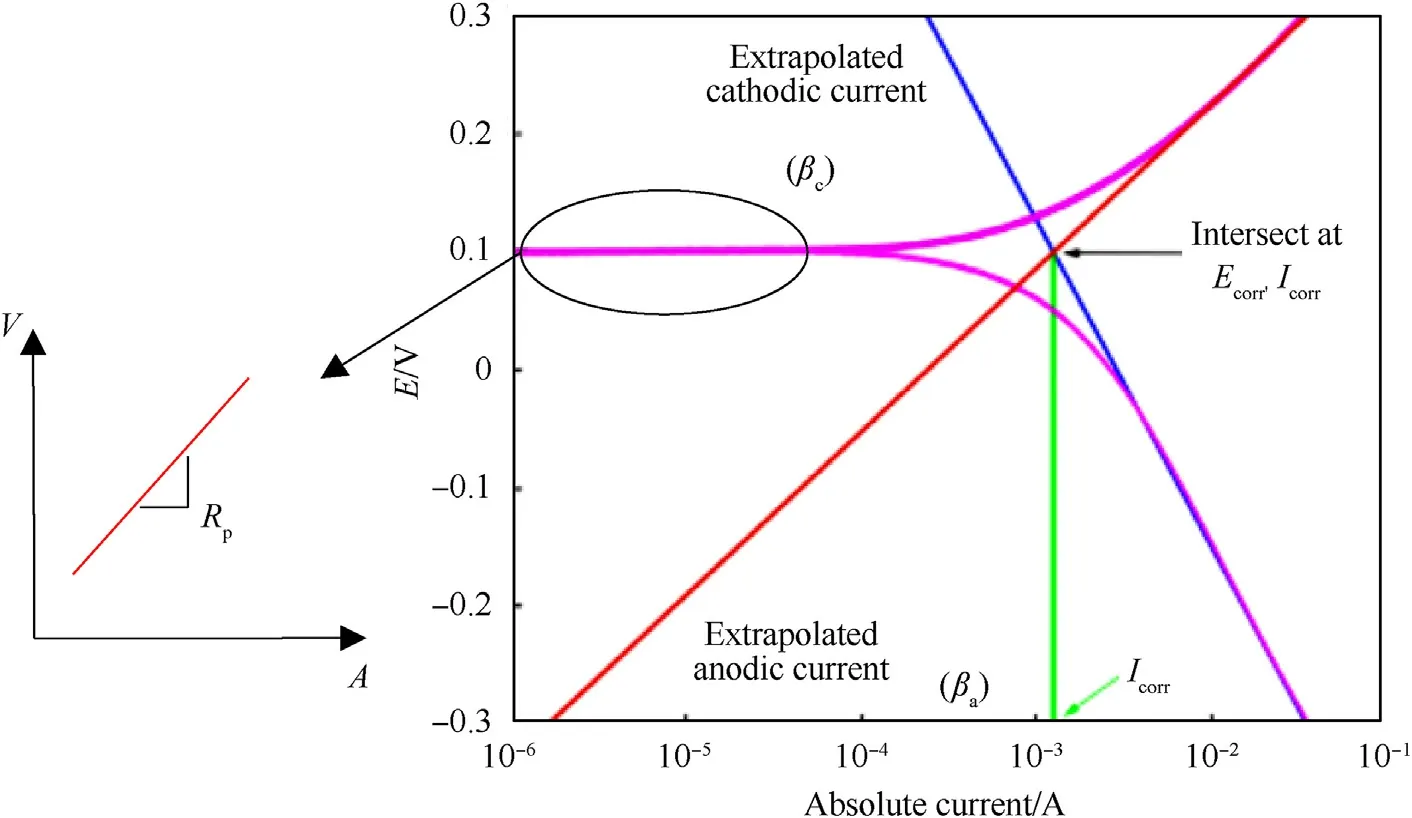
Fig.12. Cyclic potentiodynamic polarization curves showing the analysis of Tafel slopes and polarization resistance in order to determine the corrosion current and voltage[41].
where A is the pre-exponential factor (μA/cm2) and Ea0is the apparent activation energy (kJ/mol). Fig. 14 and Table 9 show a comparison of the corrosion current density of Alloy 709 and other common austenitic stainless steels used as dry cask canister materials, such as 304 and 316 austenitic stainless steels. As shown,Alloy 709 is the least corroded steel among other stainless steels in both acidic and basic solutions, in which 316 stainless steel is the most corroded steel shown and 304 stainless steel falls in between.At 20°C,Alloy 709 exhibits corrosivity close to 304 stainless steel in acidic environment,while 304 stainless steels exhibits the highest corrosion current density in basic environment compared to other steels.The high corrosion resistance of Alloy 709 in both corrosive solutions and at all tested temperatures is attributed to its chemical composition, in which the additives of Nb, B and especially Ti contribute in enhancing the corrosion resistance of the alloy and therefore, propose the use of Alloy 709 as a canister material in spent nuclear fuel dry casks.

