Oxidation treatment of carbon aerogels supports to modulate Ru/CA catalysts for Fischer-Tropsch synthesis
ZHANG Lin-na,ZHANG Juan,WANG Guo-fu,ZHAO Wen-tao,CHEN Jian-gang,*
(1. State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. University of Chinese Academy of Sciences, Beijing 100049, China;3. Beijing Sanju Environmental Protection & New Materials Co., Ltd. Beijing 100049, China)
Abstract: Oxidation treated carbon materials for exploiting highly efficient and stable loaded catalysts have been proven to be valid. In this work,the surfaces of carbon aerogels (CA) were functionalized with different oxidizing agents,i.e.,H2O2 and HNO3. A series of Ru-supported catalysts on carbon aerogels (CA) with/without functionalized were prepared by the impregnation strategy. The impact of oxidation treatment on the texture features of carbon aerogels,the types and contents of formed surface oxygen-containing functional groups,the metal-support interactions and the Fischer-Tropsch synthesis reaction performances of the catalysts were systematically investigated. Our results showed that Ru/CA catalyst without oxidation treatment displayed the highest initial activity but the poor stability,while the Ru/CA-H2O2 catalyst exhibited excellent activity and C5+ selectivity. The oxidation treatment increased the carbon aerogels defects,thereby broadening the specific surface area. The increased content of oxygen-containing functional groups on the surface enhanced the interaction between the support and Ru nanoparticles and improved the stability of the catalyst. Nevertheless,the excessive oxygencontaining functional groups on the surface decreased the activity and the C5+ selectivity of carbon aerogels-loaded Ru catalysts.
Key words: carbon aerogels;chemical oxidization;surface oxygen containing groups;metal-support interaction;supported ruthenium catalyst;Fischer-Tropsch synthesis
Fischer-Tropsch synthesis (FTS) is an economically efficient and environmentally friendly approach to convert syngas derived from natural gas,coal or biomass into high value-added chemicals and fuels[1,2]. Recently,the severe energy crisis has prompted a great deal of investigations on the development of efficient and stable FTS catalysts.Iron,cobalt and ruthenium are the catalytically active species for the major FTS reactions[3-5],with Co and Fe being commercially available catalysts for FTS.Nevertheless,Ru catalysts provide an ideal platform to investigate the functionalization and reaction mechanism of the catalysts. Because Ru catalysts possess unique properties for FTS: a high intrinsic activity; the capability to work in high water pressure or other oxygenated atmospheres; being particularly essential for the conversion of biomass syngas[6,7]. The supported Ru-based catalysts are designed to minimize the cost and explore the possibility of metal-support interaction,in view of the fact that the microstructure and surface properties of the supports can affect the reactivity and selectivity through metalsupport interaction and reactant-product mass transfer behavior[8]. High specific surface supports such as alumina,zirconia,silica,molecular sieve and carbon materials are the preferred candidates for obtaining highly dispersed Ru nanoparticles[6,9-13]. Acidic sites on the surface of the metal oxide supports can significantly affect the product distribution of the catalysts[14,15]. Carbon materials are considered to be more inert than metal oxide,favoring the reduction of metal precursors to the zero valence state and facilitating metal-promoter interactions rather than metal-support interactions[16,17]. Among these,carbon aerogels are a distinctive class of materials with high porosity,specific surface area and extremely low density that have been employed as supports in various reactions[18-20]. Metal-support interaction is quite critical for loaded catalysts. The weak interaction of metal-support can lead to metal nanoparticle agglomeration during the chemical reactions[21]. To modulate the metal-support interaction (MSI),several strategies have been proposed[22,23]. In particular,surface oxidation for carbon materials has been proven to be an effective approach to overcome such a difficulty[24-27]. Kumi et al.[28]investigated the influence of nitric acid treatment on the CO hydrogenation performances of porous carbon sphere-loaded Ru catalysts and discovered that the acid treatment increased the surface defects,raised the specific surface area,and enhanced the dispersion of Ru,due to the increase of the oxygen-containing functional groups on the support,which affected the reduction behavior of Ru nanoparticles. Li et al.[29]reported that oxidation treatment could restore oxygen-containing groups on the surface of activated carbon,which was removed by high-temperature H2,thereby restoring and improving the activity for FTS. Overall,oxidation treatment can effectively modulate the catalyst performances by regulating the metal-support interactions. Nevertheless,the modulation of oxygencontaining groups on the surface of carbon aerogels supports has been rarely reported. Herein,we adopted carbon aerogels with high specific surface area and porosity as the supports and introduced oxygencontaining functional groups using different oxidants to investigate the effects of oxidation treatment on the weave properties,Ru reduction behavior and finally FTS performance.
