Highly dispersed CoPx nanoparticles supported on carbon cloth for the enhanced catalytic performance of methanol electro-oxidation
ZHANG Jian-yuan,XING Shuang-feng,ZHAO Shi-chao,XIONG Mi,ZHANG Bian-qin,TONG Xi-li,QIN Yong,GAO Zhe,*
(1. State Key Laboratory of Coal Conversion, Institute of Coal Chemistry, Chinese Academy of Sciences, Taiyuan 030001, China;2. Center of Materials Science and Optoelectronics Engineering, University of Chinese Academy of Sciences, Beijing 100049, China)
Abstract: Direct methanol fuel cell (DMFC) is a potential commercial fuel cell technology that is presently hindered by the expensive noble metal materials of the anode. Developing a method to obtain a uniformly dispersed metal phosphide catalyst with narrow size distribution is still a challenge. In this work,cobalt oxide was deposited on carbon cloth (CC) through atomic layer deposition (ALD),then cobalt phosphide was obtained after the phosphorization process. By changing the number of ALD-based ozone pulses (ALD-O3) for CC,the nucleation and growth modes of cobalt oxide (ALD-CoOx) on the CC were regulated,and CoPx nanoparticles with small particle size and uniform distribution were obtained. The optimized CoPx-based catalyst with 40 cycles of ALD-O3 treatment (CoPx/40-CC) exhibits excellent activity (153 mA/cm2) toward methanol electrocatalytic oxidation reaction in the alkaline solution,which is higher than the catalyst prepared by impregnation (Imp-CoPx/CC),although the CoPx loading of CoPx/40-CC is lower than that of Imp-CoPx/CC. The results indicate that the enhanced activity benefits from the small particle size and the uniform CoPx distribution,which promote the electron-transfer and mass transport kinetics of the methanol electro-oxidation process.
Key words: atomic layer deposition;cobalt phosphide nanoparticles;methanol electrocatalytic oxidation reaction
Considering the energy crisis and global warming caused by fossil fuel usage,direct methanol fuel cells(DMFCs) may serve as a potential green energy conversion technology,because methanol can be easily obtained,stored,and transported[1,2]. However,commercial catalysts such as platinum-based noble metal catalysts are expensive and easy to poison by CO,which considerably hinders the applications of DMFCs[3-7]. Therefore,it is essential to design high efficiency and low-cost electrocatalysts with long-term stability for the methanol electrocatalytic oxidation reaction (MOR) for the largescale commercialization of DMFCs in the future.
Non-noble metal catalysts,such as oxides and alloys provide certain advantages because of the inexpensiveness and relatively non-toxicity,however,they are limited by their low activity[8-11]. Non-noble metal phosphide performance for the MOR offers promising electrocatalytic activity,owing to their specific electronic structure,long-term stability,and wide pH application range[8,12-16]. Recently,several methods have been reported for the fabrication of various nanostructures metal phosphides,such as nanoarrays,nanowall arrays,and hollow porous nanostructures to obtain a large surface area and potential electrocatalytic activity[17-19]. However,it is still a challenge to synthesize small and uniformly dispersed metal phosphide nanoparticles for high methanol electro-oxidation activity.
Owning to the precisely control of the size/thickness and perfect uniformity[20-23],atomic layer deposition (ALD) is a powerful technique for depositing single atoms,nanoparticles,thin films,as well as catalytic materials at the atomic level[23-26]. In this work,ALD was introduced to synthesize CoOxnanoparticles on carbon cloth (CC),and then NaH2PO2was used as a phosphorization reactant to obtain CoPxnanoparticles.By controlling the number of ALD-O3pulses in the pretreatment process,the defect sites on the CC surface were modified. As a result,the nucleation and growth of the nanoparticles during ALD-CoOxsynthesis were tuned. For the MOR reaction,the CoPx-based catalyst with 40 cycles of ALD-O3treatment (CoPx/40-CC)presents the highest current density (153 mA/cm2)among all the catalysts prepared by ALD and impregnation. Catalytic activities also change with the number of ALD-O3pulses,resulting in volcano-like behavior. Detailed analyses suggest that the enhanced activity benefits from the small particle size and the uniform CoPxdistribution,which promote the electrontransfer and mass transport kinetics of the methanol electro-oxidation process.
