Urbanization strengthens the edge effects on species diversity and composition of woody plants in remnant forests
Zijin Wang,Jingyi Yang
College of Forestry,Guizhou University,Guiyang,550025,China
Keywords:Remnant forest Urbanization Edge effect Species diversity and composition Niche width Guiyang
A B S T R A C T Background:Urban remnant forests are embedded in urban areas and are threatened directly and indirectly by urbanization.The direct effects(e.g.,loss of area)have been well studied.However,knowledge about the indirect influence of urbanization through edge effects on the biodiversity in remnant forests is limited.
1.Background
Urban remnant forests are important natural resources in cities,as they are biodiversity hotspots and native species pools(Zipperer,2002;LaPaix et al.,2012;Foo,2016).These forests are embedded in urban areas and usually,severely threatened by urbanization(Huang et al.,2013).Urban remnant forests are not only directly damaged by urban expansion(e.g.,loss of area)(Salghuna et al.,2018)but also indirectly affected by urban environments(e.g.,changes in the conditions of habitats)(Huang et al.,2012).Remnant forest patches and the surrounding urban landscapes can be seen as typical patch-matrix frameworks(Fern′andez et al.,2018).The environmental conditions of the habitats located at the edge of remnant patches can be altered by a surrounding urban matrix.Previous studies have examined the impact of urbanization on the biodiversity in remnant forests(Stiles and Scheiner,2010;Ramalho et al.,2014;Yang et al.,2021).However,few studies have focused on the combined effects of urbanization and edge effects on the plant diversity of remnant forests(Guerra et al.,2017).
Limited evidence of these effects in urban ecosystems hinders the management of edge habitats and the conservation of biodiversity in urban remnant forests.Edge effects alter the spatial allocation of matter and energy in fragmented habitats and increase the difference in biodiversity patterns between edge and interior sites(Krishnadas et al.,2019).The interactions among different groups of species,their dispersal routes and mortality rates and the community trophic structure can be influenced by edge effects(Happer et al.,2005).For example,edges lead to increased mortality of canopy trees but improved the access of organisms and enhanced the dispersal of pollen and seeds(Cadenasso and Pickett,2001).The edge effects of habitat patches have been proven to be mediated by both patch attributes and the surrounding matrix(Porensky and Young,2013).For example,patch size increased edge-to-interior differences in carabid beetle richness and abundance(Soga et al.,2013).In addition,a surrounding matrix that improved connectivity among bird populations reduced edge effects in embedded native forest patches(Hatfield et al.,2019).
The levels of urbanization influence the intensity of edge effects by changing the degree of contrast between habitat patches and the surrounding matrix(Magrach et al.,2013).High levels of urbanization result in high contrast between forest edges and adjacent environments(Guerra et al.,2017).In addition,regions with high levels of urbanization often have high human population densities(Gao and O'Neill,2020).The edge habitats of remnant patches embedded in these regions are more likely to be severely disturbed by human activities(e.g.,trampled underfoot,injuries to trees,light and air pollution)(Bagnall,1979;Carreiro and Tripler,2005;Heckmann et al.,2008).Although many edge effects have been observed in natural forest landscapes,such as the replacement of plants with ubiquitous or pioneer tree species in edge habitats(Liu et al.,2019),few surveys have been conducted in urban areas.The levels of urbanization surrounding habitat patches and edge effects are both vital determinants of plant species diversity in urban remnant patches.Nevertheless,the combined effects of urbanization and edge effects have seldom been tested.Meanwhile,patch size also influences the edge effects through mediating the perimeter/area ratios of habitat patches(Fletcher et al.,2007).Generally,decreasing patch size increases the proportion of edge habitats(Laurance,2008).
Species diversity and composition are good predictors for exploring the difference between the edge and interior habitats of urban remnant forests.First,existing studies on urban remnant forests have shown that species diversity is sensitive to the levels of urbanization(Aronson et al.,2014;Ozkan et al.,2016).Species diversity indices can help to compare study results with those in other regions(Hahs and McDonnell,2007;Lugo-Perez and Sabat-Guernica,2011;Ramalho et al.,2014).Second,variation in species composition between different sites can indicate how edge habitats have changed compared to interior sites(Anderson et al.,2006).Moreover,the species composition measurement may be more sensitive to changes in environmental conditions than species diversity because it considers compositional dissimilarity rather than just species number and evenness(Legendre and De Caceres,2013).However,this measurement is rarely applied in studies of urban remnant forests.Moreover,few studies have differentiated the tree species in urban remnant forests into adult trees and saplings/seedlings and may thus overlook the legacy effects of urbanization on remnant forests,as saplings/seedlings represent the future compositions of these forests(Yang et al.,2021).