1 Experimental
1.1 Chemicals
Resorcinol (C6H6O),formaldehyde (HCHO,37%),Sodium carbonate (Na2CO3),acetic acid(CH2COOH),tert-butanol (C4H10O),hydrogen peroxide(H2O2,30%),nitric acid (HNO3,68%) were obtained from Sinopharm Chemical Reagent Co. Ltd.Ruthenium nitrate (N4O10Ru,12.8%) was produced at Guiyuan Platinum Co. Ltd. All chemicals were analytical reagents and had not been further processed.
1.2 Synthesis of Ru/CA catalyst
1.2.1 Preparation and functionalization of carbon aerogels
Carbon aerogels (CA) were prepared by hydroxyaldol condensation reaction of resorcinol and formaldehyde. Specifically,resorcinol and formaldehyde(37%) were mixed (mole ratio = 1∶2) and added into distilled water. And then the admixture solution was kept under magnetic stirring for 30 min at 85 °C under an inert atmosphere to produce a wet gel with the sodium carbonate as a catalyst. The wet gel was acidified in an acetic acid solution (3%) that was replaced once a day for three days. Subsequently,the wet gel was exposed to solvent replacement in a tertbutanol solution for three days (daily replacement of the solution). The wet gels were freeze-dried and calcined in a tube furnace at 1000 °C for 5 h under Ar atmosphere to obtain carbon aerogels.
Carbon aerogels were functionalized with two different oxidants,including H2O2and HNO3. One was to reflux the carbon aerogels with 35% H2O2at 60 °C for 12 h,followed by washing with distilled water to neutrality,separation,and desiccation to obtain a sample,denoted as CA-H2O2. The other oxidation method was to condense the reflux with HNO3(68%) at 60 °C for 2 h,followed by cooling the suspension,filtration,washing,and drying,named as CA-HNO3.
1.2.2 Preparation of Ru/CA catalyst
Catalysts with 5% Ru loading on metal basis were prepared using the impregnation method. The carbon aerogel supports with and without functionalization were dispersed into a certain amount of distilled water at ambient temperature,doped with ruthenium nitrate as a metal precursor,magnetically stirred for 24 h. And then the resulting product was dried at 120 °C overnight. The obtained catalysts were named as Ru/CA,Ru/CA-H2O2and Ru/CA-HNO3,respectively.
1.3 Characterizations
Texture properties including specific surface area,average pore size,and pore volume were determined on a Micrometrics ASAP 2420 physisorption instrument. Prior to the experiments,the samples were degassed at 300 °C for 3 h to exclude the effects of moisture and other impurities. The pore size distribution curve was calculated using the Barrett-Joyner-Halenda (BJH) formula based on the desorption branch of the isotherm. The specific surface area and pore volume of the catalysts were calculated based on the Brunauer-Emmett-Teller (BET) formula.
Raman spectra were measured using a Bruker Optics Senterra Raman confocal microscope spectrometer with an excitation wavelength of 532 nm.
X-ray photoelectron spectrometer (XPS) was conducted on a Thermo Scientific ESCALAB 250Xi(USA) spectrometer,using C 1sas the internal standard element,minus the charge effect.
The elemental contents of Ru in the catalyst samples were determined using an Agilent ICPOES720 inductively coupled plasma emission spectrometer,USA.
X-ray diffraction patterns (XRD) were obtained using a 30 mA,40 kV CuKα X-ray source on a D/max-RA diffractometer with a scan speed of 4 (°)/min and a scan range of 5°-100°.
The reduction behaviours of the active metal in the catalysts were analyzed by H2-temperature programmed reduction (H2-TPR) measurements.Approximately 20 mg of catalyst samples were loaded into quartz tubes and warmed up from 50 to 800 °C,the ramp rate was kept at 10 °C/min and the hydrogen consumption was recorded by a thermal conductivity detector (TCD).