1 Experimental section
1.1 Chemicals
The ALD precursor of bis (cyclopentadienyl)cobalt (Cp2Co,98%) was obtained from Alfa Aesar,and the O3precursor was obtained by an ozone generator. The CC (WOS1011) was purchased from Cetech Co.,Ltd.,NaH2PO2was acquired from Shanghai Aladdin Bio-Chem Technology Co.,Ltd.,and cobaltous nitrate hexahydrate (Co(NO3)2·6H2O) was obtained from Sinopharm Chemical Reagent Co. ,Ltd.All chemical reagents were used as received,and all aqueous solutions were prepared using deionized water,which was produced by an ultrapure water system.
1.2 Catalyst synthesis
Before ALD,the raw CC was cut into 1 cm × 4 cm pieces and was then cleaned by ultrasonication for 30 min. Afterward,the samples were washed with deionized water and anhydrous ethanol three times,respectively. Finally,the CC pieces were dried in an oven at 80 °C for 1 h.
The ALD process was carried out in a hot wall and closed chamber. In the first step,several cycles of O3pulses were used to treat the CC at 250 °C,with pulse,exposure,and purge times of 1,15 and 27 s,respectively. The samples were denoted asn-CC (nrefers to the number of ALD-O3cycles)[27]. Then,the CoOxnanoparticles were deposited onto the CC with the Cp2Co and O3precursors,which were denoted as CoOx/n-CC[28]. The reaction equation to form CoOxis as follows[29]:
During the reaction process,the temperature of Cp2Co was maintained at 70 °C,accompanied by valving the parameters of pulse,exposure,and purge times of 0.5,16 and 25 s,respectively. In addition,the time for the corresponding O3precursor treatment was 0.1,12 and 30 s,respectively. Afterward,the CoOx/n-CC samples were added into the combustion boat with 0.5 g of NaH2PO2·H2O,then the temperature was increased from room temperature to 300 °C at 2 °C/min in the tubular furnace in an Ar atmosphere and finally maintained for 120 min. In the end,the samples were washed with deionized water and ethanol three times and dried in an oven at 80 °C for 30 min.
Synthesis process for the impregnated sample,denoted as Imp-CoPx/CC,was as follows. The clean CC was soaked with 5.5% cobalt nitrate ethanol solution in a beaker and stirred for 30 min. Then,the sample was dried in the oven for 30 min and placed in a furnace,with a programmed heating rate of 2 °C/min from room temperature to 300 °C. Then the temperature was maintained for 180 min at 300 °C to obtain Imp-CoOx/CC. Finally,the Imp-CoOx/CC sample was used for the same phosphide process as the ALD samples.
1.3 Materials characterization
Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images were collected using a JEOL-2100F microscope. For TEM analysis,the samples were physically crushed with scissors and a mortar,and then dispersed in ethanol solution to prepare a highly dispersed suspension. A small amount of liquid was dropped onto the microgrid and allowed to dry naturally at room temperature. X-ray diffraction(XRD) patterns were obtained using a Bruker D8 Advance X-ray diffractometer with CuKα radiation(λ= 1.540 nm),and 2θwas ranging from 5° to 90°. The X-ray photoelectron spectra (XPS) were obtained by an ES-300 photoelectron spectrometer (KRATOS Analytical) with AlKα excitation (1486.6 eV). Co and P content in the samples were determined by inductively coupled plasma optical emission spectrometry (ICP-OES) analysis (Thermo ICAP 6300),and the samples were annealed at 800 °C to remove the CC before ICP-OES analysis.
1.4 Electrochemical measurements
All electrochemical measurements were performed on a CHI760D electrochemical workstation(Shanghai,China). The conventional three-electrode system was equipped with a graphite rod electrode as the counter electrode and a saturated calomel electrode(SCE,saturated KCl solution with a salt bridge) as the reference electrode. For the self-supporting electrode,CoPx/n-CC or Imp-CoPx/CC on a glassy carbon electrode clip was used as the working electrode(0.50 cm × 1.0 cm). Cyclic voltammetry (CV) and chronoamperometric measurements were conducted in 1 mol/L methanol + 1 mol/L KOH solution to study the activity and stability of catalyst. Linear sweep voltammetry (LSV) measurements were performed in 1 mol/L KOH solution with or without the addition of 1 mol/L methanol to evaluate the MOR and the oxygen evolution reaction (OER). Electrochemical impedance spectroscopy (EIS) was measured at a potential of 1.48 V(vs RHE) in 1 mol/L methanol + 1 mol/L KOH solution in a frequency range from 10 kHz to 0.1 Hz.Additionally,all experiments were tested at (25 ± 2) °C,and the solutions were exposed to the air. The current densities are given in terms of geometrical area(mA/cm2).
