Effect of Zn on performance of Ni/SiO2 for hydrodeoxygenation of anisole
WANG Dan-dan,GU Xiao-yu,SHI Hao-nan,CHEN Ji-xiang
(Tianjin Key Laboratory of Applied Catalysis Science and Technology, Department of Catalysis Science and Engineering,School of Chemical Engineering and Technology, Tianjin University, Tianjin 300350, China)
Abstract: Herein,SiO2 supported metallic Ni (Ni/SiO2) and bimetallic Ni-Zn (NixZn/SiO2) (x represents the Ni/Zn atomic ratio) catalysts were prepared by the incipient wetness impregnation method and their activities were tested in vapor phase hydrodeoxygenation (HDO) of anisole under 0.1 MPa. The characterization results show that Ni-Zn alloy forms in NixZn/SiO2 after reduction at 550 °C,and a suitable Ni/Zn atomic ratio (30) leads to smaller alloy particle size and consequently more H2 adsorption amount than others. In the HDO reaction,the formation of Ni-Zn alloy facilitates the direct deoxygenation pathway and suppresses CO methanation and C-C bond hydrogenolysis,which is ascribed to the isolation effect of the Ni atoms by the oxophilic Zn ones. Ni30Zn/SiO2 gives not only higher anisole conversion but also higher selectivity to benzene than Ni/SiO2.Therefore,the introduction of a suitable amount of oxophilic Zn in Ni/SiO2 promotes the HDO of anisole to benzene. Finally,we propose that the Ni30Zn/SiO2 deactivation is related to the oxidation of Ni-Zn alloy and carbon deposition on the catalyst surface.
Key words: Ni-Zn alloy;hydrodeoxygenation;direct deoxygenation;methanation;C-C bond hydrogenolysis
The excessive consumption of fossil resources has caused to some serious environmental problems. It is urgent to develop sustainable alternatives[1]. Biomass has attracted wide attention because of its renewable,abundant and carbon-neutral characteristics[2,3]. Biomass can be converted to bio-oil through fast pyrolysis;however,bio-oil cannot be directly used due to its high oxygen content[4]. To upgrade bio-oil,hydrodeoxygenation (HDO) is a feasible strategy[5,6].The bio-oil derived from lignin contains amounts of phenolics (such as phenol,anisole,guaiacol,etc.). The HDO of phenolics generally involves two routes,i.e.,direct deoxygenation (DDO) and hydrogenationdeoxygenation (HYD). DDO route is a desirable reaction pathway because phenolics are converted into important BTX (benzene,toluene and xylene) and this route can maximally lower the consumption of expensive H2.
A lot of catalysts have been researched for the HDO of phenolics. Noble metal catalysts (Ru[7,8],Rh[9],Pd[10],Pt[11,12],etc.) exhibit high HDO activities;however,the scarcity and high cost limit their commercial application. Metallic Ni catalyst is more promising because of its low cost and high activity in comparison with noble metal catalysts. Unfortunately,it is also active for benzene hydrogenation,C-C bond hydrogenolysis and CO methanation,which increases H2consumption and reduces the yields of BTX[13-15]. To circumvent this shortage,it is effective to modify the electronic and geometric property of metallic Ni by introducing a second metal. The second metal can form alloy or intermetallic compound (IMC) with metallic Ni. Especially,when the second metal is oxophilic(such as Fe[16],Re[17],Ga[18]),it can adsorb the oxygen atom in phenolic compounds,promoting the cleavage of the CAR-O bond (CARdenotes the carbon in benzene ring). In the HDO of guaiacol,PdFe/C catalyst has higher selectivity to aromatics than Pd/C,which is ascribed to the synergistic effect between Pd and Fe atoms[19]. Similar cases have been also found for NiRe and NiGa catalysts in the HDO of phenolics[20,21].
