Exogenous gonadotropin releasing hormone (GnRH) modulates scrotal and testicular biometrics, libido, endocrinological and heamatological profiles in Ganjam goat under humid tropical coastal ecosystem of Odisha
Jibanjyoti Nayak, Anil Kumar Nahak, Purna Chandra Mishra, Dillip Kumar Karna, Chinmoy Mishra,Perumal Ponraj
1OUAT-College of Veterinary Science and Animal Husbandry, Bhubaneswar, Odisha, India
2ICAR-Central Island Agricultural Research Institute, Port Blair-744105, Andaman and Nicobar Islands, India
ABSTRACT
Objective: To assess the effect of exogenous gonadotropin releasing hormone (GnRH) in libido, scrotal and testicular biometrics,endocrinological and heamatological profiles of Ganjam goat buck during winter and pre-monsoon seasons.
Methods: Forty eight healty Ganjam goat bucks of 3-4 years old were equally divided into the control and treatment groups. The control group received distilled water as placebo, while the treatment group received GnRH injection (4 µg Buserelin acetate/mL) once a week for four months. Body weight, scrotal circumference and testicular parameters (testicular volume, testicular weight and testis index), endocrinological profiles [follicle stimulating hormone(FSH), luteinizing hormone (LH), and testosterone], sex behavioural profiles (libido score, mating ability score, and sex behavioural score) and heamatological profiles (red blood cells, white blood cells, haemoglobin, and packed cell volume) were estimated.
Results: The libido, scrotal and testicular biometrics, and endocrinological profiles significantly differed between the control and GnRH-treated bucks within and between the seasons within the two groups (P<0.05). Body weight, scrotal circumference, testicular volume, testicular weight, testis index, FSH, LH, testosterone,libido score, mating ability score, sex behavioural score, red blood cells, haemoglobin and packed cell volume were significantly higher in the GnRH-treated bucks compared to the control bucks in pre-monsoon and winter seasons (P<0.05). Body weight,scrotal circumference, testicular volume, testicular weight, testis index, FSH, LH, testosterone, libido score, mating ability score,sex behavioural score, red blood cell counts (in treatment), and haemoglobin were significantly higher in pre-monsoon compared to winter season in the experimental groups (P<0.05). The white blood cell counts neither differed between seasons nor between the two groups.
Conclusions: Exogenous GnRH supplementation and premonsoon season have higher beneficial effects in improvement of endocrinological profiles with cascading beneficial effects on scrotal circumference, testicular volume, testicular weight, and sex behavioural profiles, which in turn will improve the sperm production and its cryo-survivability and fertility rate in Ganjam goat.
KEYWORDS: Hormone; GnRH; Ganjam goat; Libido; Scrotal parameters; Testicular parameters
1. Introduction
Ganjam goat (Capra hircus; INDIA_GOAT_1500_GANJAM_06008), one important breed, is found in tropical humid coastal area of Southern Odisha and bordering of Andhra Pradesh,India. It is a dual purpose breed and is mostly used for meat and milk. Natural mating is a preferred breeding practice in the field;however, it is associated with many drawbacks including disease transmission, poor body confirmation of kids born from the natural mating of inbred parents and inbreeding depression; therefore,production and reproduction performances were decreased in Ganjam goat. Scrotal and testicular parameters are not only the critical components to evaluate the breeding soundness, but also help indirectly to measure the endocrinological profiles[1], spermatogenic capabilities[2], semen production capacity and birth weight of progeny in the breeding males[3].
