L-arginine alleviates postmenopausal complications in female rats by stimulating ovarian dopamine beta hydroxylase
Fatemeh Lakzaei, Manizheh Karami✉, Mohammadreza Jalali Nadoushan
1Department of Biology, Faculty of Basic Sciences, Shahed University, Tehran, Iran
2Department of Pathology, Faculty of Medicine, Shahed University, Tehran, Iran
ABSTRACT
Objective: To evaluate the levels of estrogen, albumin and gonadotropins (luteinizing hormone, follicle-stimulating hormone)as well as the activity of dopamine beta hydroxylase (DAβ H) in aged female rats treated with nitric oxide precursor L-arginine and neuronal nitric oxide synthase antagonist L-NAME.
Methods: A total of 224 Wistar rats (36 weeks old, weighing 250 g) based on a random sampling were divided into the control and experimental groups after Pap smear test. The control group received only saline (1 mL/kg) intraperitoneally (i.p.). The experiential groups were treated with L-arginine (5, 25 and 50 mg/kg, i.p.) and L-NAME (5 and 25 mg/kg, i.p.) for 3 to 21 days, once a day. Blood samples were taken from the rats and the levels of estrogen and albumin and gonadotropins in the serum were monitored by enzyme-linked immunosorbent assay kit, and the ovaries were examined immunohistopathologically for DAβH activity.
Results: L-arginine (5 mg/kg) significantly increased estrogen level (P<0.05), which was associated with DAβH activation in the ovaries. L-NAME reduced this effect when administered prior to L-arginine dose. L-arginine caused no significant change in the levels of luteinizing hormone and follicle-stimulating hormone. Except for the lowest dose of L-arginine in the shortest period, albumin levels significantly decreased in other treatments compared to the control group (P<0.05).
Conclusions: L-arginine is likely to reduce postmenopausal problems due to an increased nitric oxide level.
KEYWORDS: Postmenopausal; Estrogen; Albumin; Dopamine beta-hydroxlase; L-arginine; Nitric oxide
1. Introduction
Many aging disorders including deep vein thrombosis[1] in females are associated with ovarian dysfunction, especially decreased estrogen levels during menopause (occurrence of reproductive aging) and decreased albumin levels in these conditions[2].
Nitric oxide (NO) has significant physiological and pathophysiological roles such as the relaxation of vascular structures and reduction of elevated blood pressure, inflammation and oxidative stress, and neurological functions and disorders[3-5]. It is a gaseous molecule produced by three different isoforms of NO synthase (NOS) and is involved in a wide range of physiological and pathophysiological processes in the body[6]. This enzyme (NOS) produces NO from the oxidation of L-arginine in the presence of molecular oxygen and nicotinamide adenine dinucleotide phosphate (NADPH) in vascular endothelial cells and tissues, including the ovaries. Regulation of gonadotropin, ovulation, sperm capacitance and ejaculation are some of the functions of this molecule in the reproductive system.NO levels appear to be regulated by endogenous estrogens because higher levels of NO have been reported in the follicular phase than in the luteal phase of the female cycle[7].
Due to the fact that many disorders of the reproductive tract in old age may be due to decreased levels and function of sex hormone(estrogen) and its carriers (albumin, a well-known sex hormonebinding globular protein that is mainly produced by the liver and is involved in the turnover of sex hormones in animals)[8], this study first aimed to answer that whether there is a meaningful correlation between NO and estrogen and albumin levels or not. It should be noted that the gonads are part of the reproductive axis. The hypothalamic-pituitary-gonadal axis (reproductive axis) plays an important role in the normalization of the level of gonadotropins.Catecholamines are neurotransmitters that have several types of receptors in the gonads and endocrine cells. Their activation,depending on the target cell, can alter blood flow, steroidogenesis and gene expression, as well as gonadotropin levels[9].
Alpha and beta adrenergic receptors have been reported to be found in theca and granulosa cells. Also, high levels of dopamine receptors are found in the human ovaries. Evidence for dopamine receptors in granulosa cells has also been presented[10]. Decreased dopamine levels due to inhibition of tyrosine hydroxylase activity indicate that dopamine is required for normal maturation and ovarian fertility[11].NO, one of the smallest synthesized molecules in the biological system[12], is of great importance in the regulation of ovarian function[13] including the secretion of luteal cells due to adrenergic factors[14]. But does NO regulate the enzyme dopamine beta hydroxylase (DAβH) which produces these factors? This glycoprotein enzyme with a molecular weight of 290 000 is found in catecholamine storage vesicles in chromaffin cells and adrenergic units in the peripheral and central nervous system, where it synthesizes noradrenaline from dopamine[15]. Can NO levels increase DAβH enzyme activity in the ovary, and can the increase in the level of estrogen and albumin be related to the increase in the activity of this enzyme in the ovaries?
2. Materials and methods
2.1. Animals
In this study, 224 virgin Wistar rats weighing approximately 250 g, and aged at 36 weeks were obtained from Pasteur Institute of Iran and kept under standard conditions in accordance with the Declaration of Helsinki. The rats were maintained under (20-25) ℃ and light cycles of 12 h with free access to water and food ad libitum.