2 Results and discussion
2.1 Characterization of catalysts
Figure 1 shows a schematic of CoPx/n-CC preparation by ALD. The CC was treated with different cycles of O3pulses by ALD to achieven-CC,where n indicates the number of O3pulses. Then,then-CC samples were deposited through 200 ALD cycles of CoOxto obtain CoOx/n-CC. The CoOx/n-CC samples were further treated with NaH2PO2to synthesize CoPx/n-CC.
The morphology and microstructure of the CoOx/n-CC catalysts were examined by TEM and HRTEM. As shown in Figure 2(a)-(c),the particle sizes of CoOx/10-CC,CoOx/40-CC,and CoOx/75-CC are 5.1,3.3 and 1.9 nm,respectively. The particle size of CoOxdecreases with an increasing number of ALDO3cycles. Additionally,the distribution of CoOxchanges with the number of ALD-O3cycles. The CoOxof CoOx/10-CC and CoOx/40-CC consist of particle films,while CoOxof CoOx/75-CC consists of dispersed particles. The measured lattice distances of CoOxin CoOx/40-CC are 0.209,0.286 and 0.246 nm (Figure 2(d)),which correspond with the (400),(220) and (311)planes of Co3O4,respectively.
After the phosphorization reaction,CoPx/10-CC,CoPx/40-CC and CoPx/75-CC were synthesized,and the morphologies of these samples are shown in Figure 2(e)-(g). The particle sizes of CoPx/10-CC,CoPx/40-CC and CoPx/75-CC are 10.0,5.2 and 8.2 nm,respectively. The size of CoPxof CoPx/n-CC is larger than the corresponding cobalt oxide sample (CoOx/n-CC).CoOx/75-CC has the smallest CoOxparticle size among CoOx/10-CC,CoOx/40-CC and CoOx/75-CC,while CoPx/40-CC possesses the smallest particle size among CoPx/10-CC,CoPx/40-CC and CoPx/75-CC. The smallest CoOxparticles do not produce the smallest CoPxparticles after phosphorization. Additionally,CoPx/40-CC exhibits the most uniform dispersion and narrow size distribution among these catalysts. ICPOES analysis indicates that the loading amounts of CoPxin CoPx/10-CC,CoPx/40-CC and CoPx/75-CC are 0.79%,0.76% and 0.6%,respectively. Figure 2(h)presents the HRTEM of CoPx/40-CC,which shows that the measured lattice distances of CoPxare 0.247 and 0.254 nm,which correspond to the (111) and (200)CoP planes,respectively.
The morphologies of the samples prepared by the impregnation method are shown in Figure 3(a) and (b).The CoOxsize of Imp-CoOx/CC is 10.7 nm,while the CoPxsize of Imp-CoPx/CC is 18.9 nm. No uniform nanoparticle distribution and morphology are observed in Imp-CoOx/CC and Imp-CoPx/CC and ICP-OES analysis indicates that the loading amount of CoPxis 3.2% for Imp-CoPx/CC.
Figure 4 shows the X-ray crystal diffraction(XRD) spectra of CoPx/10-CC,CoPx/40-CC,CoPx/75-CC and Imp-CoPx/CC,where the peaks at 22.8° and 43.2° represent graphite carbon[30]. The peaks of Imp-CoPx/CC at 2θof 31.6° and 48.1° are designated as(011) and (211) facets of CoP (PDF#29-0497)[31].However,there are no obvious CoPxpeaks in the samples prepared by ALD (CoPx/10-CC,CoPx/40-CC,and CoPx/75-CC),owing to low content and/or high dispersion of CoPx.