Recently,some researchers have modified metallic Pt and Ni with the oxophilic Zn,and investigated the effect of Zn on the catalyst performance for the HDO of oxygenates (not just phenolics). In the HDO process of 5-hydroxymethylfurfural,PdZn alloy exhibits higher activity and selectivity to 2,5-dimethylfuran than monometallic Pd[22],attributed to that the oxophilic Zn favors the activation of C-O bond. In the HDO of anisole,the aromatic ring parallelly adsorbs on Pt (111)surface,while anisole is bonded to the Zn site via oxygen on Zn/Pt (111) surface,and the benzene ring is tilted away from the surface[23]. This adsorption configuration beneficiates the cleavage of the CAr-O bond and inhibits the hydrogenation of the benzene ring on Zn/Pt (111). In the hydrogenolysis of glycerol,NiZnAl catalysts shows higher reactivity and selectivity to 1,2-propanediol than NiAl catalysts due to the formation of Ni-Zn alloy[24]. The Ni-Zn alloy is beneficial to adsorb hydroxyl groups in glycerol,promoting C-O bond cleavage but suppressing C-C bond hydrogenolysis. Similarly,in the deoxygenation of methyl laurate,Ni-Zn alloy and their intermetallic compounds possess very lower activity for C-C bond hydrogenolysis and methanation[25].Based on the above,we speculate that,in the HDO of phenolics,the modification of metallic Ni with oxophilic Zn may promote the CAR-O bond cleavage.To the best our knowledge,this topic has not been reported.
In this work,we prepared SiO2supported Ni-Zn alloy and studied the effect of Zn on the performance of Ni/SiO2for the HDO of anisole as a model compound. It was found that the formation of Ni-Zn alloy promoted the DDO pathway of anisole and suppressed the C-C bond hydrogenolysis and CO methanation.
1 Experimental
1.1 Materials
All chemicals were analytically pure and directly used without any further purification after purchasing from the commercial suppliers. Ni(NO3)2·6H2O and Zn(NO3)2·6H2O were purchased from Adamas and Macklin,respectively. Anisole and ethylbenzene were from Aladdin. Octane was supplied by Kermel.SiO2was obtained from Qingdao Haiyang Chemical Co.,Ltd.
1.2 Catalyst preparation
Ni/SiO2and NixZn/SiO2(xrepresents the Ni/Zn atomic ratio,x= 10 and 30) catalysts with nominal Ni loading of 15% were prepared by the incipient wetness impregnation method. SiO2was incipiently impregnated with an aqueous solution of Ni(NO3)2and/or Zn(NO3)2at room temperature for 48 h,and then dried at 120 °C for 12 h and calcined at 500 °C for 4 h.Before the reaction,all samples were reduced by H2at 550 °C for 2 h. To avoid deep oxidation,before some characterizations (XRD,N2adsorption-desorption,ICP-OES and TEM),the reduced catalysts were passivated at room temperature with a 0.5% O2/N2flow(320 mL/min) for 4 h.
1.3 Catalyst characterization
H2-TPR and H2-TPD were carried out on a homemade apparatus to characterize the reducibility and the exposed Ni sites on the reduced catalysts,respectively.X-ray diffraction (XRD) patterns were collected on a D8 Focus power X-ray diffractometer using CuKα radiation (λ= 0.1541 nm) at 40 kV and 40 mA. N2adsorption-desorption was carried out on a Quantachrom QuadraSorb SI instrument at -196 °C.The specific surface area (SBET) was determined by the Brunauer-Emmett-Teller (BET) equation.Transmission electron microscopy (TEM) was carried out on a JEOL JEM-2100F instrument (200 kV).Thermogravimetric analysis (TGA) was conducted on a Mettler Toledo TGA 1/SF instrument. The Ni and Zn contents in the fresh and spent Ni30Zn/SiO2catalysts were measured on SHIMADZU ICPE-9000 by Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES).