The sample size was calculated based on the value of "E" which is the degree of freedom of variance analysis. The value of E should be between 10 and 20. E was measured by the following formula[16]:
E = total number of animals - total number of groups
According to this formula, the size of each sample was 6 rats and the total number was 240 rats. However, we had mortality of rats(especially during the 21 day period), so the actual total number was 224 rats.
2.2. Pap smear and female cycle
In order to determine the stages of the rat’s female cycle, the vaginal smear was taken by using the sterile dropper (Pasteur pipette), and the samples were fixed on the slide with 3% ethanol.After this step, the stabilized samples were washed with running water for 5 s and then stained with hematoxylin for about 2 min.After this time, the samples were immediately rinsed with running water, and after exposure to 3% ethanol (3 s), they were stained with Orange G6 dye to differentiate the cytoplasm. After that, the samples were immersed in 3% ethanol. Then, the clarification was performed in 4 steps with xylene. At the end, the samples were mounted on the slide with the aid of Entellan (Merck, Germany) and examined with a light microscope (Olympus, Japan) at 40× magnification.
2.3. Grouping and treatments
Regarding the smears, animals in the diestrus cycle were specifically selected. This smear preparation also confirmed that the virgin rats at this age are actually old. Because, as already been stated in the documents[17], the older animals are in the stage of diestrus. These animals were classified into the control and treatment groups.
The control group received intraperitoneal (i.p.) injection of saline (1 mL/kg), the physiological serum (0.9%), throughout the procedure (3, 5, 9, and 21 days). The treatment groups were divided into the L-arginine dose groups and the NG-nitro-L-arginine methyl ester (L-NAME) plus L-arginine dose groups. Regarding previous findings[18,19], the L-arginine dose groups received different doses of L-arginine (5, 25, and 50 mg/kg) for several time periods (3, 5, 9,and 21 days, i.p., once per day)[18,19]. The L-NAME plus L-arginine groups were first exposed to L-NAME (5, and 25 mg/kg) and after 20 min, L-arginine (5, 25, and 50 mg/kg) was administered during the time periods (3, 5, 9, and 21 days, i.p., once per day).
According to the current experimental plan, first, a 3-day treatment with the lowest dose of L-arginine (5 mg/kg, i.p., once a day) was performed, then other doses (25 and 50 mg/kg) were examined in this period, and finally, longer periods (5, 9, and 21 days) from low dose to higher doses were studied. Six rats per treatment dose were used for each time period.
2.4. Serology
Twenty four hours after the last injection, blood samples from the hearts of rats (under anesthesia with ketamine 100 mg/kg and xylazine 20 mg/kg) were taken, and after half an hour centrifuged at 2 000 ×g for 10 min to separate the blood clot and serum, and the serum samples were stored in the freezer (-80 ℃). These samples were finally evaluated by measuring the serum concentrations of estrogen(Abcam, UK), albumin (Abcam, UK), follicle stimulating hormone(FSH) [LSBio, USA; detection range: 0.625–40 ng/mL, sensitivity:typically less than 0.375 ng/mL, intra-assay CV (<11.07%); interassay CV (<9.57%)] and luteinizing hormone (LH) [LSBio, USA;detection range: 0.313–20 U/mL, sensitivity: typically less than 0.188 U/mL, intra-assay CV (<15%); inter-assay CV (<15%)] using enzyme linked immunosorbent assay kits.
2.5. Pathology
After blood sampling, a longitudinal incision was made in the rat’s abdominal surface and ovaries were biometrically examined. At the end, the tissues were collected in 10% formalin. These tissues after 48-72 h were cut in sections (3-4 µm) by a rotary microtome (Leica,Germany) and then stained by hematoxylin-eosin and bromocresol purple. The slices were eventually evaluated and compared with the control.
2.5.1. Hematoxylin-eosin staining
First, the fixed sample (with the help of 10% formalin) entered the xylol bath (3 times, each for 5 min) and alcohol solutions with decreasing degree (4 times, each for 1-2 min) and then washed with distilled water. After that, the sample were immersed in hematoxylin 20% (10-15 min), washed with distilled water, and placed in acidalcohol solution. It was then passed through a solution of calcium carbonate (for differentiation) and immersed in eosin (for a few seconds). It was subsequently rinsed with distilled water, immersed in alcohol (4 times, with increasing degree, 15-30 s each), cleared with xylol (2 times, 3-5 min each), and finally mounted (with the help of Entellan), and finally covered.
2.5.2. Bromocresol purple staining
The instructions for preparing this dye were as follows: 0.5 g of bromocresol powder should be dissolved in 0.92 mL of 0.1 M sodium hydroxide and 20 mL of ethanol (95%) and then its volume should be increased to 100 mL with distilled water. Tissue samples were first deparaffinized and washed with various alcohols and then placed in bromocresol purple stain. After 6 to 7 h, the slides were dehydrated with different alcohols and the image was observed under a microscope (Olympus, Japan) at 40× magnification.