XPS was performed to analyze the surface chemical states of CoPx/10-CC,CoPx/40-CC,CoPx/75-CC,and Imp-CoPx/CC. For all samples,the peaks locate at 779.0 and 794.0 eV in the high-resolution spectrum(Figure 5(a)) are assigned to metallic Co 2p3/2and 2p1/2of CoPx,respectively[31,32]. An obvious peak at 781.4 eV is attributed to Co2+2p3/2of Imp-CoPx/CC;however,it is inconspicuous in CoPx/10-CC,CoPx/40-CC,and CoPx/75-CC[31]. This indicates that Imp-CoPx/CC contains more Co2+than the other samples. The high-resolution P 2pspectra are shown in Figure 5(b).The CoPx/10-CC,CoPx/40-CC,CoPx/75-CC and Imp-CoPx/CC have prominent peaks at 130.4,130.5,130.2 and 130.4 eV,which are ascribed to phosphide P 2p3/2,and the peaks at 131.0,131.3,131.0 and 131.2 eV are attributed to P 2p1/2[32]. P in CoPx/40-CC shows the highest binding energy of all the ALD samples. In all of the samples,the weak peak around 134 eV is assigned to the contamination of phosphate P on the catalyst surfaces[33].
2.2 Electrochemical activity of the catalysts
The electrocatalytic performances of the asprepared catalysts (CoPx/n-CC and Imp-CoPx/CC) for MOR were tested in 1 mol/L methanol + 1 mol/L KOH solution. The electrocatalytic activities of CoPx/n-CC(n= 0,10,20,30,40,50 and 75) and Imp-CoPx/CC were first studied by CV at a scan rate of 100 mV/s within a potential range of 1.068-1.868 (V vs RHE) in the 1 mol/L KOH and 1 mol/L methanol electrolyte. CoPx/40-CC shows the highest current density (153 mA/cm2at 1.7 V vs RHE) and the lowest overpotential (1.39 V at 10 mA/cm2) among all catalysts,revealing the highest electrooxidation activity of MOR (Figure 6(a)). Also,the current densities of the electrocatalysts at 1.7 V (vs RHE) in the positive sweep of the CV curves are summarized in Figure 6(b). With increasing ALD-O3cycles,the catalytic activities of CoPx/n-CC exhibit a volcano-like trend. In addition,CoPx/40-CC exhibits higher electroactivity than Imp-CoPx/CC (107 mA/cm2),although the CoPxloading of CoPx/40-CC (0.76%) is lower than that of Imp-CoPx/CC (3.2%). And CoPx/40-CC shows the excellent MOR electroactivity compared to other catalysts reported in the literatures (Table 1).
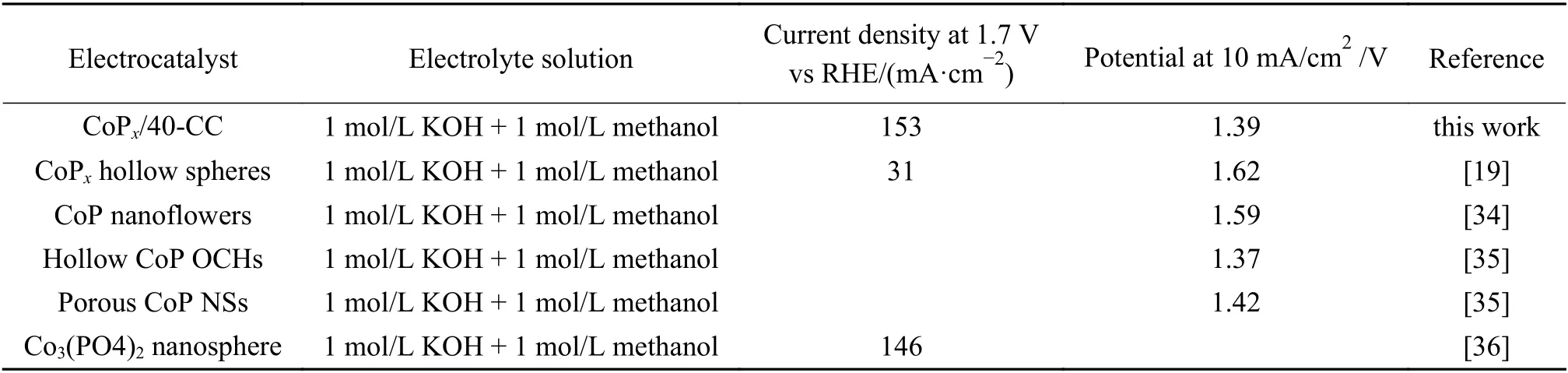
Table 1 Comparison of the MOR performance of CoPx/40-CC with those of other catalysts reported in the literatures.