The testes and scrotum are sensitive in the alteration of environmental temperature as either high or low temperature triggers the testicular degenerative changes; thereby, scrotal circumference,testicular volume, testicular weight are reduced and testicular consistency is altered in goats exposed to extreme summer or winter seasons[4]. Day length, temperature humidity index (THI) and rainfall are used to determine the seasonality and secretory pattern of reproductive and metabolic hormones[5]. Longer photoperiod and higher THI in summer compared to winter season cause considerable reduction of melatonin secretion (short day breeder); reduced concentration of melatonin stimulates increased prolactin secretion,and thereby prolactin inhibits the secretion of gonadotropin releasing hormone (GnRH), gonadotropins and gonadal hormones, which in turn adversely affects the testicular and scrotal development, libido and semen production profiles[6].Administration of GnRH analogue improved the libido and semen quality profiles in bubaline species[7]. Bulls provided with additional energy in the diet combined with weekly administration of GnRH had increased the testosterone level and scrotal circumference compared to those bulls that were fed with the additional energy only in the diet[8]. Bharath Kumar et al[9] observed higher testosterone in the bull calves after administration of GnRH. Breeding males require threshold level of testosterone to exhibit their sexual activity[10]and GnRH increases the plasma testosterone concentration through luteinizing hormone (LH) mediated stimulation in the Leydig cells. Hence, libido improvement in the poor libido males might have occurred due to higher testosterone following GnRH therapy.GnRH induces higher testosterone which is well documented.Testosterone has a significant effect on haematopoiesis through direct action on the bone marrow cells, especially on pluripotential hematopoietic stem cells[11]. Though the previous studies indicated that exogenous GnRH had improved the hormone profiles, scrotal and testicular biometrics and libido in different species (rat[12],goat[13,14], rabbit[15], sheep[16], camel[17], dog[18], bull[19], buffalo[20],stallion[21,22]), perusal of available literature on similar line of studies revealed no study reports were available in Ganjam goat. Therefore,the present study was designed to evaluate the effect of exogenous GnRH on scrotal and testicular attributes and their associated hormone profiles, sex behavioural scores and heamatological profiles in the Ganjam goat under humid tropical coastal ecosystem of Odisha, India.
2. Materials and methods
2.1. Area of the study
The present study was conducted during the period from January to April in the year 2020 and 2021 at ICAR-AICRP on Goat Improvement Organised Farm, Rambha, Ganjam District, Odisha,India which is located at 19.52°N 85.1°E with elevation of 87 m mean sea level. The study was conducted in two seasons, winter(January-February; THI: 73.06/day, sunshine hours: 9.14 h/day and rain fall: 16.13 mm) and pre-monsoon (March-April; THI:80.81/day, sunshine hours: 8.61 h/day and rain fall: 24.63 mm).
2.2. Animals
Forty eight healthy (body condition score: 3-4 out of 5, 1:extremely thin to 5: extremely fat, with 0.5 point-increment;classified as good) Ganjam buck of 3-4 years of age (average:22.80 months) were selected randomly from the flock. Bucks were maintained under uniform feeding, housing and other standard managemental practices as per the farm schedule. Male goats were equally divided into the control and treatment groups. Animals in the treatment received GnRH injection, 4 µg Buserelin acetate/mL once a week for two months in each season. The control animals received distilled water as placebo.
2.3. Collection of blood and estimation of experimental profiles
Blood samples were collected by venipuncture of jugular vein in heparin tubes (20 IU of heparin per mL of blood) from the experimental Ganjam goats at 4-hour interval throughout the day(one day; at 08:00, 12:00, 16:00, 20:00, 24:00, and 04:00 h) during the different seasons. Blood samples were collected on day 51 (21st February) of GnRH administration (day 1: 1st January; day 59:28th February) for the winter season and on day 51 (21st April) of GnRH administration (day 1: 1st March; day 61: 30th April) for the pre-monsoon season for two years (4 replicates) for analysis of endocrinological profiles as it was texted that the period required to form the sperm from spermatogonium A is 47.70 days in goat. The blood samples were centrifuged at 1 200×g for 15 min at 4 ℃. The plasma samples were separated rapidly, labelled properly and preserved at -80 ℃ in deep freezer for further endocrinological profiles analysis.