2.6. Immunohistochemical findings
First, ovarian samples were prepared from animals and fixed in formalin and molded in paraffin, and then deparaffination and hydration steps were followed. The slides were then placed in a citrate buffer, covered in a container, and placed in a 600-Watt microwave oven for 20 min. The samples were then placed in the laboratory for 20 min to cool. The slides were then placed in 0.03% Tris-buffered saline (TBS) and Triton X-100 twice for 5 min. They were then washed with normal saline or 1% bovine serum albumin in TBS for 2 h at room temperature. The slides were subsequently washed for 3 min in 0.03% TBS and Triton X-100. Then, the slides were placed in a dark room to inhibit peroxidase: 1 mL H2O2+ 9 mL ddH2O (double distilled water) for 10 min. They were then tested with primary antibodies under these conditions: 4 h at 37 ℃ and overnight at 4 ℃. The next steps were as follows:
The slides were washed 3 times each for 5 min with TBS plus 0.03% Triton X-100, then incubated with streptoavidin-horseradish peroxidase (enzyme) (pink) secondary antibody for 10 min in vitro,and the slides were then rinsed with TBS plus 0.03% Triton X-100 three times each for 1 min, followed by 3, 3 -diaminobenzidine(DAB) exposure (1 µL chromogen DAB + 20 µL DAB substrate)for 10 min, then washed three times each time for 1 min with TBS plus 0.03% Triton X-100 , and immersed in hematoxylin for 2 min at laboratory temperature, then dehydrated in 80%, 90% and 96% alcohols (for 5 min) and 100% alcohol (3 times), and then finally cleared two times in xylene for 30 min. At the end, the slides were glued with Entellan (Merck, Germany) and covered with coverslip.Immunohistochemistry scoring was also provided using an image analyzer (ImageJ, free, version 4.1.) and was shown as the difference of positive reaction to the specific test in the experimental group compared to the control group.
2.7. Statistical analysis
All data were analyzed using SPSS software (version 21, IBM SPSS Statistics). First, the normality of the data was checked with the Kolmogorov Smirnov test, and if confirmed, the data were calculated with one-way analysis of variance (ANOVA). Turkey’s post hoc analysis was used to examine the differences between the groups. The data were shown as mean±standard deviation(mean±SD). A P value <0.05 was considered to be statistically significant.
2.8. Ethics statement
This study was approved by the Ethics Committee of Shahed University (IR.SHAHED.REC.1399.106), and all experiments were conducted in accordance with the principles of the Helsinki Declaration.
3. Results
3.1. Pap smear
Pap smears were obtained from all rats to examine the cycle, and only those animals that were in the diestrus stage were specifically selected. It should be noted that small leukocytes are predominant in this phase (Figure 1 A). In Figure 1, the ovary of the control animal was compared with that treated with L-arginine (3 days of treatment with the lowest dose of L-arginine, 5 mg/kg), which significantly showed that the number of follicular cysts in the sample treated with L-arginine (low-dose, short-term) decreased compared to the control(P<0.05). Higher doses (25, 50 mg/kg), even in short time periods,did not have significant effect on cyst reduction.
3.2. Serological findings (levels of estrogen, albumin and gonadotropins) related to 3 to 21 days of treatment with L-arginine
In the shortest period (3-day treatment) at the lowest dose of L-arginine (5 mg/kg), the estrogen level increased significantly compared to the control group (P<0.0.5), but this effect was reversed by pre-injection of L-NAME (Figure 2). In the lowest dose of L-arginine (5 mg/kg), the albumin level did not change significantly in the periods of 3 to 5 days compared to the control group (Figure 3). The level of albumin under the doses of 5 and 25 mg/kg of L-arginine had a significant decrease compared to the control group during the 9 and 21-day period. The highest dose of L-arginine (50 mg/kg) showed a decreasing effect on albumin levels compared to the control group during 5, 9 and 21-day period. This reduction effect was stronger for the highest dose in the longest period and thus the effects were both dose and period dependent. Gonadotropins (FSH, LH) did not show significant changes compared to the control group without dependence on dose and period (Figure 4). The levels of these gonadotropins in the groups treated with L-arginine (5-50 mg/kg) in periods of 3 to 21 days did not show a significant decrease compared to the control group. In the groups where L-NAME was injected before L-arginine,no significant response was observed compared to the control group.
3.3. Findings of measurement of sex hormone binding protein in the ovary (albumin)
At the lowest doses of NO precursor L-arginine (5 mg/kg), the level of ovarian sex hormone binding protein increased compared to the control group (Figure 5), as obtained by specific staining with bromocresol purple. Immunohistochemistry score was compared between different groups. The percentage of postive staining was obtained as the difference between immunohistochemically positive areas and control areas. This study showed that only the 3-day treatment group had a significant difference (70%) compared to the control group.