In this study,we aimed to assess how levels of urbanization of surrounding matrix affect remnant forests through edge effects.We selected a subtropical climate city with recent rapid urbanization–Guiyang,China–as a case study.The specific objectives of this study were(1)to examine the combined effects of levels of urbanization and edge effects on the species diversity and composition of woody plants in remnant forests,and(2)to analyse whether high levels of urbanization and stronger edge effects increase the abundance of generalist species.We expected that edge effects in remnant forest patches would be stronger with increasing levels of urbanization.Species with wider niche widths were expected to be more abundant in edge habitats with high levels of urbanization.
2.Methods
2.1.Study area
The study area was the metropolitan area of Guiyang,which is located in the middle of Guizhou Province in China(Fig.1).Guiyang is a famous mountainous city in China due to its karst landform.This area has a subtropical monsoon humid climate,and the main natural vegetation is subtropical evergreen broadleaf forests(Bai et al.,2009).The natural forests in this city have been impacted by urban expansion since 1996.The population of the city was 1.35 million in 1996 and increased to 3.59 million in 2018.Many remnant forest patches have been retained during the process of urbanization because of the area's steep topography.These remnant forest patches are highly fragmented and scattered throughout the whole city(Yang and Wu,2022).
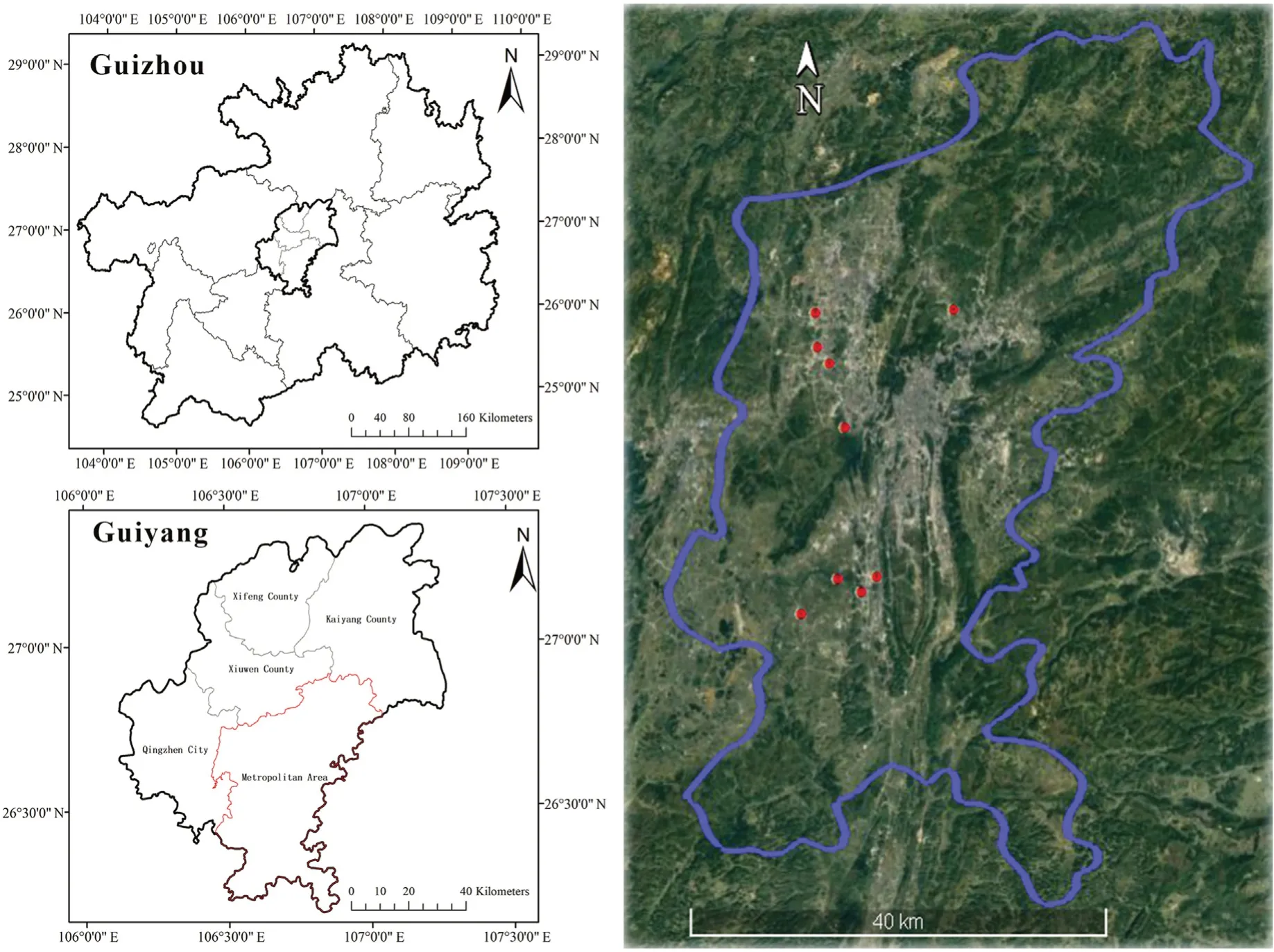
Fig.1.The study area,including the sample patches(numbered).