1.4 Catalytic tests
The Fischer-Tropsch synthesis reaction evaluation of the catalysts were performed in a fixed-bed reactor with a reaction tube diameter of 10 mm. The electrically heated vertical stainless steel tubular reactor was wrapped with a 50 cm copper cylinder,and the gas stream was controlled by a Brooks 5850E mass flow controller. Pressure of the reaction system was controlled by a pressure setter,and the reaction products passed through a heat trap at 130 °C and a cooling trap at 5 °C under reaction pressure. The gas phase products were measured online by gas chromatography. 0.8 g of catalyst was placed in the isothermal zone of the reactor,followed by a purge of the reaction system with hydrogen gas to ensure that the reaction system was free of impurity gases. The hydrogen flow rate was adjusted to 50 mL/min,and the temperature was increased to the reduction temperature of 250 °C at a heating rate of 2 °C/min,and the reduction temperature was maintained for 6 h to reduce the metal active components. Subsequently,when the reaction temperature was lowered to the reaction temperature,the synthesis gas was passed in and the reaction was carried out. The Fischer-Tropsch synthesis reaction conditions were set to H2/CO = 2,p= 2.0 MPa,t= 240 °C and WHSV = 2 L/(gcat·h). The products obtained from the reaction were collected by the hot trap (130 °C) and cold trap (10 °C),respectively,and the non-condensable gas was analyzed by gas chromatography (Agilent Technologies 7890A,60/80 Carboxen 1000 column)online.
2 Results and discussion
2.1 Crystalline phase and structure
The crystalline phases and structures of the fresh catalysts were analyzed by X-ray diffractometer and the results were displayed in Figure 1. Two broad diffraction peaks at around 24.5° and 43.0° can be clearly observed,which indicated the presence of large carbon grains and the carbon aerogels were assigned to the amorphous carbon. In addition,the absence of the diffraction peaks of Ru might probably be attributed to the low content and uniform dispersion of Ru nanoparticles on the surface of the carbon aerogels.Both the untreated and oxidized catalysts showed similar spectra,implying that the functionalization process did not alter the structure of the carbon aerogels support. To further investigate the effect of oxidative treatment of carbon aerogels on the catalysts FTS performance,XRD patterns of the spent catalysts were also performed. Diffraction peaks corresponding to SiO2appeared at 21.5°,23.9° and 29.42°,respectively,which was due to the inevitable admixture of the reduced catalysts into the quartz sand that filled the reaction tube during the recovery process. No diffraction peaks of Ru were observed,which was probably attributed to no aggregation from the low Ru loading.
2.2 Morphological study
In order to further analyze the morphological structure of the catalysts,TEM characterization of the catalysts was carried out. The TEM images of the reduced catalysts were presented in Figure 2(a),(c),and (e). The Ru nanoparticles were uniformly distributed on the CA support for the reduced catalysts.The particle sizes of Ru on the surface of Ru/CA,Ru/CA-H2O2,Ru/CA-HNO3catalysts were 1.01,0.88 and 0.86 nm,respectively. Furthermore,the oxidation treatment led to the decrease of Ru particle size on the CA surface. The TEM images of the spent catalysts were presented in Figure 2(b),(d) and (f). The particle sizes of Ru on the surface of Ru/CA,Ru/CA-H2O2,Ru/CA-HNO3catalysts after the reaction were 3.47,3.39 and 3.15 nm,respectively. It revealed that the particle size of Ru nanoparticles on the spent catalysts surface increased,which may be caused by the aggregation of Ru nanoparticles during the reaction.Moreover,the Ru nanoparticles on the surface of the spent Ru/CA-H2O2catalysts could maintain a high degree of dispersion (Figure 2(d)).
2.3 Graphitization degree
Raman spectra provided structural information about carbon materials. As shown in Figure 3,the two characteristic peaks were located at about 1350 and 1596 cm-1in the D and G bands,respectively. The D band (sp3carbon) was associated with graphene defects caused by pentagonal or heptagonal shapes and represented the surface defects and the degree of disorder in the carbon support,while the G-band corresponded to the in-plane stretching vibration ofsp2carbon atoms,indicating that the carbon support had a graphitic structure with regard to the order of C atoms.Seen from Figure 3 and Table 1,theI(D)/I(G) ratio decreased as follows: CA-H2O2(2.90) > CA-HNO3(2.72) > CA (2.61). The highI(D)/I(G) values of the functionalized samples could be attributed to the high level of defects due to the oxidation treatment that destroyed the surface graphite structure of the CA.