Table 8 Corrosion current density of Alloy 709 in acidic (pH = 2.2) and basic (pH = 8.22) corrosive solutions at various temperatures.
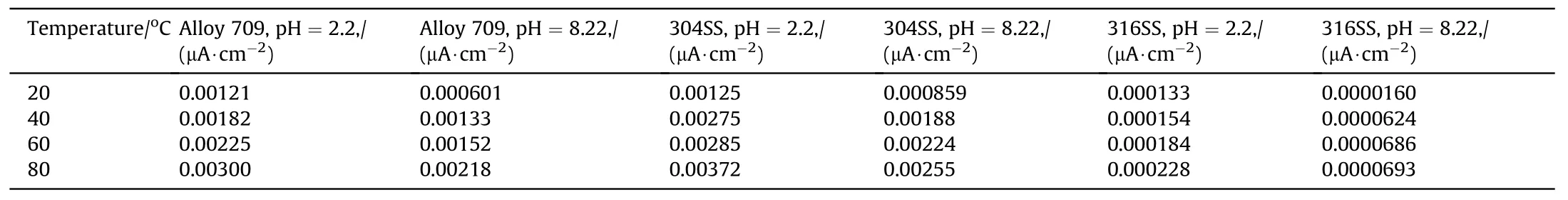
Table 9 Comparison of the corrosion current density of Alloy 709 and other stainless steels in acidic (pH = 2.2) and basic (pH = 8.22) corrosive solutions at various temperatures.
4. Conclusions
Nuclear waste produced from nuclear power plants is more important than the waste produced from other industries, and must be monitored carefully to protect working personnel and the surrounding environment. Therefore, to prevent transmission of radiation from nuclear waste into the surroundings, radiation shielding properties as well as corrosion properties play an important role in the design and operation of spent nuclear fuel storage systems.In this work,shielding and corrosion properties of the Alloy 709 advanced austenitic stainless steel have been investigated as a candidate canister material in spent fuel dry casks.The results revealed that experimental and computational data of the linear and mass attenuation coefficients of Alloy 709 appeared in good agreement, in which the attenuation coefficient values decreased with increasing photon energy. Also, Alloy 709 was shown to exhibit the highest linear attenuation coefficient against gamma rays when compared to 304 and 316 stainless.On the other hand,the results of circulating salt brines corrosion testing showed that Alloy 709 exhibited no considerable weight change over a 69-day period, while electrochemical polarization corrosion testing showed an exponential increase of corrosion current density with temperature in acidic and basic corrosive solutions. An interesting observation of the corrosion current density of Alloy 709 whencompared to common austenitic stainless steels used as dry cask canister materials, such as 304 and 316, showed that Alloy 709 is the least corroded steel among other stainless steels in both acidic and basic solutions. The optimistic results of radiation shielding and corrosion properties of Alloy 709 due to its chemical composition, suggest employing it as a canister material in spent nuclear fuel dry casks.
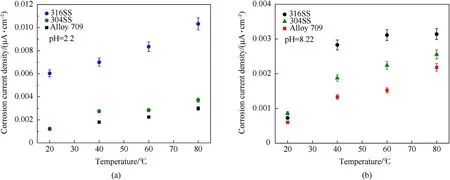
Fig.14. Comparison of the corrosion current density of Alloy 709, 304 and 316 stainless steels in acidic (pH = 2.2) (a) and basic (pH = 8.22) (b) corrosive solutions, using electrochemical polarization testing at various temperatures.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Work supported by the Russell Family Foundation Grant for research on High Level Waste Packages and Dry Casks, and additional support from the Department of Nuclear Engineering at North Carolina State University. The authors would like to express their gratitude to Sean Kerrigan for his help in conducting the corrosion experiments.
- Defence Technology的其它文章
- Evaluation method and optimization strategies of resilience for air &space defense system of systems based on kill network theory and improved self-information quantity
- Angular disturbance prediction for countermeasure launcher in active protection system of moving armored vehicle based on an ensemble learning method
- Study on jet formation behavior and optimization of trunconical hypercumulation shaped charge structure
- Trans-scale study on the thermal response and initiation of ternary fluoropolymer-matrix reactive materials under shock loading
- Camouflaged people detection based on a semi-supervised search identification network
- A super resolution target separation and reconstruction approach for single channel sar against deceptive jamming