2.2.Selection of sample patches
Remnants patches were identified from the previous study(Yang et al.,2021).From this survey we selected only patches within 5 and 10 ha,because previous studies have provided estimates of core areas of forest patches are at least 100 m from patch boundary(Wei and Hoganson,2005).Therefore,patches that are too small cannot be differentiated into interior and edge sites.We only included broadleaf forests in our study because they were dominant vegetation type in the study area.To quantify the levels of urbanization in the surrounding landscape,the percentage of impervious surfaces within 500 m of the remnant patches that met our two selection criteria was derived from a land cover survey with a ground resolution of 30 m obtained from Zhang et al.(2021).A buffer of 500 m has been proved to be the best distance for detecting the relationship between urban matrix and plant diversity of habitat patches(Vakhlamova et al.,2014).The percentage of surrounding impervious surfaces was divided into three levels(less than 20%,20%–50%,or more than 50%)and categorized as low,medium and high levels of urbanization,respectively(Wickham et al.,2010).Subsequently,we randomly selected three patches from each level of urbanization as our sample patches.The distance between pairwise sample patches was over 1 km.Thus,a total of nine remnant patches were selected(Fig.1),and their characteristics can be found in Table 1.

Table 1Characteristics of the sample patches.
2.3.Field survey
We divided each sample patch into an edge area and an interior area.The edge area was defined as the area of the patch located within 25 m of the boundary.The reason is that many studies observed the species composition in 25 m inside urban woodlands began to be different from that at exterior parts within 25 m of the boundary(Honnay et al.,2002;Vallet et al.,2010).The interior area was defined as the area of the patch at least 100 m from the boundary and extending to the centre of the patch(Fig.2).We marked a sample site(20 m×20 m)at each of the cardinal points in the interior and edge areas.A total of eight sample sites were created in each remnant patch.Thus,the nine sample patches were divided into 72 subsample plots,which were categorized into six groups that combined the three levels of urbanization(high,medium or low)and the two habitat types(interior or edge habitat):the high-interior(HI),high-edge(HE),medium-interior(MI),medium-edge(ME),low-interior(LI)and low-edge(LE)groups.
We conducted a field survey from September to December 2021.In this survey,we recorded the species name and abundance of all the woody plants.Whether the recorded species was a tree or shrub was determined by referring to theFlora of Guizhou(Chen,2004).Tree species were additionally classified as adult trees or saplings/seedlings(<3 cm in diameter at breast height)according to Fang et al.(2009).We only focused on the woody plant species because they represent late-state plant successions in the study area(Tu and Li,1986).Further differentiating the ontogenetic stage of tree species was due to different responses of adult tree and sapling/seedling to urbanization(Yang et al.,2021,2022).

Fig.2.Schematic of the sample sites within each patch.
2.4.Data analysis
The alpha diversity of the woody plants in each sample site was estimated using the Shannon–Wiener index(hereafter the Shannon index),the Simpson index and the Pielou index.Alpha diversity is a local diversity,refers to the number and evenness of species in a single site(Thukral,2017).We used a nonparametric test,Wilcoxon signed-rank test(hereafter Wilcoxon test),to compared differences in alpha diversity of the woody plants between pairwise groups,which can deal with dependent samples(Ng et al.,2013).We also determined the difference in the alpha diversity of the woody plants between the edge and interior habitats of each patch at the different levels of urbanization using Wilcoxon paired test(Baringhaus and Gaigall,2018).