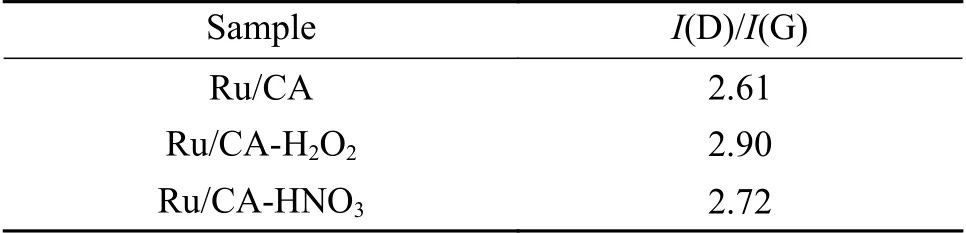
Table 1 Ratio of I(D) to I(G) of the CA-X supports from Raman spectra
2.4 Textural properties and elemental analysis
The physical adsorption and desorption behaviours of the fresh catalysts were performed by BET and the results were displayed in Figure 4 and Table 2. The adsorption and desorption isotherms of the catalysts showed no obvious difference,and all these displayed type IV Langmuir isotherms with H3 type hysteresis loop (Figure 4(a)),indicating that both functionalized and non-functionalized catalysts were typical mesoporous materials. The weave characteristics of the catalysts before and after oxidation treatment of carbon aerogels were essentially identical,further confirming that the oxidation treatment would not damage the physical structure of carbon aerogels. The reduction of catalysts pore size and the increase of pore volume after oxidation treatment implied that further functionalization of CA produced more pores,causing the functionalized catalysts producing higher surface area (from 531.42 m2/g up to 594.27 m2/g). This result was consistent with that from the Raman data that the oxidation treatment led to the destruction of the graphitic carbon structure and generated more defects.

Table 2 Textural properties and Ru elemental analysis of the Ru/CA-X catalysts
To further confirm the accomplishment of Ru loading,ICP was employed to determine the content of Ru metal elements in the catalysts,and the data were summarized in Table 2. The content of elemental ruthenium ranged between 4% and 5%,which was approximated to the theoretical content. The slightly lower than the theoretical content may be attributed to the loss of trace elements during the impregnation process.
2.5 Surface functional groups
Figure 5 depicted the FT-IR spectra of the catalysts samples before and after functionalization.The enhanced absorption of the functionalized catalysts at 1172 cm-1corresponded to the C-OH stretching vibration in phenols[25]. Moreover,compared to the FTIR spectrum of Ru/CA,enhanced absorption peaks can be distinctly observed on the functionalized catalysts at 1576 cm-1,corresponding to the aromatic ring vibration,and additionally at 1730 cm-1(C=O stretching vibration of the carboxyl or carbonyl group). The C=O absorption peak indicated the presence of carbonyl or carboxyl groups on the catalysts surface. Unfortunately,carbonyl and carboxyl groups were difficult to distinguish on FT-IR spectra. The absorption peak at 3440 cm-1was associated with the H-O stretching vibration of water adsorbed on CA. From the above analysis,it can be concluded that the samples before and after functionalization exhibited obvious absorption peaks of C-OH and C=O,which indicated the presence of certain content of oxygen-containing groups on the surface of carbon aerogels support.
2.6 Reduction behavior
H2-TPR was applied to probe the reduction behavior of the catalysts and explore the degree to which the active metal interacted with the support. TPR profiles of untreated and oxidation-treated carbon aerogels supported catalysts were depicted in Figure 6.All catalysts exhibited two reduction peaks between 200 and 300 °C,corresponding to a two-step reduction process from RuO2to Ru0. The H2consumption around 300 °C may be assigned to the reaction with carbon aerogels at high temperature to form methane[28].Compared to the Ru/CA catalyst,Ru/CA-H2O2exhibited two distinct reduction peaks and a significantly lower reduction temperature in the first step. It likely resulted from the enhanced content of oxygen-containing functional groups on the surface after the oxidation treatment,which improved the dispersion of the active metal on the surface and reduced the particle size of Ru nanoparticles,thus dropping the reduction temperature. Nevertheless,the reduction temperature of Ru/CA-HNO3obviously raised,which may be explained by the excessive interaction between the surface groups of the carbon aerogels support and Ru nanoparticles,leading to an elevation of the reduction temperature. The difference in the interaction between the support and the active metal probably accounted for the different performances of the catalysts.