1.4 Catalytic test
The vapor-phase HDO of anisole on Ni/SiO2and NixZn/SiO2catalysts was evaluated on a quartz tube fixed-bed reactor (inner diameter of 8 mm) at atmospheric pressure. Prior to the reaction,the catalyst was reduced by a H2(100 mL/min) flow at 550 °C for 2 h,and then cooled to the reaction temperature.Subsequently,anisole was fed using a micro pump,vaporized and mixed with H2before entering the reactor. The H2/anisole molar ratio was kept at 25 for all runs. The liquid effluent was absorbed byn-octane in an ice bath. If no specified,the product at the 4thhour was analyzed on gas chromatography (GC).
The liquid samples were analyzed on a SP-3420 GC equipped with a flame ionization detector (FID)and a DB-1 capillary column (60 m × 0.32 mm × 3.0 μm).Ethylbenzene was used as an internal standard for quantitative analysis. The C1gaseous products (CH4,CO and CO2) were quantitively analyzed on an online 102 GC equipped with a TCD and a TDX-101 packed column,and N2was used as an internal standard. The C2-C5gaseous products were on line analyzed on a SP-3420 GC equipped with an FID and a HP-AL/S capillary column (50 m × 0.535 mm × 15 μm).
Here,the conversion of anisole (x) and the selectivity to producticontaining 6-8 carbon atoms(si) and selectivity to productjcontaining 2-5 carbon atoms (sj) were calculated by the following formulas:
wheren0andndenote the moles of the fed anisole and the unreacted anisole,respectively;niandnjdenote the moles of productiandj,respectively;krepresents carbon atom numbers in the productj.
2 Results and discussion
2.1 Catalyst characterization
2.1.1 Calcined catalysts
The XRD patterns of calcined catalysts are shown in Figure 1. All the catalysts give four diffraction peaks at 2θ= 37.3°,43.3°,62.9° and 75.4°,ascribing to the(101),(012),(110) and (113) reflections of facecentered cubic NiO (PDF#71-1179),respectively. No ZnO is detected,indicating that the Zn species are highly dispersed,and Zn2+ions may enter into NiO lattice and form oxide solid solution because the radii of Zn2+(0.074 nm) and Ni2+(0.069 nm)[26].
Figure 2 shows the H2-TPR profiles of the calcined catalysts. In the profile of Ni/SiO2,a main peak at 350 °C and a shoulder at 460 °C are ascribed to the reduction of NiO and nickel silicate[27],respectively. In comparison,Ni30Zn/SiO2and Ni10Zn/SiO2give two reduction peaks at higher temperature,which is more remarkable with lower Ni/Zn ratio. Therefore,the presence of Zn suppresses the reduction of Ni species. Particularly,the peak at low temperature (350 and 360 °C) is stronger than that at high temperature for Ni/SiO2and Ni30Zn/SiO2,while the peak area at high temperature (493 °C)surpasses that at low temperature (390 °C) for Ni10Zn/SiO2. It is known that metallic Zn has higher affinity to oxygen than metallic Ni,and ZnO is more difficultly reduced than NiO[25]. On the other hand,metallic Zn possesses lower ability for activating H2than metallic Ni. Thus,the presence of ZnO restrains the reduction of Ni species. In addition,the peak at high temperature also involves the reduction of ZnO,and it becomes strong at high Zn content (i.e.,low Ni/Zn atomic ratio).
2.1.2 Reduced catalysts
The physical properties of reduced catalysts are summarized in Table 1. With decreasing the Ni/Zn atomic ratio,the BET surface area and pore volume decrease from 409 m2/g and 0.78 cm3/g for Ni/SiO2to 244 m2/g and 0.51 cm3/g for Ni10Zn/SiO2. However,all the catalysts have similar pore diameters (5.7 nm).Because the nominal Ni contents are same for all the catalysts,the introduction of Zn species leads to the increase of total metal content,which aggravates the pore blockage,and then reduces the BET surface areas and pore volumes.