The differences in the Bray–Curtis distances between the edge and interior habitats for each sample patch were calculated.Bray–Curtis distances represent the compositional dissimilarities between pairwise sites.Then we compared the Bray–Curtis distances of remnant patches at the three levels of urbanization.To test the significance of the difference in mean plant compositions between edge and interior habitats in each sample patch,we applied a permutational multivariate analysis of variance(PERMANOVA).To test whether there is a significant difference in species composition among the six groups,we conducted an analysis of similarities(ANOSIM)that is a nonparametric multivariate analysis ofone-way ANOVA(Clarke,1993).Then,we used principal coordinate analysis(PCoA)axes to visualize the multivariate homogeneity of the dispersions of the six groups.
Furthermore,to test whether high levels of urbanization and edge effects increased the abundance of species with wide niche widths,we quantified the niche widths of all the recorded species using the Shannon index developed by Zhang(2018),which was calculated using Eq.(1):

wherePijis the percentage of number of speciesiin the communityjin the total number of this species.ris the number of communities that speciesiis occupied.Biis the niche width of speciesi.A highBivalue indicated a wide niche width of speciesi.When the numbers of speciesiinrcommunities are equal,theBivalue is high.When all the individuals of species are concentrated in only one community,theBivalue is low.
Then we used an abundance-weighted mean of niche width to measure the comprehensive niche width for all the woody plant species in each community and compared the abundance-weighted mean of niche width of three types of woody plants among six groups by Wilcoxon test.
All data analysis in our study was carried out in R version 3.6.2(R Core Team,2019).The Shannon index,Simpson index,Pielou index and Bray–Curtis distance were calculated using theveganpackage,which was also used to conduct the PERMANOVA and ANOSIM(Oksanen et al.,2018).PCoA was conducted using theapepackage(Paradis et al.,2004).Niche widths were calculated with thespaapackage(De C′aceres,2013).
3.Results
A total of 177 species of woody plants were recorded in 72 subsample plots within the nine selected patches.Of these species,92 were found in the adult tree layer,88 species in the sapling/seedling layer and 75 species in the shrub layer.In the interior habitats,we recorded 78(84.8%),71(80.7%)and 59(78.7%)species in the adult tree,sapling/seedling and shrub layers,respectively.In the edge habitats,we recorded 72(78.3%),72(81.8%)and 67(89.3%)species in the adult tree,sapling/seedling and shrub layers,respectively.24 species were only found in the interior habitats,while 28 species were only found in the edge zone.The species information recorded in the field survey can be found in the supplementary material(Table S1).
3.1.The alpha diversity of remnant patch groups
As shown in Fig.3,the results of Wilcoxon test indicated that the Shannon and Simpson index of species in the adult tree layer in the LI group was significantly higher than that in the HI and HE groups(Fig.3a and b).However,the species in the shrub layer showed the opposite trend:the Shannon and Simpson indices in the LI group were significantly lower than those in the HE group(Fig.3g and h).For the species in sapling/seedling layer,the Shannon,Simpson and Pielou indices in the LI layer were the highest,which were significantly higher than those in the MI group.
The significant differences in the Shannon and Simpson indices of species in the sapling/seedling layer between the pair of interior and edge habitats under low urbanization were detected in the analysis(Table 2).The mean Shannon and Simpson index values of species in the sapling/seedling layer at the interior sites(2.110±0.300 and 0.841±0.054,respectively)were higher than those at the edge sites(1.771±0.273 and 0.762±0.075,respectively)under low urbanization.There were no other significant differences.