Red blood cells (RBC), haemoglobin, packed cell volume (PCV)and white blood cells (WBC) were estimated with the use of veterinary fully automatic hematological analyzer (Prokan, PE-6800). Follicle stimulating hormone (FSH; 500710; analytical sensitivity: 0.6 mIU/mL; intra- and inter-assay coefficients of variation: 6.78% and 9.65%, respectively), LH (500720; analytical sensitivity: 0.5 mIU/mL; intra- and inter-assay coefficients of variation: 6.55% and 9.87%, respectively) and testosterone (582701;analytical sensitivity: 6 pg/mL; intra- and inter-assay coefficients of variation: 5.84% and 9.34%, respectively) were estimated using commercially available enzyme-linked immunosorbent assay kits(Cayman Chemical Company, Ann Arbor, MI, USA) as per the instruction of manufacturer with use of 96-well clear polypropylene microplate using a MRC Microplate Reader (UT-2100C, Israel).
2.4. Measurement of scrotal circumference and testicular parameters
Testicular parameters and scrotal circumference were measured by using a caliper (Mitutoya Digimatic Caliper, Japan) and a measuring tape following proper restraining of the buck[23]. Testicular volume was estimated by using the following formula for volume of an ellipsoid as described by Mbaeri et al[24] (4/3πabc, a=thickness/2;b=width/2 and c=length/2). Weight of the testes was calculated by multiplying 1.038 with volume as 1.038 is the approximate density of testicular tissue[25]. Testis index was calculated from testis volume and body weight of the bucks as testis index (cm3/kg): testis volume (cm3)/body weight (kg). Scrotal circumference and testicular parameters were measured by the same operator at winter and premonsoon seasons for the control and treatment groups.
2.5. Sex behavioural profiles
Sex behavioural profiles such as libido score, mating ability score and sexual behavior score of Ganjam buck were estimated as described by Ponraj et al[26].
2.6. Statistical analysis
To determine any possible differences in the observed body weight,scrotal circumference, testicular volume, testicular weight, testis index, FSH, LH, testosterone, libido score, mating ability score,sex behavioural score, RBC, haemoglobin and PCV with respect to between seasons within the same experimental group and between the two experimental groups within the season, repeated measures of two-way analysis of variance (ANOVA) was applied using PROC GLM multivariate model of Statistical Analysis Software (SAS,Version 9.3.1; SAS Institute, Inc., Cary, NC, 2011) and for multiple comparison, Tukey test was applied for the experimental parameters.The data used in the study was tested for normality before analysis using Shapiro Wilk statistics and the outliers were removed.All the data was normally distributed. Data were expressed as mean±standard deviation (mean±SD). Differences were considered significant if P<0.05.
2.7. Ethics statement
The experimental procedures were approved by the Institutional Animal Ethics Committee of OUAT-College of Veterinary Science and Animal Husbandry, Bhubaneswar, Odisha, India with approval No. 18192C04/2020. All these animal experiments were performed according to the International Guidelines on Ethical Use of Animals.
3. Results
3.1. Body weight, scrotal circumference and testicular biometrics
Body weight, scrotal circumference, testicular volume, testicular weight and testis index of the bucks were significantly higher in the GnRH-treated bucks compared to the control bucks in premonsoon and winter seasons (P<0.05). Body weight, scrotal circumference, testicular volume, testicular weight and testis index were significantly higher in pre-monsoon compared to winter season in the control and GnRH-treated bucks (P<0.05) (Figure 1; Figure 2).
3.2. Endocrinological profiles
FSH, LH and testosterone concentrations were significantly higher in the GnRH-treated bucks compared to the control bucks in premonsoon and winter seasons (P<0.05). FSH, LH and testosterone were significantly higher in pre-monsoon compared to winter season in the control and GnRH-treated bucks (P<0.05) (Figure 3).