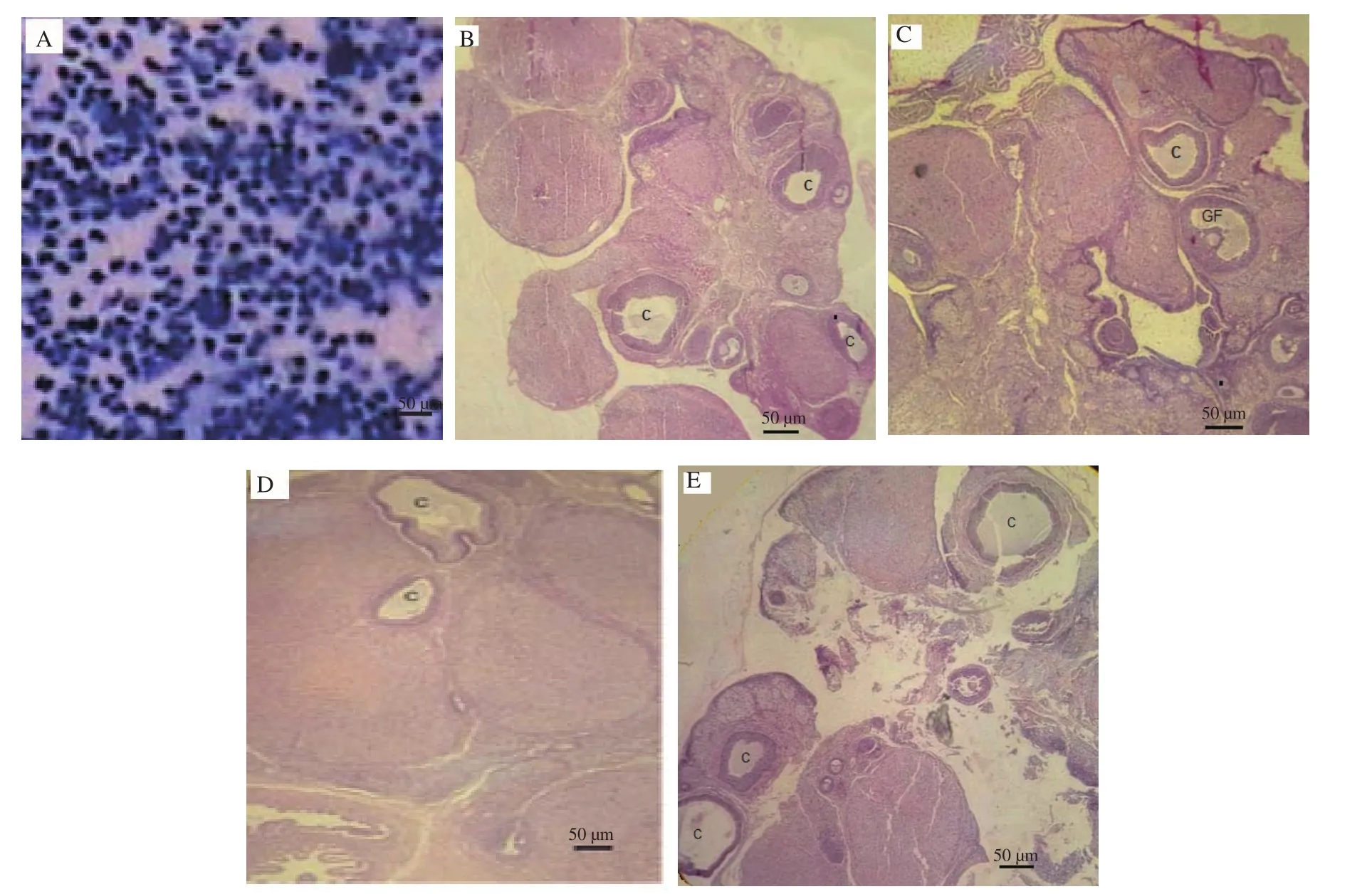
Figure 1. The pap smear shows that the control rat is in the diestrus (full of small leukocytes) stage (A). Hematoxylin-eosin staining reveals that the ovaries of the control animal have a number of marginal cysts (B). Normal follicular activity is prominently observed in the shortest period (3 days) with L-arginine treatment at a low dose (5 mg/kg), and the number of cysts is reduced (C). Higher doses (D: 25 mg/kg, E: 50 mg/kg) have no significant effect on cyst reduction, even in short periods. (Other periods are not shown due to lack of significant responses). C: Cyst; GF: Graafian follicle. Scale bar is 50 µm.