Figure 3 presents the XRD patterns of the Ni/SiO2and NixZn/SiO2reduced at 550 °C. H2-TPR of the reduced Ni/SiO2and NixZn/SiO2indicate that the Zn species in NixZn/SiO2can completely be reduced at 550 °C (Figure 2). Ni/SiO2gives three diffraction peaks at 2θ= 44.5°,51.8° and 76.3°,ascribing to the (111),(200) and (220) reflections of face-centered cubic Ni(PDF#04-0850),respectively. Likewise,NixZn/SiO2catalysts also possess three peaks,while the peaks slightly shift to low angles,which become more obvious with the decrease of Ni/Zn atomic ratio (i.e.,Ni10Zn/SiO2). This indicates the incorporation of the large Zn atoms (0.133 nm in radius) into the metallic Ni lattice (0.125 nm in radius),causing the Ni unit cell to expand and forming Ni-Zn alloy. Based on the Ni(111) lattice plane,the crystalline sizes are estimated as 11.0,10.4 and 12.5 nm in Ni/SiO2,Ni30Zn/SiO2and Ni10Zn/SiO2(Table 1),respectively. The crystalline sizes were also measured from the TEM images(Figure 4). The average size of Ni particles on Ni/SiO2are about 14.7 nm,and the Ni-Zn alloy particles sizes on Ni30Zn/SiO2and Ni10Zn/SiO2are 12.1 and 17.6 nm,respectively. In all,Ni30Zn/SiO2possesses the smallest Ni-Zn alloy particles.
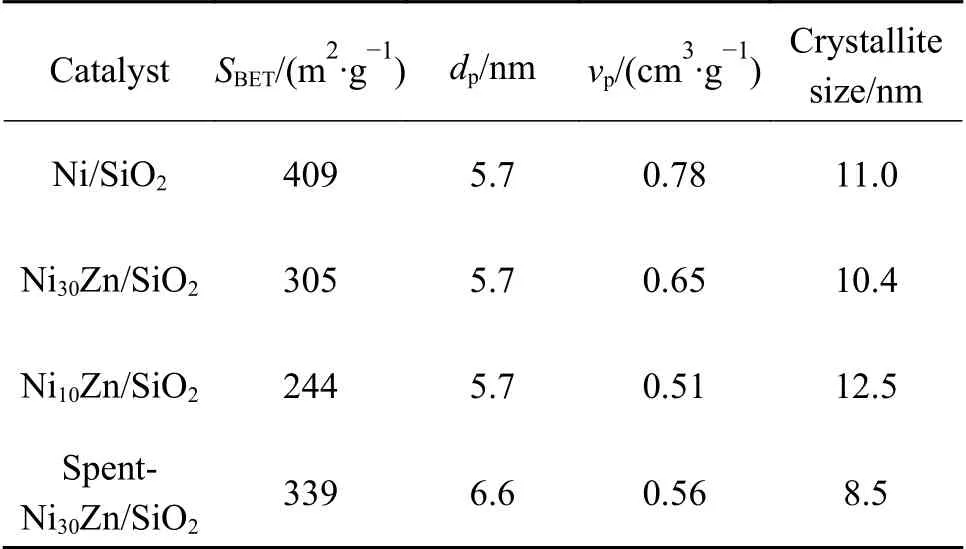
Table 1 Properties of different catalysts
The H2-TPD profiles of the catalysts are shown in Figure 5. For all the catalysts,only one peak appears below 400 °C,corresponding to the desorption of hydrogen on metallic Ni[27-29]. Compared with Ni/SiO2,Ni10Zn/SiO2has a very low H2uptake. This is reasonable because metallic Zn is nearly inactive for hydrogen adsorption,and the formation of Ni-Zn alloy reduces the numbers of exposed Ni sites[25]. Interestingly,Ni30Zn/SiO2gives higher H2desorption amount than Ni/SiO2,that is,the introduction of a small amount of Zn forming Ni-Zn alloy increase H2adsorption amount.This is related to smaller Ni-Zn alloy particle size in Ni30Zn/SiO2(Table 1) than others.