For MOR kinetics,the electrochemical impedance spectroscopy (EIS) results of the MOR on the CoPx/n-CC and Imp-CoPx/CC electrodes are shown in Figure 6(c).The electron-transfer resistance of the MOR decreases when the ALD-O3cycle increases from 0 to 40 and then increases when the ALD-O3cycle increases from 40 to 75,which is consistent with the CV results shown above. CoPx/40-CC shows the lowest electron-transfer resistance,suggesting the best improvement of electron-transfer kinetics in all the electro-catalysts.CoPx/10-CC,CoPx/40-CC,CoPx/75-CC,and Imp-CoPx/CC were selected for Tafel analysis. As shown in Figure 6(d),the CoPx/40-CC electrode exhibits the lowest Tafel slope (163 mV/dec),indicating that CoPx/40-CC has low electron and mass transport barriers in the MOR process.
To further investigate the catalytic performance of CoPx/40-CC,linear sweep voltammograms (LSVs)were measured at a scanning rate of 5 mV/s (Figure 6(e))in the absence/presence of 1 mol/L methanol in 1 mol/L KOH. CoPx/40-CC shows approximately 200 mV negative potential of MOR compared to the oxygen evolution reaction,revealing the favorable electrochemical response of CoPx/40-CC for the MOR and the increase of current is due to the addition of methanol. Furthermore,Figure 6(f) is CV curves of CoPx/40-CC at different scanning rates. The inset of Figure 6(f) shows that CoPx/40-CC exhibits a linear relationship between the current densities values at 1.7 V vs RHE and the square root of the potential scan rates,indicating that the MOR on the surface of this catalyst is a diffusion-controlled process.
To investigate the stability of the four catalysts,CoPx/10-CC,CoPx/40-CC,CoPx/75-CC,and Imp-CoPx/CC,chronoamperometric (CA) measurements were conducted in 1 mol/L KOH and 1 mol/L methanol at 1.57 V (vs RHE) for a duration of 10000 s. The results are displayed in Figure 6(g). CoPx/40-CC exhibits the highest catalytic activity after 10000s among four samples.
Finally,the electrocatalytic activities ofmCoPx/40-CC (mrefers to the number of ALD-CoOxcycles,m= 0,50,100,150,200,250 and 300) were studied by CV under the same conditions to investigate optimal Co loading. The CV curves (Figure 6(h)) and bar graph(Figure 6(i)) suggest that CoPx/40-CC (200 cycles of CoOx) is the best electrocatalyst for MOR,and the activities of these catalysts show volcano-like behavior.
2.3 Discussion
The results show that enhanced MOR catalytic performance of CoPx/n-CC can be obtained by changing ALD-O3cycles in CC pretreatment. The high catalytic activity of CoPx/40-CC nanocatalyst benefits from the small particle size and the uniform CoPxdistribution. The ALD-O3pretreatment produces oxygen containing functional groups on CC,which act as the nucleation sites of CoOx[37]. When the number of ALD-O3pulses is only 10,the nucleation sites on CC are insufficient. CoOxnanoparticles grow larger at these nucleation sites. With the increase of ALD-O3cycles,more nucleation sites on CC are obtained. The size of CoOxnanoparticles is decreased and the dispersion of CoOxnanoparticles on support is improved. During the phosphorization process,CoOxnanoparticles are transformed into CoPxnanoparticles,and the particles grow up. Due to the optimal dispersion of as-prepared CoOxparticles and the suitable interaction between the particles and support,after phosphorization,the CoPxnanoparticles of CoPx/40-CC exhibit small particle size and uniform distribution,which promote the electron-transfer and mass transport kinetics of MOR reaction.
3 Conclusions
In summary,ALD was used to pretreat CC with O3pulses and to deposit CoOxnanoparticles on CC. By optimizing the number of ALD-O3pulses,the nucleation and growth modes of CoOxwere regulated.After phosphorization,CoPxnanoparticles on CC with uniform dispersion and narrow size distribution were successfully prepared. The results show that the CoPx/40-CC nanocatalyst corresponds to an average CoPxnanoparticle size of 5.3 nm,and exhibits the best catalytic activity (153 mA/cm2at 1.7 V vs RHE) among all of the CoPx/n-CC and Imp-CoPx/CC synthesized in this work. The current density of the catalysts changes as a function of the ALD-O3pulse number and exhibits volcano-like behavior. Overall,ALD could provide an alternative approach for non-noble catalysts to enhance the catalytic performance of the MOR.