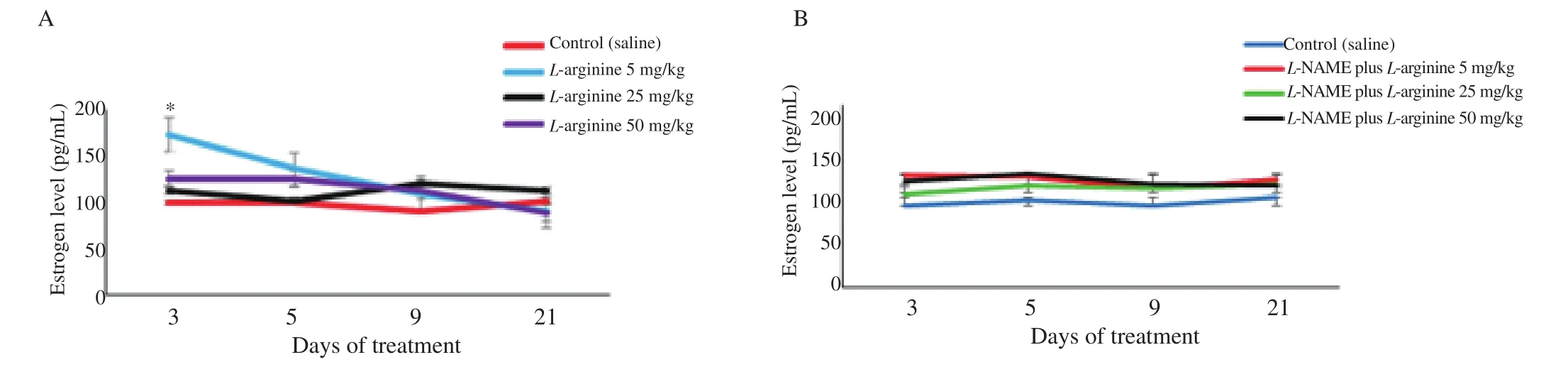
Figure 2. Level of estrogen in the control and L-arginine-treated rats (5-50 mg/kg) (A) and effect of L-NAME pretreatment on estrogen levels in L-argingine treated rats (B). Data are shown as mean±SD. *P<0.05 indicates a significant difference between the lowest dose group (5 mg/kg) and the control group in the 3-day period based on the post hoc test. Furthermore, L-NAME blocks this effect when injected before L-arginine. Comparison between other groups does not show any significant difference.
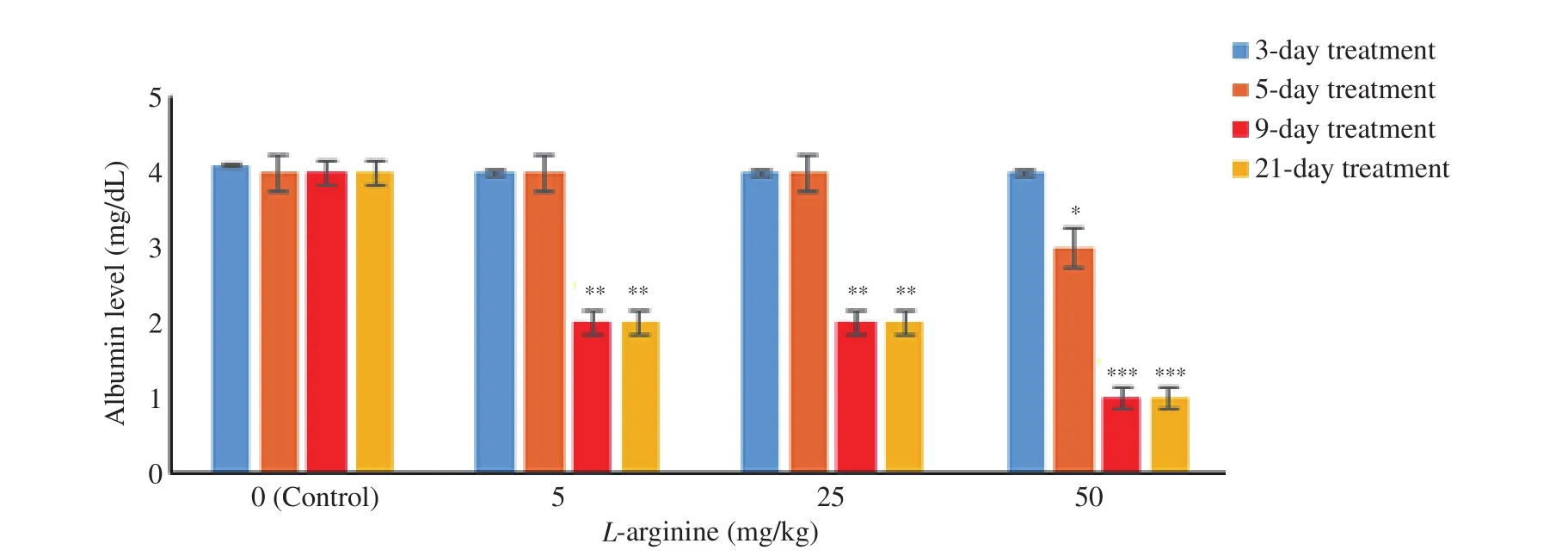
Figure 3. Level of albumin in the control and L-arginine-treated rats (5-50 mg/kg). Data are shown as mean±SD. *P<0.05, **P<0.01, and ***P<0.001 indicate a significant difference between the L-arginine dose groups used in the period of 3 days to 21 days and the control group based on the post hoc test.