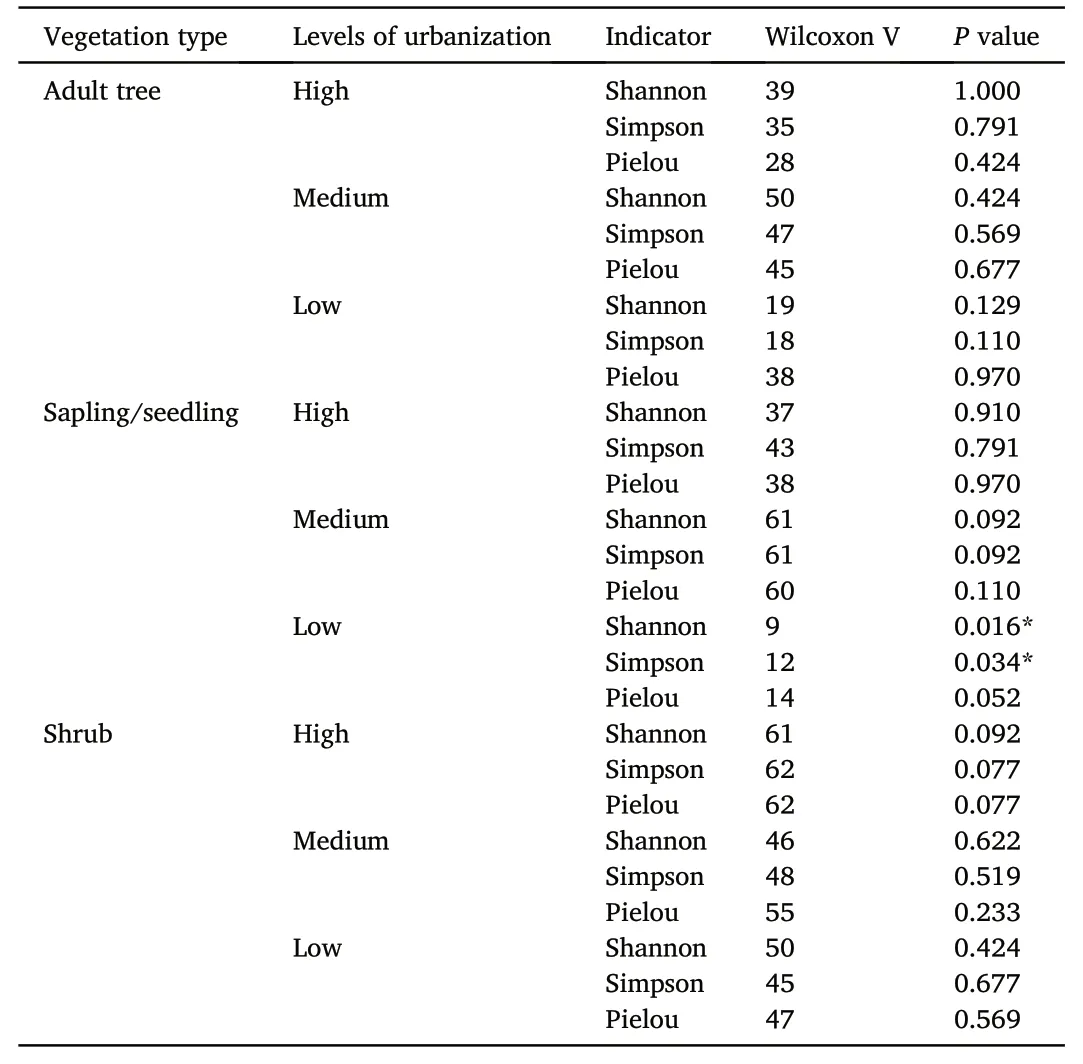
Table 2The results of Wilcoxon paired test for the alpha diversity of woody plant species.
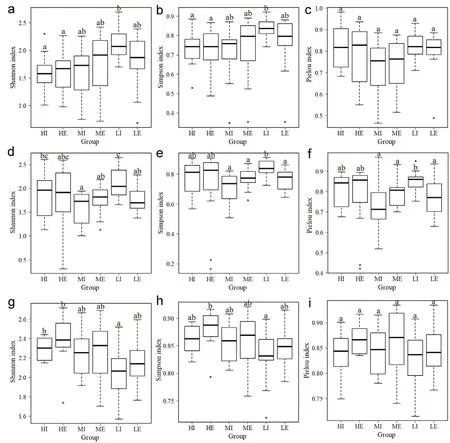
Fig.3.Alpha diversity of species in the adult tree(a–c),sapling/seedling(d–f),and shrub layer(g–i).Same characters represent non-significant differences(P>0.05)and different characters represent significant differences(P<0.05).HI:interior habitats under high urbanization;HE:edge habitats under high urbanization;MI:interior habitats under medium urbanization;ME:edge habitats under medium urbanization;LI:interior habitats under low urbanization;LE:edge habitats under low urbanization.