3.3. Sex behavioural profiles
Sex behavioural profiles such as libido score, mating ability score,and sex behavioural score were significantly higher in the GnRH-treated bucks compared to the control bucks in pre-monsoon and winter seasons (P<0.05). Libido score, mating ability score, and sex behavioural score were significantly higher in pre-monsoon compared to winter season in the control and GnRH-treated bucks(P<0.05) (Figure 4).

Figure 1. Effect of exogenous GnRH on body weight (A), scrotal circumference (B) and testis index (C) of buck in winter and pre-monsoon seasons. Winter:January-February and pre-monsoon: March-April. Vertical bar on each point represents standard deviation of mean. Vertical bar with capital letters (A, B)indicates significant difference between seasons within the group (P<0.05) and small letter (a, b) indicates significant difference between the control and treatment groups within the season (P<0.05).
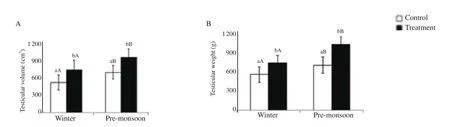
Figure 2. Effect of exogenous GnRH on testicular volume (A) and testicular weight (B) of buck in winter and pre-monsoon seasons. Winter: January-February and pre-monsoon: March-April. Vertical bar on each point represents standard deviation of mean. Vertical bar with capital letters (A, B) indicates significant difference between seasons within the group (P<0.05) and small letter (a, b) indicates significant difference between the control and treatment groups within the season (P<0.05).
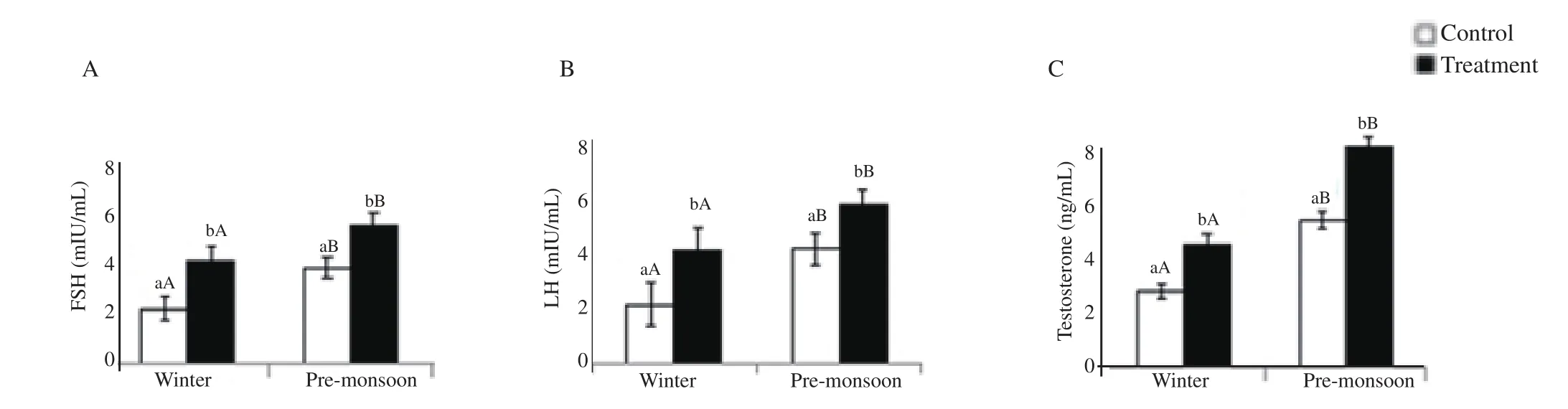
Figure 3. Effect of exogenous GnRH on follicle stimulating hormone (FSH; A), luteinizing hormone (LH; B) and testosterone (C) of buck in winter and pre-monsoon seasons. Winter: January-February and pre-monsoon: March-April. Vertical bar on each point represents standard deviation of mean. Vertical bar with capital letters (A, B) indicates significant difference between seasons within the group (P<0.05) and small letter (a, b) indicates significant difference between the control and treatment groups within the season (P<0.05).