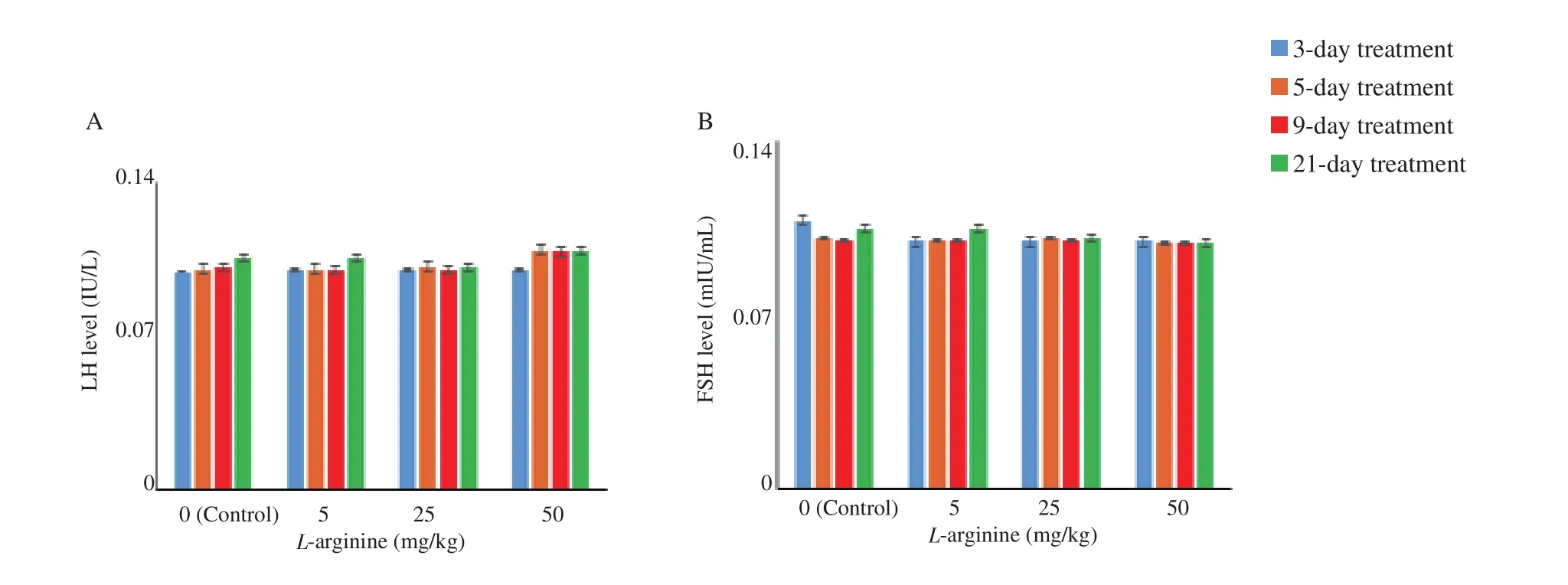
Figure 4. Level of luteinizing hormone (LH) and follicle stimulating hormone (FSH) in the control and L-arginine-treated rats (5-50 mg/kg). Data are shown as mean±SD. No significant difference is observed between the doses in the same period or between the doses of the 3-day to 21-day treatments and the control group. A: LH; B: FSH.
3.4. Findings on DAβH specific marking(immunohistochemistry)
At the lowest dose of L-arginine (5 mg/kg), the ovarian DAβH showed increased activation compared to the control group (Figure 6),which corresponded to enhanced levels of albumin. By calculating the immunohistochemistry score between different groups and examining percentage of postive staining as the difference between immunohistochemically positive areas and control areas, it was found that only the 3-day treatment group had a significant difference (78%) compared to the control group. Others did not show any significant difference compared to the control group.
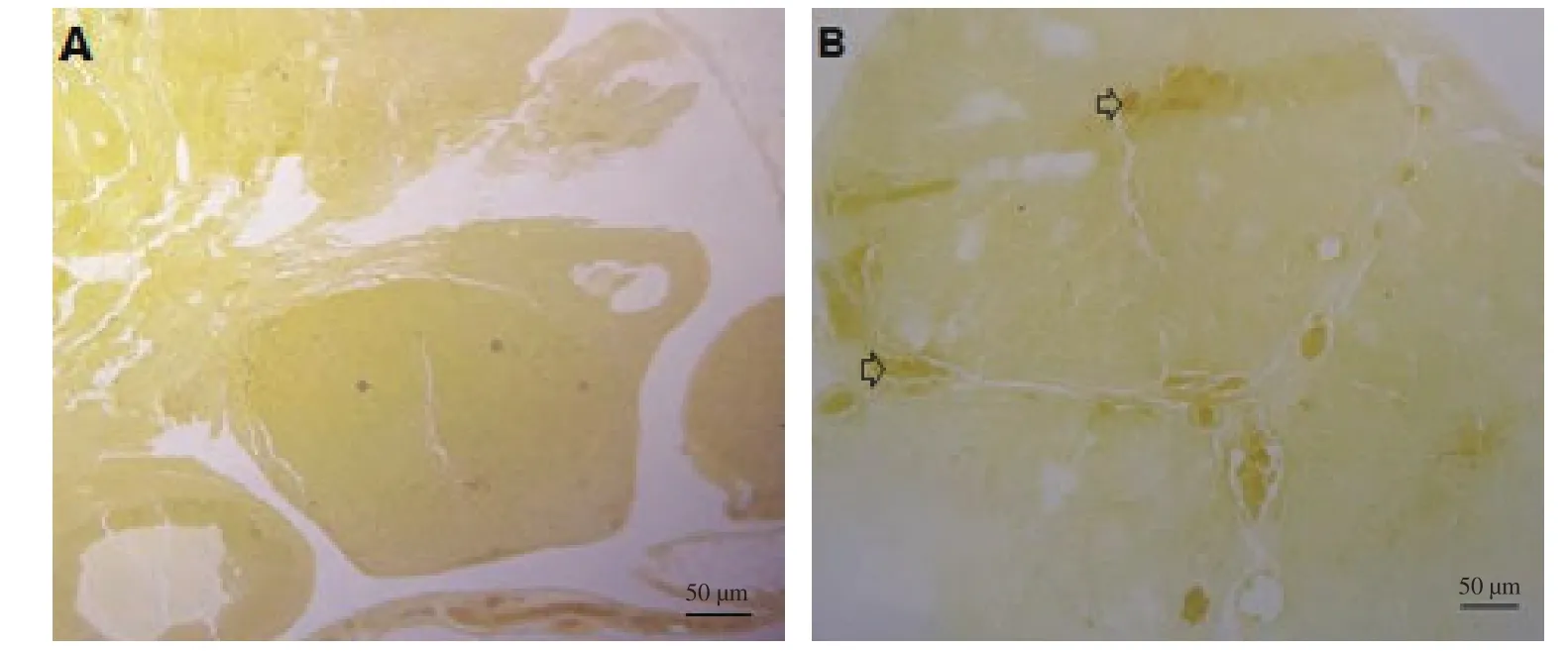
Figure 5. Result of staining by bromocresol purple. Ovarian sex binding element, especially in the stroma, shows an obvious increase with L-arginine (5 mg/kg) during the 3-day treatment period (B) compared to the control group (A). (The image of the group that is significantly different from the control group is shown, while other groups are not shown due to lack of meaningful responses). Arrows indicate ovarian albumin measured by bromocresol purple method. Scale bar is 50 µm.
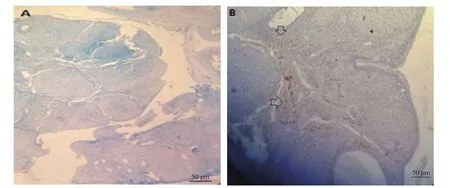
Figure 6. Immunohistochemical staining. Ovarian dopamine beta hydroxylase (DAβH) shows an obvious increase with L-arginine (5 mg/kg) during the 3-day treatment period (B) compared to the control group (A). (The image of the group that is significantly different from the control group is shown, while other groups are not shown due to lack of meaningful responses). Arrows indicate the positive immunoreaction of DAβH in the ovary. Scale bar: 50 µm.
4. Discussion
The study evaluated the effect of short-term and long-term injection of L-arginine in different doses on serum estrogen levels and its consequences. According to the results, the lowest dose of L-arginine in the shortest period, at the highest significant level, increased the level of estrogen and activated ovarian DAβH without having side effects on the level of serum albumin.