2.2 Catalyst performance
The reactivity of Ni/SiO2and NixZn/SiO2catalysts for the HDO of anisole were evaluated at 300 °C and 0.1 MPa. CO,CH4and cracked products C2-C5exist in gaseous effluent. The liquid products include benzene,cyclohexane,cyclohexanone and phenol,and there is a small amount of cyclohexene,cyclohexanol,and hexane and hexene. Given the product distribution and the related literatures[30,31],the HDO pathway is proposed in Figure 6. The HDO pathway of anisole mainly includes: direct deoxygenation (DDO) and HYD. The DDO pathway involves the direct cleavage of CAr-OCH3bond,producing benzene and methanol. Methanol can be further decomposed to CO and H2. In the HYD pathway,the hydrogenation of the benzene ring proceeds firstly to produce cyclohexanone and cyclohexanol,and then cyclohexanol is converted to cyclohexane via dehydration-hydrogenation. In addition,benzene can also be further converted to cyclohexane via hydrogenation,and cyclohexane is converted to C2-C5alkanes via C-C bond hydrogenolysis. CH4can be produced from three ways: (1) CO methanation; (2) CArO-CH3bond hydrogenolysis; (3) C-C bond hydrogenolysis.
2.2.1 Performance comparison of Ni/SiO2 and NixZn/SiO2 in HDO of anisole
Figure 7(a) presents the anisole conversion and product selectivity on Ni/SiO2and NixZn/SiO2(x= 10 and 30) catalysts. The anisole conversion on Ni/SiO2,Ni30Zn/SiO2and Ni10Zn/SiO2catalysts are 45.1%,48.6%,and 41.7%,respectively. That is,Ni30Zn/SiO2has higher activity than other catalysts,which is related to its higher H2adsorption amount (Figure 5). The main liquid product on all the catalysts is benzene.However,Ni/SiO2gives lower selectivity to benzene(67.5%) than Ni30Zn/SiO2(89.4%) and Ni10Zn/SiO2(73.2%),and higher selectivity to phenol (3.23%) than Ni30Zn/SiO2(1.5%) and Ni10Zn/SiO2(0.79%). This indicates that the direct cleavage of CAr-OCH3bond(i.e.,DDO pathway) may be dominant on both Ni/SiO2and NixZn/SiO2. Significantly,the formation of Ni-Zn alloy promotes the DDO pathway,favorably producing benzene. This is confirmed by the gaseous products.
As shown in Figure 6,the cleavage of CAr-OCH3bond in anisole produces benzene and methanol.Methanol can decompose to CO and H2,and CO is further hydrogenated to CH4. Figure 7(b) shows the molar ratios of the formed CH4,CO and methanol to the converted anisole (expressed as nCH4/nΔAnisole,nCO/nΔAnisoleand nCH3OH/nΔAnisole,respectively). No methanol forms on all the catalysts,indicating that methanol easily decompose to CO. Since CO is only generated from the decomposition of methanol,a high nCO/nΔAnisolemolar ratio can give the following information: (1) the DDO pathway via cleavage of CAr-OCH3bond is promoted; (2) methanation is suppressed. No CO detected on Ni/SiO2is ascribed to the high activity of metallic Ni for methanation. In contrast,CO forms on NixZn/SiO2,and lower Ni/Zn atomic ratio gives rise to higher nCO/nΔAnisolemolar ratio.Thus,the formation of Ni-Zn alloy not only promotes the DDO of anisole but also inhibits CO methanation.Particularly,Ni/SiO2gives the nCH4/nΔAnisolemolar ratio of 1.8,higher than 1.0,indicating that apart from the hydrogenolysis of O-CH3group,CH4is also generated from benzene ring via hydrogenation and C-C bond hydrogenolysis. This is confirmed by the formation of C2-C5hydrocarbons. The nC2-C5/nΔAnisolemolar ratio reaches 0.35 on Ni/SiO2,while they are 0.13 and 0.008 on Ni30Zn/SiO2and Ni10Zn/SiO2,respectively. The nCH4/nΔAnisolemolar ratios are 1.0 and 0.40 on Ni30Zn/SiO2and Ni10Zn/SiO2,respectively. Thus,the formation of Ni-Zn alloy also restrains the C-C bond hydrogenolysis. The effect of Zn on the performance of Ni/SiO2is ascribed to the high oxophilicity of Zn and the isolation of Ni atoms with Zn.