3.4. Heamatological profiles
RBC, haemoglobin and PCV were significantly higher in the GnRH-treated bucks compared to the control bucks in pre-monsoon and winter seasons (P<0.05). RBC (in treatment), and haemoglobin(in treatment and control) were significantly higher in pre-monsoon compared to winter season in the two experimental groups (P<0.05).The WBC neither differed between seasons nor between the two experimental groups (Figure 5).
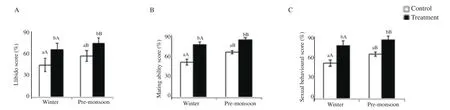
Figure 4. Effect of exogenous GnRH on libido score (A), mating ability score (B) and sexual behavioural score (C) of buck in winter and pre-monsoon seasons. Winter: January-February and pre-monsoon: March-April. Vertical bar on each point represents standard deviation of mean. Vertical bar with capital letters (A, B) indicates significant difference between seasons within the group (P<0.05) and small letter (a, b) indicates significant difference between the control and treatment groups within the season (P<0.05).
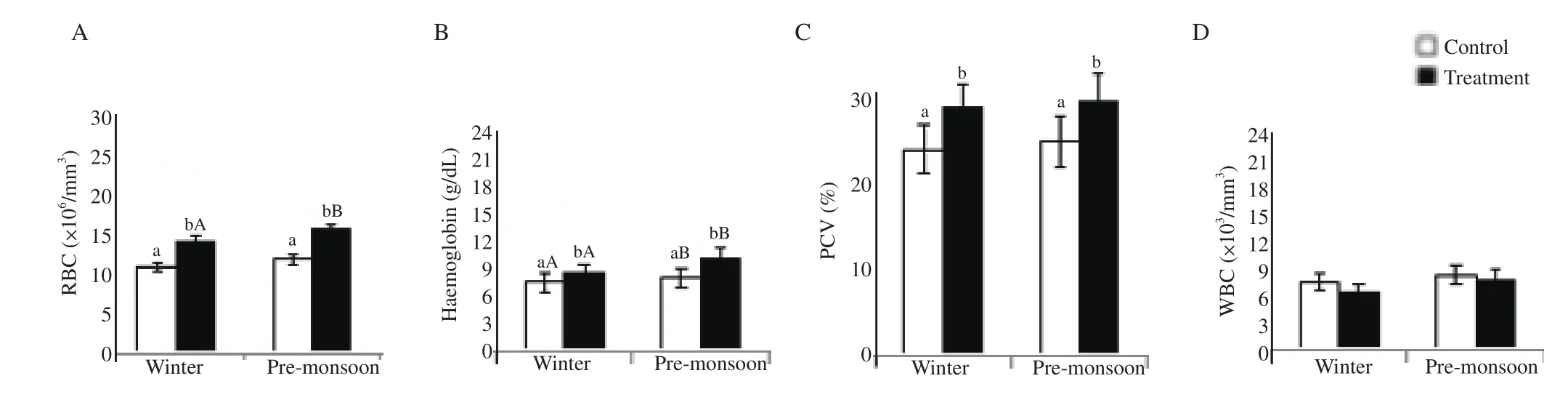
Figure 5. Effect of exogenous GnRH on haematological profiles of buck in winter and pre-monsoon seasons. Winter: January-February and pre-monsoon:March-April. Vertical bar on each point represents standard deviation of mean. Vertical bar with capital letters (A, B) indicates significant difference between seasons within the group (P<0.05) and small letter (a, b) indicates significant difference between the control and treatment groups within the season (P<0.05). A: red blood cell counts, RBC; B: haemoglobin; C: packed cell volume, PCV; D: white blood cell counts, WBC.