During reproductive aging, the ovaries mainly stop producing estrogen and progesterone, and ovulation does not occur, and levels of FSH and LH increase[20]. Decreased levels of estrogen and progesterone provide feedback in the pituitary gland to increase levels of FSH and LH[20]. As indicators of menopause, FSH levels are greater than 40 mIU/mL and estrogen levels are less than 32 pg/mL[21]. FSH stimulates primary follicles in the ovaries to begin to grow and progress to estrogen-producing secondary or antral follicles[22], and LH promotes follicle maturation and ovulation. The corpus luteum in the luteal phase of the menstrual cycle produces estrogen and progesterone[23]. These ovarian events stop at menopause, which is the period of ovarian depression. Also,at this stage, the ovaries become cystic[24,25]. Menopause in animals such as Wistar rats has human-like side effects[26]. So far, the most common treatment for menopausal problems has been estrogen therapy[27,28], which, of course, has side effects such as a high risk of cardiovascular disease[29], and that increases the serum concentration of cholesterol and triglycerides.
Estrogen also affects the activity of the NO system, which triggers the metabolism of free radical NO[30,31]. The gas molecule, NO, as a major endogenous mediator, is involved in many physiological and pathological phenomena in the body. Some authors have written that steroid hormones can regulate NO production in women and have shown that lower estrogen levels in postmenopausal women may reduce NO levels during this period[32]. Therefore, there is a relationship between estrogen and NO levels. Hence, can increasing NO levels increase the level of estrogen? In fact, with our results,this is possible. But by what mechanism? As our results show, there are two ways, one is to activate ovarian DAβH and the other is to maintain albumin.
L-arginine, based on previous history, is effective in restoring human health[33]. In addition, L-arginine has positive effects on estrogen levels[34-36]. Therefore, our aim was to use this amino acid in different amounts and in wide range period to treat postmenopausal problems in aged rats. L-arginine typically accounts for approximately 5%-7% of the amino acid content of a healthy adult diet. This means an average of 2.5-5.0 g/day, which meets only the minimum needs of the body for tissue repair, protein synthesis and immune cell maintenance[37]. Therefore, the minimum dose of 5 mg and more was chosen for our study that was conducted in an animal model.
We know that with age, female sex hormones such as estrogen decrease sharply, and this is one of the reasons for adverse outcomes such as ovarian cystogenesis, etc[38]. Estrogen, as one of the most important female sex hormones, plays an important role in the reproductive system. This hormone may activate the NO synthase system by activating the catecholamine system in the ovary.Noradrenergic agents, which are produced by DAβH enzyme, have been shown to regulate GnRH secretion, and norepinephrine has a modulatory role in LH secretion[39,40].