It has been reported that the large Ni ensembles favor the adsorption of benzene ring and C-C bond[32,33].In the Ni-Zn alloy,the continuous Ni atoms are isolated by the oxophilic Zn ones,leading to smaller Ni ensembles. In addition,metallic Zn has weaker interaction with carbon than metallic Ni. As a result,the isolated Ni atoms on Ni-Zn alloy is not favorable for the adsorption of aromatic ring and C-C bond,and the hydrogenation of benzene and C-C bond hydrogenolysis are suppressed. In addition,the electron transfer from Ni to Zn occurs in Ni-Zn alloy[25,34]. The reduced electron density of Ni atoms can weaken the interaction between Ni and CO,and CO methanation is restrained on NixZn/SiO2[35,36].
A possible reaction mechanism on Ni-Zn alloy is presented in Figure 8. In the Ni-Zn alloy,the continuous Ni atoms are isolated by the oxophilic Zn ones[25]. The O atom in anisole can preferentially absorbs on oxophilic Zn. As a result,the dissociation barrier of CAr-OCH3bond is reduced,and the direct cleavage of CAr-OCH3bond is facilitated.
In short,in comparison with the monometallic Ni catalyst,the formation of Ni-Zn alloy in NixZn/SiO2catalysts facilitates the DDO pathway,and inhibits CO methanation and C-C bond hydrogenolysis. Among the as-prepared catalysts,Ni30Zn/SiO2shows the best performance.
2.2.2 Effects of WHSV and reaction temperature on performance of Ni30Zn/SiO2
As mentioned above,Ni30Zn/SiO2has the best performance among the concerned catalysts. In order to get optimal reaction conditions,the effects of weight hourly space velocity (WHSV) and reaction temperature on the performance of Ni30Zn/SiO2were investigated.
Figure 9 presents the performance of Ni30Zn/SiO2at different WHSV. As shown in Figure 9(a),as anisole WHSV is increased from 1 to 4 h-1,the anisole conversion decreases from 83.9% to 33.0% due to the reduced contact time. The selectivity to benzene reaches the maximum value (89.4%) at the anisole WHSV of 2 h-1. The variation tendency of selectivity to cyclohexane with WHSV is similar to that of benzene.When the anisole WHSV is increased from 1 to 4 h-1,the selectivity to phenol gradually increases (from 0.1% to 2.7%),and nCO/nΔAnisolemolar ratio increases while nCH4/nΔAnisolemolar ratio decreases,indicating that the increase in WHSV is unbeneficial to the methanation of CO.