3.5. Interaction of season and GnRH on bucks
Season × treatment interactions were statistically different for scrotal circumference, testosterone and haemoglobin (P<0.05), while difference was found between the treatment groups for body weight,scrotal circumference, testicular volume, testicular weight, testis index, FSH, LH, testosterone, libido score, mating ability score, sex behavioural score, RBC, and haemoglobin (P<0.05) and between the seasons for body weight, scrotal circumference, testicular volume,testicular weight, testis index, FSH, LH, testosterone, libido score,mating ability score, sex behavioural score, RBC (in treatment), and haemoglobin in Ganjam goat bucks (P<0.05).
4. Discussion
Beneficial effects of exogenous GnRH on endocrinological profiles,scrotal and testicular biometrics and libido were studied in different species (rat[12]; goat[13,14]; rabbit[15]; sheep[16]; camel[17]; dog[18];bull[19]; buffalo[20]; stallion[21,22]), but similar line of investigations were lacking in Ganjam buck in tropical humid coastal ecosystem of Odisha, India. However, in our study, the GnRH enhanced the gonadotropins, gonadal hormones, scrotal and testicular biometrics,libido and haematological profiles in Ganjam goat. Similar reports were documented in bubaline species that GnRH analogue administration has significantly improved the endocrinological profiles, libido and semen quality profiles[7]. In another study,GnRH administration at weekly interval has significantly enhanced the scrotal circumference and gonadal androgen concentration in bovine species[8]. Similarly, Bharath Kumar et al[9] also documented that significantly higher testosterone concentration was obtained in the bovine species after administration of exogenous GnRH.In general, breeding males require optimum or threshold level of testosterone to exhibit their libido or sexual activity[10] and the GnRH supplementation increases the plasma testosterone through LH mediated stimulation of Leydig cells in the testis. Hence, libido improvement in poor libido males might have occurred due to higher testosterone secretion in the GnRH administered males. GnRH stimulates higher testosterone secretion. Funabashi et al[12] reported that subcutaneous GnRH injection (450 ng/100 g body weight) in male rats has not only increased the scrotal circumference, testicular volume and testicular weight, but also enhanced the libido, FSH,LH and testosterone concentration. Similarly, Wagner and Claus[27]also documented that immunization against GnRH has declined the testicular weight (67.0%) and area of tubuli (60.6%). Additionally,Leydig cell size was remarkably reduced in the GnRH antagonist treated boars. In goat bucks, daily administration of GnRH analogue during non-breeding season increased the serum testosterone concentration[13] and testicular length, width, total testicular volume and testicular weight and scrotal circumference[14]. Similarly,intramuscular injection of GnRH (20 mg) has increased the libido in New Zealand White rabbit bucks[15]. On the other hand, single intravenous injection of GnRH (0.042 mg) in aged stallion during breeding season has not shown significant improvement in the libido parameters compared to the control animals[28].
Trigg and Yeates[29] and Junaidi et al[30] documented that GnRH implantation triggered an acute increase of testosterone concentrations within 60-120 min; however, the testosterone was undetectable in the majority of dogs at day 12-17 of post implantation. Slow releasing subcutaneous GnRH implant has slightly increased the testosterone level on day 47 and became significantly higher on day 71 of implantation in Awasi rams[16].Similarly, AL-Ameri et al[31] found that intramuscular injection of GnRH in Cyprus bucks during breeding and non-breeding seasons induced higher plasma testosterone concentration at 20, 30, 40 and 50 min after treatment. Giriboni et al[14] also documented that bucks treated subcutaneously with Deslerolin had higher serum testosterone concentration and this higher testosterone concentration was observed during the first hours after its administration. The administration of 100 µg of Gonadorelin (GnRH analogue) has temporarily increased the blood testosterone concentration in equine species to stimulate the libido and mating ability[21,22]. A similar observation was reported in the Ganjam goat in the present study.