3.2.Compositional dissimilarity among remnant patch groups
The Bray–Curtis distance,a measure of species composition dissimilarity,in the adult tree layer was significantly higher between interior and edge sites under high urbanization than those under moderate and low urbanization(Fig.4).There were no significant differences in the compositional dissimilarity of species in the sapling/seedling and shrub layers at different levels of urbanization.

Fig.4.The Bray–Curtis distance measuring species composition differences in the adult tree layer between interior and edge sites.
Significant differences in the species composition of the adult tree layer were observed between interior and edge sites in Patches 1 and 4,which were under high urbanization(Table 3).Another patch under high urbanization(Patch 3)did not show significant differences in species composition between the interior and edge sites.
The results of the analysis of similarity revealed significant differences in species composition among the groups for species in the adult tree(R=0.203,P=0.001,Fig.5a),sapling/seedling(R=0.177,P=0,001,Fig.5c)and shrub layers(R=0.196,P=0.001,Fig.5e).The variation in species composition was the greatest in remnant patches surrounded by moderate urbanization(Fig.5b,d,f).The range of species composition of all the groups overlapped in the adult tree layer(Fig.5b).For species composition in the sapling/seedling layer,the ranges of the LI and HE groups had no overlap(Fig.5d).For species in the shrub layer,the range of species composition in the two groups under high urbanization(HI and HE)did not overlap with that of the LE group(Fig.5f).
3.3.Analysis of niche width
According to the results of Wilcoxon test,the abundance-weighted mean of niche width of shrub species was significantly higher than that of adult tree and sapling/seedling(Fig.6).However,there were no significant differences in the abundance-weighted mean of niche width among six groups for the same type of woody plants.
The range of niche widths for all species collected in this study was from 0 to 4.04.The niche width of each species can be found in the supplementary material(Table S2).The species with the largest niche width in the adult tree layer(Ligustrum lucidum)had the highest abundance in the HE group(Table 4).The species with the second largest niche width in both the adult tree and sapling/seedling layers(Celtis sinensis)accounted for the highest percentage in the HE group(Table 4).
The percentage of shrub species with the two largest niche widths in the groups under moderate and high urbanization was higher than that in the groups under low urbanization(Table 5).

Table 3Significant differences in species composition between the interior and edge sites of each patch according to the PERMANOVA test.

Table 4The abundance of tree species with large niche widths in the six groups,presented as a proportion of the total tree species.
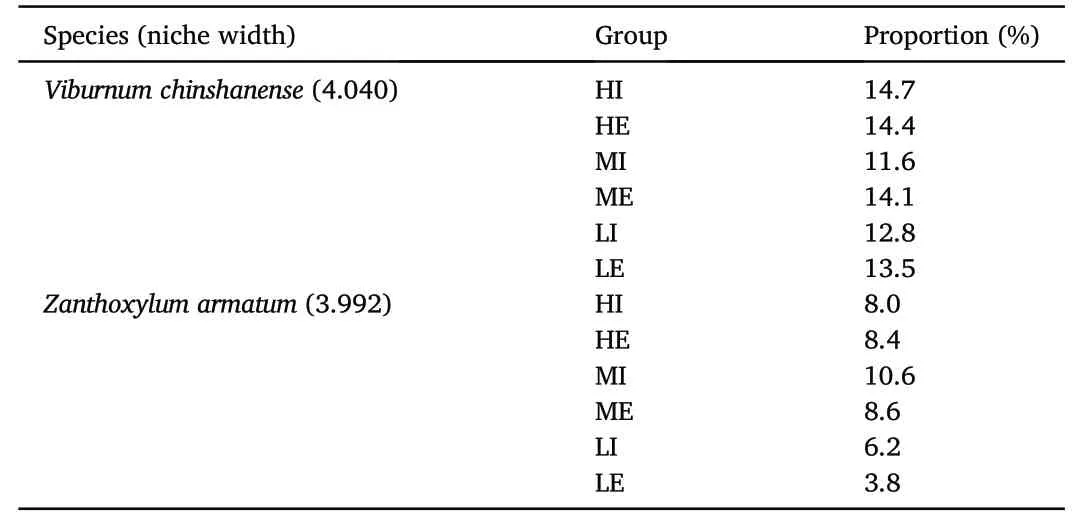
Table 5The abundance of shrub species with large niche widths in the six groups,presented as a proportion of the total shrub species.