Figure 10 provides the effect of reaction temperature on performance of Ni30Zn/SiO2. When the reaction temperature increases from 260 to 300 °C,the anisole conversion maintains about 50% (Figure 10(a)),when further increasing to 320 °C,the anisole conversion decreases to 37.5%. In terms of kinetics,increasing the reaction temperature improves the reaction rate; however,from the view of the thermodynamic,the HDO of anisole is exothermic,and increasing the reaction temperature is not conducive to reaction. As the reaction temperature increases,the selectivity to benzene first increases and then decreases,while the selectivity to phenol shows an opposite change tendency. The highest selectivity to benzene (89.4%) and the lowest selectivity to phenol(1.5%) are obtained at 300 °C. This indicates that,below 300 °C,raising the reaction temperature promotes the cleavage of the CAr-OCH3bond,while high reaction temperature may favor the breakage of the CArO-CH3bond,consistent with the kinetic results on Cu/γ-Al2O3[37,38]. This is also reflected by reducednCO/nΔAnisolemolar ratio (from 0.34 to 0.09),and the increasednCH4/nΔAnisolemolar ratio (from 0.42 to 1.24)with raising the reaction temperature from 260 to 320 °C(Figure 10(b)). As the reaction temperature increases from 260 to 320 °C,the selectivity to cyclohexane decreases from 8.0% to 2.2%. This is related to that the benzene hydrogenation is exothermic. In addition,the raised reaction temperature promotes the C-C bond hydrogenolysis reaction,causing thenC2-C5/nΔAnisolemolar ratio increasing from 0.01 to 0.24.
2.2.3 Stability of Ni30Zn/SiO2 in HDO of anisole
The stability of Ni30Zn/SiO2was investigated at 300 °C,0.1 MPa and WHSV of 2 h-1. As shown in Figure 11,the anisole conversion decreases from 70.0%to 34.9% after reaction for 12 h,while the selectivity to benzene maintains at 75%. As mentioned below,the surface oxidation of Ni-Zn alloy might decrease the density of Ni sites,which can reduce the activity.However,the existence of Zn2+can still provide Lewis acid sites to adsorb oxygen[39,40],so the selectivity to benzene is not changed. The initial selectivities to phenol and cyclohexane are 1.0% and 1.78%,respectively,and they slightly increase with the reaction time. These results suggest that Ni30Zn/SiO2is deactivated during the HDO of anisole. To reveal the reason,the fresh and spent Ni30Zn/SiO2catalyst were characterized by XRD,TG,and N2adsorption-desorption.
As indicted in Figure 12,Ni-Zn alloy still exists in the spent Ni30Zn/SiO2,and its crystallites size (8.5 nm) is close to that in the fresh catalyst. Therefore,there is no sintering of Ni-Zn alloy crystallites. Interestingly,the peak due to Ni-Zn alloy is less sharp in the spent catalyst in comparison with the fresh one,meaning that the crystalline degree of Ni-Zn alloy is reduced. This may be related to the surface oxidation of Ni-Zn alloy particles by the formed water because metallic Zn is easily corroded by water. This case has been reported by Friedrich et al.[41]. In addition,the TG results indicate that there is 1.3% carbon deposition on the spent catalyst.As listed in Table 1,the pore volume decreases on the spent Ni30Zn/SiO2. The actual Ni and Zn contents in fresh catalyst are 16.4% and 0.70%,respectively,which are still 16.0% and 0.67% in the spent catalyst,respectively.That is,the leaching of Ni and Zn is not obvious during the reaction. Thus,we speculate that the catalyst deactivation is related to the surface oxidation of Ni-Zn alloy and the carbon deposition.
3 Conclusions
Metallic Ni and Ni-Zn alloy form in Ni/SiO2and NixZn/SiO2,respectively. Particularly,Ni30Zn/SiO2has smaller metal particle size and higher H2adsorption amount than other catalysts. As a result,Ni30Zn/SiO2gives higher activity and selectivity of benzene than Ni/SiO2and Ni10Zn/SiO2in the HDO of anisole. At 300 °C and WHSV of 2 h-1,the anisole conversion and the selectivity to benzene reach 48.6% and 89.4% on Ni30Zn/SiO2,respectively. The formation of Ni-Zn alloy promotes the DDO pathway due to the oxophilicity of Zn,and suppresses the C-C bond hydrogenolysis and methanation owing to the isolation of Ni atoms by Zn ones. Additionally,the Ni30Zn/SiO2deactivation is ascribed to the surface oxidation of Ni-Zn alloy and carbon deposition.