2.7 Surface chemical states of the catalysts
The elemental composition and the chemical state of Ru were further determined by X-ray photoelectron spectroscopy (XPS). As shown in Figure 7(a),the XPS spectra confirmed the presence of all relevant elements(including C,O and Ru elements). In the C 1sspectrum,the binding energy peaks were located at 284.8,286.0,287.3,288.8,291.5 eV,respectively corresponding to graphitic carbon (C-C),carbonoxygen bond (C-O),carbonyl group (C=O),carboxyl group (-COOH),and carbon in aromatic compounds(π-π*)[24,29,30]. The spectra around 281.0 and 284.0 eV were derived from Ru 3dand rarely applied for spectroscopic analysis due to the interference with C 1s[30]. The O 1sspectrum can be deconvoluted into five peaks,at 531.4 eV (C=O),532.5 eV (C-OH),533.4 eV(-O-),534.8 eV (-COOH),and 536.4 eV (H2O),respectively[29]. The relative contents of the groups and the surface O/C ratios calculated by peak fitting were listed in Table 3. Both oxidants enhanced the surface oxygen content,with HNO3exhibiting the highest degree of oxidation up to the highest O/C (0.36→0.52).The types and contents of the generated oxygencontaining functional groups varied,which naturally had distinct effects on Ru anchoring,electronic structure and thus led to different FTS reaction performances. Nevertheless,the oxygen-containing functional groups might be removed during the hightemperature reduction process. Therefore,we further performed XPS characterization of the reduced catalysts. Figure 7(e) and f illustrated the C 1sand O 1sspectra of the reduced catalysts,respectively. The C 1sand O 1sspectra indicated that a high content of oxygen-containing functional groups were still present on the catalyst surface after reduction. Therefore,the oxidation treatment is effective,and the oxygencontaining functional groups added by this procedure were still well retained after the high temperature reduction process. The Ru 3pspectrum had two spinorbit splitting of Ru 3p3/2and Ru 3p1/2(Figure 7(d)).Specifically,the peaks at 463.2 and 485.4 eV can be attributed to Ru3+on the surface[22,31]. The Ru 3ppeaks of the oxidized catalysts were shifted to higher binding energy compared to the Ru/CA catalyst,indicating a strong electronic effect between Ru and the functionalized carbon aerogels. In particular,Ru/CAHNO3exhibited the highest binding energy,indicating the strongest interaction between Ru and CA-HNO3support,which was consistent with the findings of H2-TPR. The functionalized carbon aerogels contained more oxygen-containing functional groups,which were beneficial for improving the stability of the catalysts.The support surface groups provided more nucleation sites for the deposition of ruthenium nitrate and facilitated the reduction of agglomeration of Ru nanoparticles at higher reaction temperatures.