4.Discussion
Our results revealed that the levels of urbanization influenced the woody plants in remnant forest patches through edge effects.The combined effects of urbanization and edge effects on the alpha diversity of shrub species and tree species were opposite.We found that shrub species were more in edge habitats under high levels of urbanization but tree species in the adult tree layer were fewer.Shrub species are more tolerant to disturbed edge environments than tree species due to their shorter size,faster regeneration and reduced living condition restrictions(K¨orner,2012).Tree species may be more likely to die at edges due to windthrow(Murcia,1995).Thus,this reduction in tall plants and increase in short species are regarded as a typical edge effect,reported by many studies(Santos et al.,2008;Guerra et al.,2017).
The alpha diversity of species in the sapling/seedling was found to be lower under moderate levels of urbanization,both in interior and edge habitats.Saplings/seedlings are more vulnerable to urbanization than adult trees(Ribeiro et al.,2016).Even moderate urbanization can affect the survival of sapling/seedling.However,at a high level of urbanization,adult trees were more affected negatively;thus,the number of sapling/seedlings increased due to the decline of canopy density.Besides,the significant differences of alpha diversity in the pair of interior and edge habitats were only detected in the sapling/seedling layer under low urbanization.The interior habitats of patches under low urbanization were rarely disturbed but the edge habitats were often affected by thesurrounding agriculture.Higher herbivory by livestock may be the cause of lower diversity of sapling/seedling in the edge(Ribeiro et al.,2015).The interior and edge sites under higher level of urbanization were both affected,so difference of diversity between interior and edge sites was not significant for saplings/seedlings.

Fig.5.Analysis of similarity and multivariate homogeneity of group dispersions.The dissimilarities in species composition between and within the six groups in the adult tree layer(a),sapling/seedling layer(c)and shrub layer(e).Principal coordinate analysis plots of six groups for species composition in the adult tree layer(b),sapling/seedling layer(d)and shrub layer(f).Increased overlap among groups indicates increased similarity in species composition.HI:interior habitats under high urbanization;HE:edge habitats under high urbanization;MI:interior habitats under medium urbanization;ME:edge habitats under medium urbanization;LI:interior habitats under low urbanization;LE:edge habitats under low urbanization.
High levels of urbanization strengthened the edge effects on species composition in the adult tree layer.The Bray–Curtis distance of species composition in the adult tree layer between interior and edge habitats was significantly higher under high levels of urbanization than medium and low levels of urbanization.Moreover,the species composition in the adult tree layer significantly differed between the interior and edge sites in the two remnant patches under high urbanization.Compared with relatively natural edges(e.g.,edges under low urbanization),highly anthropogenic edges are surrounded by a high-contrast matrix(Capmourteres and Anand,2016).A high contrast of habitat patches and surrounding urban matrix enhances edge effects in remnant vegetation(Harper et al.,2015).However,edge effects on species composition were only detected in the adult tree layer in the present study.This may be because the patches under high urbanization were located in the earliest urbanized regions of the study area.Adult trees often have a long lifespan,so the influence of long-term human disturbance is more obvious in them than it is in younger trees(Ramalho et al.,2014).These findings indicate that the influence of the combination of urbanization and edge effects on plant diversity may vary by the ontogenetic stage of tree species.For example,Jin et al.(2021)did not find edge effects on tree species diversity in an urban forest in East China.Not differentiating between the ontogenetic stages of trees may obscure edge effects because of the different responses of adult trees and saplings/seedlings to urbanization.

Fig.6.Abundance-weighted mean of niche width for woody plant species.HI:interior habitats under high urbanization;HE:edge habitats under high urbanization;MI:interior habitats under medium urbanization;ME:edge habitats under medium urbanization;LI:interior habitats under low urbanization;LE:edge habitats under low urbanization.
The combined effects of urbanization and edge effects were a major driver of compositional dissimilarity in woody plants of remnant patches.We identified significant differences in species composition among the six groups for all three types of woody plants(i.e.,adult trees,saplings/seedlings,and shrubs).Further analysis indicated that the species composition in HE sites was very different from that in LI sites.This is similar to the findings for alpha diversity.The LI group was the least affected by human activities among the six groups.The HE group was most affected by disturbance of the edge sites.This significant difference in species composition was in line with our expectations.Edge effects are found in both natural and urban environments(Soga et al.,2013).The compositional dissimilarity between edge and interior sites was previously attributed to the difference in the amount of exposure to sunlight(Murcia,1995).However,in urban landscapes,due to proximity to a highly modified matrix,changes in microenvironmental conditions are often forced by high levels of urbanization,especially in edge sites(Angulo et al.,2016;Guerra et al.,2017).If we did not categorize by levels of surrounding urbanization,there were no significant differences in species composition between interior and edge sites in remnant patches.These results indicated that urbanization had both a direct effect on species diversity in remnant forest patches(Huang et al.,2013)and an indirect effect through edge effects(Soga et al.,2013).