GnRH is secreted in the hypothalamus and entered into anterior pituitary through hypothalamic-hypophyseal portal system, where it induces the release of two pituitary gonadotropins namely, LH and FSH. LH acts on the Leydig cells to stimulate testosterone production and FSH targets the Sertoli cells in the seminiferous tubules for spermatogenesis. FSH and LH act together in synergy with testosterone for the production of regulatory molecules and nutrients that are required for the maintenance of spermatogenesis and stimulation of the development of the reproductive system.Testosterone has considerable effects on haematopoiesis through two factors. First, the erythropoietin arrives from the kidneys and shows considerable response in erythropoiesis[32]. The another factor is direct effect of androgens or their metabolites on the bone marrow cells through the stimulant effect on pluripotential or hematopoietic stem cells[11]. Further, it was reported that androgens promote the differentiation of erythroid colony-forming units into erythropoietinresponsive cells. Hemoglobin synthesis is enhanced via increases in intestinal iron absorption and the incorporation of iron into red blood cells following testosterone secretion and release. Inverse relationship between testosterone and leukocyte count was reported in other species[33]. Therefore, GnRH induced testosterone has reduced the concentration of total white blood cells in the present study.Thus, the GnRH administration stimulates testosterone production which in turn acts on the hematopoietic system to stimulate the production of blood cells. Similar results were observed in the present study in which GnRH treatment enhanced the blood profiles in the Ganjam goat.
However, the present study has some limitations. In this study, we examined the effect of GnRH on scrotal and testicular biometrics,endocrinological and haematological profiles and sex behavioural profiles. We used only one group of GnRH (4 µg Buserelin acetate/mL). Therefore, we need to conduct further studies on effect of different doses of GnRH on scrotal and testicular biometrical and endocrinological profiles to select more suitable or optimum dosage for Ganjam goat to get higher values in the selected experimental parameters. Further, for GnRH effect on the semen production and its quality profiles, in-vitro or in-vivo fertility trials are needed to be conducted to confirm the present findings.
In conclusion, exogenous GnRH and pre-monsoon season have significantly higher testosterone secretion resulting in escalated scrotal circumference and testicular biometrics and sex behavioural profiles in Ganjam buck. However, further study is needed to analyse the possible associations between hormone production,sex behavioural profiles, semen production and fertility profiles in Ganjam buck during different seasons to confirm the present findings.
Conflict of interest statement
Authors declare that there is no conflict of interest involved in the present work.
Acknowledgements
The authors are thankful to AICRP on goat improvement, ICAR,New Delhi for providing necessary facilities to carry out this research.
Funding
The study received no extramural funding.
Authors’ contributions
Jibanjyoti Nayak and Anil Kumar Nahak contributed to conceptualization; Dillip Kumar Karna and Chinmoy Mishra contributed to data curation; Dillip Kumar Karna and Chinmoy Mishra contributed to formal analysis; Jibanjyoti Nayak, Anil Kumar Nahak and Purna Chandra Mishra contributed to investigation;Jibanjyoti Nayak, Anil Kumar Nahak and Purna Chandra Mishra contributed to methodology; Anil Kumar Nahak and Purna Chandra Mishra contributed to project administration; Perumal Ponraj contributed to original draft writing, review and editing.
Publisher’s Note
The Publisher of the Journal remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Asian Pacific Journal of Reproduction2022年6期
Asian Pacific Journal of Reproduction2022年6期
- Asian Pacific Journal of Reproduction的其它文章
- Ovarian hyperstimulation syndrome following the use of GnRH agonist trigger of final oocyte maturation and freeze-all strategy: A case report and review of the literature
- L-arginine alleviates postmenopausal complications in female rats by stimulating ovarian dopamine beta hydroxylase
- Predictors of readiness for discharge in mothers of preterm infants: The role of stress,self-efficacy and perceived social support
- Awareness about transmission and preventive measures of COVID-19 from mother to child: A cross-sectional study among pregnant women
- Prevalence and risks of reproductive tract infections among women of urban slums in North India: A cross-sectional study
- Clinical pregnancy rate of women with unexplained infertility with or without cervical mucus aspiration before intrauterine insemination: A randomized controlled trial