There is not much information about the regulatory effect of NO on the DAβH enzyme. A previous finding has indicated an inhibitory effect by studying the treatment of human neuroblastoma cells (SKN-MC) with diethylamine/NO. It reduced cellular DAβH activity without affecting their growth rate[41]. Authors have recently shown that the enzyme tyrosine hydroxylase can also be S-nitrosylated by NO. S-nitrosylation is a reversible change of cysteine residue in protein and is known as an emerging signaling mechanism mediated by NO and tyrosine hydroxylase can be S-nitrosylated at cysteine 279 by NO. These results provide a new mechanism of how tyrosine hydroxylase enzyme activity is modulated by NO through S-nitrosylation, which is consistent with the present study[42].
According to the results of this study, this enzyme (DAβH)is activated in the ovary with L-arginine (low-dose, and shorttreatment), which at least confirms the role of catecholamines in improving the condition (since the number of ovarian cysts decreased under this treatment and depressed ovaries returned to activity).
Activation of this enzyme (DAβH) leads to the activation of the ovaries and the production of sex hormones (increased estrogen levels), which requires an increase in the level of hormone carriers.But what effect did it have on gonadotropin levels? We did not have a negative effect, so the question is whether the level of ovarian estrogen in old age is a regulator factor or maybe another factor plays a role in this phenomenon? But which factor? Whether it is catecholamine or NO. As our data showed, increasing NO levels by short-term low-dose L-arginine treatment can terminate some postmenopausal problems.
Our results may provide significant support that this molecule is an essential regulator in ovarian steroidogenesis. NOS has previously been shown to be expressed in human granulosa-luteal cells and inhibits estradiol secretion[43]. Conversely, according to this study,L-arginine-induced NOS activation is likely responsible for increased levels of estrogen (not gonadotropic hormones). It is possible that this is an aging-related mechanism that needs to be elucidated.
Considering the lack of significant difference in the findings of 9 and 21 days, it can be stated that the results of this research show that the effects of frequent consumption of L-arginine follow a certain pattern after a certain period. This may indicate that long-term use of L-arginine can lead to stimulation of certain pathways, such as inflammatory pathways, which, contrary to many recommendations[44], strictly limit long-term use of L-arginine as a supplement. Inflammatory conditions include animal weight loss,ovarian size change (not shown), and ovarian cyst formation, which is beyond the scope of this article. It has previously been suggested that inflammatory processes may contribute to ovarian aging[45].Of course, more studies are needed to determine whether there is a causal relationship between inflammation and the frequency of L-arginine intake, which we suggest as a future study.
There are some limitations in this study. Because the amino acid L-arginine is involved in many metabolic pathways and serves as a precursor for the synthesis of polyamines, proline, glutamate,creatine, agmatine and urea[46], further studies are needed to provide a definitive view of the prophylactic effect of L-arginine on polycystic ovary syndrome (PCOS) due to increased ovarian DAβH activity by NO. NO levels decrease in postmenopausal women[47,48]due to decreased estrogen levels. Therefore, according to the findings of this research, the use of L-arginine in a low dose and in a short period of time can be suggested to improve estrogen levels, but whether this substance should be used alone or together with other supplements, this research currently cannot answer and needs further study.
In conclusion, L-arginine in low doses can increase estrogen levels and stimulate ovarian DAβH. Therefore, high levels of NO due to short-term treatment with low-dose L-arginine can reduce some postmenopausal problems.
Conflict of interest statement
There is no conflict of interest to declare.
Acknowledgements
The authors thank the Vice Chancellor for Research at Shahed University for supporting the educational programs.
Funding
The study received no extramural funding.
Authors’ contributions
Manizheh Karami proposed the research plan and designed the experiments. Fatemeh Lakzaei completed the research. Manizheh Karami and Fatemeh Lakzaei analyzed the data. Mohammadreza Jalali-Nadoushan assisted in laboratory and pathology tests.All authors reviewed and agreed the final manuscript prior to submission.
Publisher’s Note
The Publisher of the Journal remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Asian Pacific Journal of Reproduction2022年6期
Asian Pacific Journal of Reproduction2022年6期
- Asian Pacific Journal of Reproduction的其它文章
- Clinical pregnancy rate of women with unexplained infertility with or without cervical mucus aspiration before intrauterine insemination: A randomized controlled trial
- Prevalence and risks of reproductive tract infections among women of urban slums in North India: A cross-sectional study
- Awareness about transmission and preventive measures of COVID-19 from mother to child: A cross-sectional study among pregnant women
- Predictors of readiness for discharge in mothers of preterm infants: The role of stress,self-efficacy and perceived social support
- Exogenous gonadotropin releasing hormone (GnRH) modulates scrotal and testicular biometrics, libido, endocrinological and heamatological profiles in Ganjam goat under humid tropical coastal ecosystem of Odisha
- Ovarian hyperstimulation syndrome following the use of GnRH agonist trigger of final oocyte maturation and freeze-all strategy: A case report and review of the literature