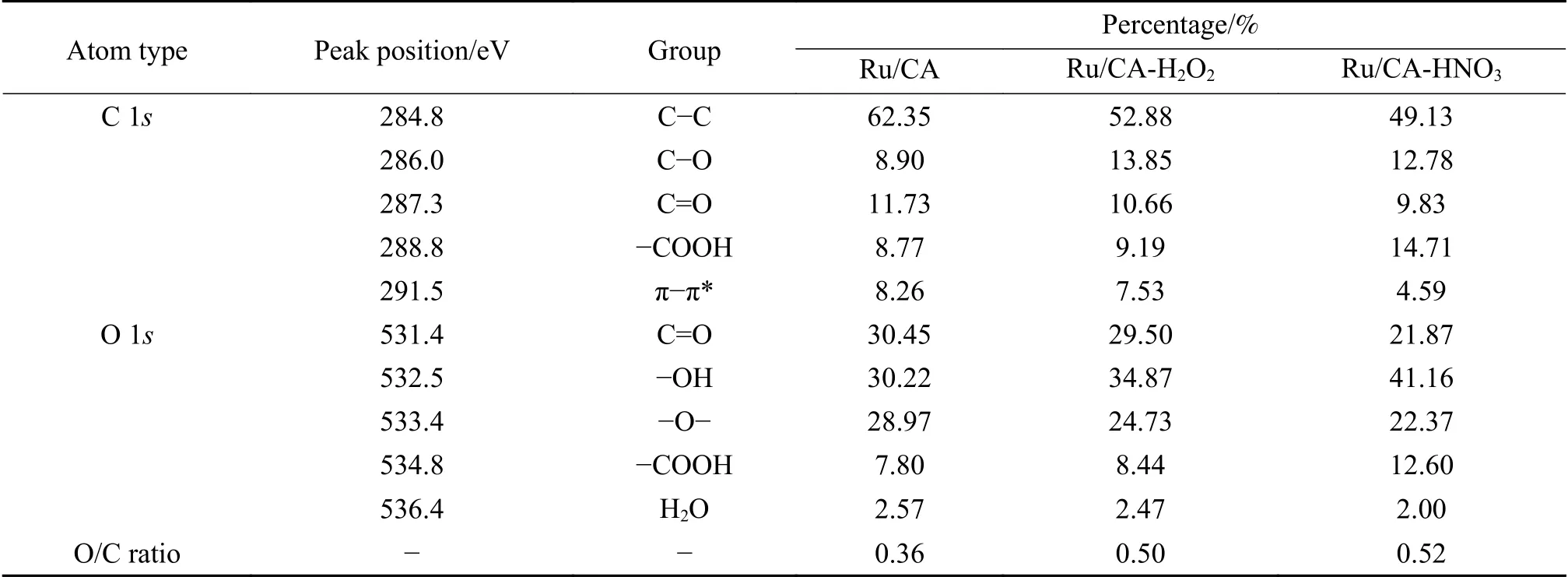
Table 3 Concentrations of surface oxygen groups of the Ru/CA-X catalysts
2.8 Catalytic performance for FTS
The Fischer-Tropsch synthesis performances on Ru/CA-Xcatalysts were shown in Figure 8. The Ru/CA catalyst exhibited the highest initial activity,whereas the CO conversion rate dropped dramatically(62.24%-37.63%) within 120 h. In comparison,the functionalized catalysts exhibited excellent stability.This can be attributed to the fact that the oxidation treatment increased the defects in the carbon aerogels,producing more pores with a higher specific surface,which suggested that the oxidation-treated supports increased the anchoring points of the Ru particles. The functionalized carbon aerogels had an increased concentration of oxygen containing groups on the surface and enhanced the interaction of Ru with the support,thereby diminishing the agglomeration of Ru nanoparticles during the reaction.
In addition,the Ru/CA-H2O2catalyst exhibited high activity and the CO conversion was maintained around 50%,while the CO conversion of the HNO3-treated catalyst was only around 35%. This may be attributed to the fact that H2O2,as a mild oxidant,moderately increased the oxygen containing group concentration on the carbon aerogels surface and enhanced the interaction between Ru and the support.The Ru/CA-H2O2catalyst maintained high activity,high C5+selectivity and low CH4and CO2selectivity.In contrast,the HNO3oxidation treatment lead to an excessive interaction between Ru and the support,resulting in lower activity and C5+selectivity[32,33]. Based on the above results,H2O2treatment proved to be the most effective surface oxidation method for carbon aerogels supports,which moderately increased the concentration of oxygen-containing groups generated on the surface.
3 Conclusions
In summary,we report a method to modulate the Fischer-Tropsch synthesis performances of carbon aerogels-loaded Ru catalysts by adjusting the concentration of oxygen-containing groups on the surface. The oxidizing agents such as HNO3and H2O2can improve the stability of the catalysts by raising the concentration of surface defects and oxygen-containing groups,and reinforcing metal-support interactions. The Ru/CA-H2O2catalyst exhibited the best activity and selectivity,which was attributed to the moderate oxidation ability,allowing the Ru nanoparticles to exhibit appropriate interactions with the carbon aerogels support. This study provides a valuable insight for tuning the performances of carbon aerogels Rubased Fischer-Tropsch synthesis catalysts.