High levels of urbanization increased the abundance of species with large niche widths in the edge sites of remnant forest patches.We found that the tree species with large niche widths were the most abundant in the HE group.This pattern can be explained by the fact that urbanization benefits generalist species that have high urban adaptability.L.lucidumhad the widest niche width;this species’natural distribution covers South China(Fang et al.,2011).The mean annual temperature of its natural distribution ranges from 1.5 to 22.4°C,and the annual precipitation ranges from 512 to 2,030 mm(Fang et al.,2011).C.sinensisalso has a wide niche width;it tolerates mean annual temperatures that range from 2.2 to 24.7°C and annual precipitation that ranges from 367 to 3,495 mm(Fang et al.,2011).These species can be regarded as generalists that can develop in a wider range of conditions,which are widespread in edge habitats under high levels of urbanization.Many studies have found that urbanization increases generalist species,leading to biotic homogenization(McKinney,2006;Winter et al.,2009;Zeeman et al.,2017).In response to the change in the disturbance regime in urban areas,increasing competition intensity becomes a strong filter and cause an increase in generalist species(Zeeman et al.,2017).In addition,the abundance-weighted mean of niche width of shrub species was found to be higher than that of tree species.This finding is accord with the conclusion of a previous study that shrub species in the study area showed a more homogenization effect compared to tree species(Yang et al.,2021).
While this study provides new knowledge on the combined effects of urbanization and edge effects on the remnant forests,some limitations should be addressed.First,we sampled the edge habitats at only one level(25 m)in this study.More levels of edges can be sampled(several sample plots in different distances from the boundary)to test the variation of edge effects with the edge width.In addition,some confounding factors,such as the spatial aggregation among urbanization levels surrounding remnants may influence the dissimilarity in species composition.Although these limitations,the current study has detected high levels of urbanization can strengthen the edge effects of remnant forest patches.
5.Conclusion
Understanding the factors that mediate biodiversity in urban remnant forests is crucial for improvements in their conservation.Little is known about the influence of urbanization and edge effects at present.In this study,we examined the combined effects of urbanization and edge effects on the species diversity and composition of woody plants in urban remnant forests.Our results confirmed that urbanization strengthens edge effects in remnant forest patches.Based on these results,we suggest strict control of the urban expansion in the surrounding regions because rapid urban expansion not only causes direct damage to the remnant forests,but also comes into the indirect effects on biodiversity through edge effects.Future studies should further explore the role of urbanization in determining edge-interior differences in the functional and phylogenetic traits of remnant vegetation.
Ethics approval and consent to participate
Not applicable.
Funding
This research was funded by the Guizhou Science and Technology Department under Grant(QKHLHZ[2016]7447)and the first-class discipline construction project of Guizhou Province under Grant(GNYL[2017]007).
Authors’contributions
ZW collected the data and drafted the manuscript;JY designed the study.JY and ZW analysed the data.All authors commented preliminary versions of the manuscript and contributed to improve the final version.The author(s)read and approved the final manuscript.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the Guiyang Ecological Environment Bureau for sharing their forest inventory data.In addition,we sincerely appreciate three anonymous reviewers'constructive comments and suggestions to improve the draft of the manuscript.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.i.org/10.1016/j.fecs.2022.100063.
- Forest Ecosystems的其它文章
- Remote sensing of bark beetle damage in Norway spruce individual tree canopies using thermal infrared and airborne laser scanning data fusion
- Replanting of broadleaved trees alters internal nutrient cycles of native and exotic pines in subtropical plantations of China
- Fine root morphology and soil properties under influence of different tree stands along an altitudinal climosequence in the Carpathian mountains
- Ground-based/UAV-LiDAR data fusion for quantitative structure modeling and tree parameter retrieval in subtropical planted forest
- Effects of forest canopy density and epixylic vegetation on nutrient concentrations in decaying logs of a subalpine fir forest
- Patterns of species diversity and its determinants in a temperate deciduous broad-leaved forest

